Summary
Stress is a widely recognized risk factor for psychiatric and metabolic disorders. A number of animal models utilizing various stressors have been developed to facilitate our understanding in the pathophysiology of stress-related dysfunctions. The most commonly used chronic stress paradigms include the unpredictable chronic mild stress paradigm, the social defeat paradigm and the social deprivation paradigm. Here we assess the potential of social crowding as an alternative chronic stress model to study the effects on affective behaviors and metabolic disturbances. Ten-week-old male C57BL/6 mice were housed in groups of four (control) or eight (social crowding; SC) in standard cage for 9 weeks. Exploration, anxiety- and depressive-like behaviors were assessed in the open field test, the elevated T-maze, the novelty-suppressed test, and the forced swim test. SC mice exhibited a modest anxiety-like phenotype without change in depressive-like behaviors. Nine weeks of social crowding did not affect the body weight, but robustly increased adiposity as determined by increased mass of fat depots. Consistent with the increased fat content, serum leptin was markedly elevated in the SC mice. Specific changes in gene expression were also observed in the hypothalamus and the white adipose tissue following SC housing. Our study demonstrates the potential of social crowding as an alternative model for the study of stress-related metabolic and behavioral dysfunctions.
Keywords: C57BL/6 mice, social stress, obesity, anxiety, white adipose tissue (WAT), Crh, Npy, Sgk1, Ucp2, Adrb1
Introduction
Chronic stress has been shown to affect metabolic, endocrine and psychological functions both in animal studies and in human population-based and clinical studies (McEwen, 2000; de Kloet et al., 2005; Block et al., 2009; De Vriendt et al., 2009; Wallis and Hetherington, 2009; Koolhaas et al., 2011). Substantial evidence indicates that chronic stress is a risk factor for affective and metabolic disorders (McEwen et al., 2012; Hunter and McEwen, 2013; Sinha and Jastreboff, 2013). In a Finnish study by Marniemi et al. (2002), monozygotic twins discordant for obesity were assessed for differences in hormonal, physiological and psychological parameters. Notably, the obese co-twins showed higher index of psychosocial stress perception compared to their lean co-twins (Marniemi et al., 2002). In humans, the effect of stress on feeding and body weight appears to be bidirectional (Gibson, 2006; Serlachius et al., 2007; Torres and Nowson, 2007; Block et al., 2009). Why some people lose and others gain weight in response to stress is not fully understood and is likely to involve many factors (Stone and Brownell, 1994; Epel et al., 2004). One explanation is the balance between an increase in β-adrenergic activation, the body’s main fat-burning mechanism (leading to weight loss), and the increased intake of sugar- and fat-rich comfort foods (resulting in weight gain) (Dallman et al., 2003; Dodt et al., 2003; Kuo et al., 2008). The observation that those who are initially overweight are more inclined to increase body weight when stressed whereas those who are of normal- or underweight do not led Dallman to propose that difference in metabolic outcomes might be the results of higher insulin concentration in people with higher body mass index (Dallman, 2010).
Similar to humans, animal models of chronic stress have produced variable and even opposite phenotypes of food intake, body weight gain and adiposity. Several studies described a pronounced anorexic phenotype following repeated stress exposure. Using an unpredictable chronic mild stress (UCMS) model, Michel and colleagues (2005) observed reduced weight gain and adiposity in stressed mice on a high-fat diet (Michel et al., 2005). A similar observation was made by Kim et al. (2003) with rats subjected to eight weeks of UCMS (Kim et al., 2003). Other types of chronic stressors, such as repetitive daily restraint, turpentine abscess, surgical stress and immobilization have also been reported to reduce food intake in rodents (Marti et al., 1994; Weninger et al., 1999). On the other hand, several studies also demonstrated that chronic psychosocial stress models such as the social defeat/sensory contact model, lead to hyperphagia and increased body weight gain and adiposity (Bhatnagar and Vining, 2003; Moles et al., 2006; Bartolomucci et al., 2009). In Syrian hamsters, social crowding (for female) and intermittent or chronic social defeat and footshock (for male) also lead to increases in food intake, body mass, and adiposity (Borer et al., 1988; Meisel et al., 1990; Foster et al., 2006; Solomon et al., 2007). Long-term social isolation has also been shown to accelerate body weight gain and adiposity in mice, although this effect appears to be strain-dependent (Nonogaki et al., 2007). Chronic stress was also found to increase consumption of more palatable food, which are typically high in fat and/or sugar content (Gibson, 2006; Zellner et al., 2006; O’Connor et al., 2008; Roberts et al., 2013) and aggravate diet-induced obesity (Kuo et al., 2007; Kuo et al., 2008). Studies of mice subjected to chronic social defeat stress identified ghrelin signaling in catecholaminergic neurons as a critical mechanism for stress-induced food-reward behavior and the associated body weight gain (Lutter et al., 2008; Chuang et al., 2011; Patterson et al., 2013). However, there were also reports of a lack of effect or even reduced body weight gain in stressed mice fed high-fat diet (Michel et al., 2005; Bartolomucci et al., 2009; Finger et al., 2012).
The highly variable metabolic phenotypes observed in the different studies have been attributed to differences in the types of stressors, diets, protocol durations, strains of animals and stress intensities. The variability also reflects the complexity of the interaction between stress and metabolic processes and highlights the importance of the development and thorough characterization of different stress models. In this study, we used high-density living as a mild form of chronic social stress due to overcrowding. We assessed the affective behaviors and body composition changes following one and two months of social crowding, respectively.
Materials and Methods
Animals
Ten weeks old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were housed in groups of four (control; 3 cages) or eight (social crowding; 2 cages) in standard cages (31 cm × 17 cm × 14 cm) for 9 weeks. All mice were kept under a 12h light/dark cycle (lights on at 0600 hr), with free access to water and standard chow diet (11% fat, 28% protein, 61% carbohydrate, caloric density 3.4 kcal/g, Research Diets). Mice were transferred to clean cages when the bedding became too soiled, which were 1–2 weeks for the control group and twice weekly for the social crowding (SC) group. Mice were weighed at week 1, 4 and 8. All use of animals was approved by the Ohio State University Animal Care and Use Committee, and was in accordance with the NIH guidelines.
Behavioral analysis
After 1 month of housing in the different conditions, mice were subjected to behavioral testing in the following order: (1) open field test on day 30, (2) elevated T-maze on day 35, (3) forced swim test on day 37, and (4) novelty suppressed feeding on day 40. All tests were conducted during the light phase.
Open field test (OF)
To assess exploration and general motor activity, mice were placed individually into the center of an open square arena (60 cm × 60 cm, enclosed by walls of 48 cm). Each mouse was allowed 10 minutes in the arena, during which time its activity was recorded and analyzed by TopScan (Clever Sys Inc, Vienna, VA, USA). Specifically, the parameters measured include distances traveled in the periphery and in the center of the arena (36 cm × 36 cm), the total distance traveled, and the time spent in the center of the arena. The total distance traveled provides a measure of exploratory activity while the time and distance ratio of arena center exploration provide an indication of anxiety level. In addition, the number of fecal boli was counted as an additional measure of physiological response to anxiety. The arena was cleaned with 30% ethanol between trials to remove any odor cues.
Elevated T-maze test (ETM)
The elevated T-maze is an ethologically based approach-avoidance conflict test targeting the natural conflict between the tendency of mice to explore a novel environment and the tendency to avoid a brightly lit open area. The T-maze consists of two open arms (30.5 cm × 15.5 cm) and an enclosed arm (46 cm × 10 cm) positioned in the shape of a ‘T’, with the enclosed arm as the stem of the T. The whole apparatus was elevated 88cm above the floor. The open and enclosed arms of the T-maze generate exploratory behavior and the avoidance of elevated open arms is considered a reflection of anxiety state. Each mouse was placed at the end of the closed arm facing toward the open arms and was allowed to explore the maze for 5 minutes. The behavior and movement of each mouse was recorded by a video camera and subsequently scored by an experimenter blinded to the experimental groups. Anxiety was indicated by the time spent on the open arms as well as the number of open arm entries. After each test, the mouse was returned to its home cage and the apparatus was cleaned with 30% ethanol.
Forced swim test (FST)
Forced swim test is one of the most commonly used rodent behavioral tests for screening antidepressant drugs (Cryan and Mombereau, 2004). Mice were placed individually in a transparent cylinder (21 cm diameter, 24 cm height) containing water (25 ± 2°C) to a depth of 15 cm for 6 minutes. At the end of each trial, mice were dried and returned to their home cage. Trials were video-recorded and a blinded experimenter scored the amount of time mice remained immobile as a measure of depressive-like behavior.
Novelty suppressed feeding test (NSF)
NSF assesses hyponeophagia, in which exposure to a novel environment suppresses feeding behavior (Samuels and Hen, 2011). NSF has been used to study anxiety- and depression-related behaviors since it is sensitive to anxiolytic and chronic antidepressant treatments. Mice were fasted overnight, with food removed at 1700 hr. The testing phase was conducted the next morning at 1000 hr. Mouse was individually placed into a brightly lit, novel open cage (40 cm × 28 cm × 20 cm). A piece of white filter paper (7 cm diameter) was placed in the center of the cage with a single pre-weighed food pellet. The latency to consumption (first bite of the food pellet) was measured. The cut-off time was 10 min. To assess if there was any difference in consummatory drive, each mouse was placed in a standard cage with the pre-weighed food pellet after its first bite or at cut-off time if it failed to eat within 10 min. The amount of food consumed in 5 min was calculated.
Metabolic chamber analysis
Following eight weeks of social crowding (or control housing), a subset of mice (n = 8 per group) was individually placed in the monitoring chambers of the Oxymax Lab Animal Monitoring system (Columbus Instruments, OH) for 3 days. Following at least 24 hours of acclimatization in the chambers, measurements of food intake, activity, heat generated and respiratory exchange ratio (RER) were recorded from day 2 to day 3.
Euthanization and tissue collection
Nine weeks after the start of SC housing, mice were culled by decapitation under isoflurane anesthesia. Trunk blood was collected and serum was isolated by centrifugation and stored at −20°C until assayed. Brown adipose tissue (BAT), inguinal (WATi), retroperitoneal (WATr), and epididymal (WATe) white adipose tissue were dissected and weighed to determine body composition and adiposity. WAT depots were collected from one side only. In addition, the hypothalamus was dissected out using a mouse brain matrix and stored at −80°C until RNA extraction. For a subset of mice, WAT was stored fresh frozen at −80°C until RNA extraction, while the rest was immersed in 4% paraformaldehyde and paraffin-embedded for subsequent morphological analysis.
WAT staining and adipocyte size analysis
Paraffin-embedded WAT was cut into 20μm sections using a cryostat and mounted on slides. Slides were stained with hematoxylin and eosin (H&E) to examine the degree of adipocyte hypertrophy. Adipocyte diameter was measured using the Quick Measure Circle Command in Stereo Investigator 7 (MBF Bioscience, Willeston, VT) using a 10x objective. Software generated 150μm grids were drawn over the entire section; the cells within every other grid were circled to estimate diameter of the circle, excluding any incomplete cells. At least 10 cells per section were measured and then averaged.
Serum biomarker analysis by ELISA
Serum was analyzed for leptin, adiponectin and IGF-1 using DuoSet ELISA Development Systems (R&D Systems, Minneapolis, MN, USA) according to manufacturer’s instruction. Serum corticosterone level was determined using Enzyme Immunoassay Kit at 1:200 dilution according to the manufacturer’s instruction (Assay Designs, Inc., Ann Arbor, MI, USA).
In a separate experiment (n = 8 mice per group), blood was collected by submandibular bleeding and serum was isolated by centrifugation for analysis of corticosterone at the early time-points of social crowding. Blood sample was taken at 1900 h (1 hour after light off) one week after social crowding began. Another blood sample was taken at 1000 h (during the light phase) following a 2 week recovery period. Serum corticosterone level was determined as described above.
Quantitative RT-PCR
Total RNA from hypothalamus and WATr tissue was isolated using RNeasy Mini Kit or RNeasy Lipid Mini Kit (Qiagen, Valencia, CA, USA), respectively, according to the manufacturer’s instruction. First-strand cDNA was generated using TaqMan Reverse Transcription Reagent (Applied Biosystems, Roche, Branchburg, NJ, USA) and quantitative PCR was carried out using a LightCycler Sequence Detection System (Roche, Indianapolis, IN, USA) with the Power SYBR Green PCR Master Mix (Applied Biosystems). Specific primers that have been previously validated (Cao et al., 2009; Cao et al., 2010b; Cao et al., 2011; Lin et al., 2011; Liu et al., 2014; McMurphy et al., 2014) were used to detect the following mouse mRNA: Actb (beta-actin), Crh (corticotropin-releasing hormone), Crh1r (corticotropin-releasing hormone receptor 1), Crh2r (corticotropin-releasing hormone receptor 2), Npy (neuropeptide Y), Npy1r (neuropeptide Y receptor Y1), Npy2r (neuropeptide Y receptor Y2), Vgf (nerve growth factor inducible), Avp (arginine vasopressin), Avpr1a (arginine vasopressin receptor 1A), Sgk1 (serum/glucocorticoid regulated kinase 1), Nr3c1 (nuclear receptor subfamily 3, group C, member 1, also known as glucocorticoid receptor), Nr3c2 (nuclear receptor subfamily 3, group C, member 2, also known as mineralocorticoid receptor), Ucp1 (uncoupling protein 1), Ucp2 (uncoupling protein 2), Ucp3 (uncoupling protein 3), Adrb1 (adrenoceptor beta 1), Adrb2 (adrenoceptor beta 2), Adrb3 (adrenoceptor beta 3), Lep (leptin), Pparg (peroxisome proliferator-activated receptor gamma), Fasn (fatty acid synthase), Gpat (glycerol-3-phosphate acyltransferase), Pomc (proopiomelanocortin), Agrp (agouti-related peptide), Mc4r (melanocortin 4 receptor). Specific primers were checked against the NCBI nucleotide database for specificity. All primer pairs generated a single melt curve product. Primer sequences are provided in Table 1. PCR data analysis was performed using the comparative 2−ΔΔCT method with Actb as endogenous reference (Schmittgen and Livak, 2008).
Table 1.
Primer sequences.
| Gene | Primer | Primer sequence |
|---|---|---|
| Actb | Forward | ACCCGCGAGCACAGCTT |
| Reverse | ATATCGTCATCCATGGCGAACT | |
| Crh | Forward | TGGCCCCAAGGAGGAAA |
| Reverse | CCACTGCAGCTCCAAATAAAAA | |
| Crh1r | Forward | TCCGCTACAACACCACAAACA |
| Reverse | TCCTGGCACTCAGAATAATTCACA | |
| Crh2r | Forward | CCGAGTACTTCAATGGCATCAA |
| Reverse | CCCGTTCTCCAGGCACTCT | |
| Npy | Forward | CTCCGCTCTGCGACACTACA |
| Reverse | AGTGTCTCAGGGCTGGATCTCT | |
| Npy1r | Forward | GCATATGACAAAGAGTTTTACATTGTGTT |
| Reverse | GGTGGTGACTGCTTTTGAAATGA | |
| Npy2r | Forward | TTGCTTGAAATTCCTGGATTCC |
| Reverse | CCAGTTCACTCTCACTTGGCTGTA | |
| Vgf | Forward | GGGCGCCCCGATGT |
| Reverse | TCAGCTACCTGCCCATTATGC | |
| Avp | Forward | CGCTCTCCGCTTGTTTCCT |
| Reverse | TGGGCAGTTCTGGAAGTAGCA | |
| Avpr1a | Forward | GTTTGGACCGATTCCGAAAA |
| Reverse | CAGCTGTTCAAGGAAGCCAGTA | |
| Sgk1 | Forward | CCCTCTCCTCCGCCAAGT |
| Reverse | TTGGCGTGAGGGTTGGA | |
| Nr3c1 | Forward | CAGCATGCCGCTATCGAAA |
| Reverse | CGCGGCAGGAACTATTGTTTT | |
| Nr3c2 | Forward | TGTCTCAGACCTTGGAGCGTTC |
| Reverse | TTGTTCGGAGTAGCACCGGAA | |
| Ucp1 | Forward | CGATGTCCATGTACACCAAGGA |
| Reverse | CCCGAGTCGCAGAAAAGAAG | |
| Ucp2 | Forward | TCATCACTTTCCCTCTGGATACC |
| Reverse | GCGCACTAGCCCTTGACTCT | |
| Ucp3 | Forward | AACGCTCCCCTAGGCAGGTA |
| Reverse | GTCCCTCCTGAGCCACCAT | |
| Adrb1 | Forward | GGACTTCGGTAGATGTGCTGTGT |
| Reverse | CGGTCCAGGGCGATGAC | |
| Adrb2 | Forward | CCTTCGCAGGTCTTCTTCGA |
| Reverse | GTCCGTTCTGCCGTTGCTA | |
| Adrb3 | Forward | GGACGCTGTTCCTTTAAAAGCA |
| Reverse | TCCATCTCACCCCCCATGT | |
| Lep | Forward | ATTTCACACACGCAGTCGGTAT |
| Reverse | AGCCCAGGAATGAAGTCCAA | |
| Pparg | Forward | GTGCCAGTTTCGATCCGTAGA |
| Reverse | GGCCAGCATCGTGTAGATGA | |
| Fasn | Forward | GATCCTGGAACGAGAACACGAT |
| Reverse | TGTCAGTAGCCGAGTCAGTCTTG | |
| Gpat | Forward | CAACACCATCCCCGACATC |
| Reverse | TGACCTTCGATTATGCGATCAT | |
| Pomc | Forward | GGCCTTTCCCCTAGAGTTCAA |
| Reverse | GGACCTGCTCCAAGCCTAATG | |
| Agrp | Forward | GCGGAGGTGCTAGATCCA |
| Reverse | AGGACTCGTGCAGCCTTA | |
| Mc4r | Forward | CACTGTGTCAGGCGTCCTCTT |
| Reverse | ATGGAAATGAGGCAGATGATGA |
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute Inc., Cary, NC, USA). Student’s t-test was used to compare the two experimental groups - control and social crowding. For activity, heat generated and RER over time, we determined the overall significance by repeated measure ANOVA followed by comparison of individual time-points using Student’s t-test. Statistical significance was set at P < 0.05. All data are presented as means ± standard error of the mean (S.E.M).
Results
Effects of social crowding on anxiety-related behaviors
In the OF, chronic SC did not affect locomotion or time traveled in the center of OF but significantly reduced the center/total distance ratio (P < 0.05; Fig. 1), suggesting an increase in anxiety-like behavior. In addition, a significant increase in defecation was observed in the SC group in the OF test (P < 0.05), which can be considered a physiological reaction reflecting the increase in anxiety. Defecation was also increased in the SC group in the ETM test (1.25 ± 0.54 vs. 0.09 ± 0.09 in control group), although the difference did not reach statistical significance (P = 0.09). Other parameters were unchanged in the ETM test (Fig. 1).
Figure 1.
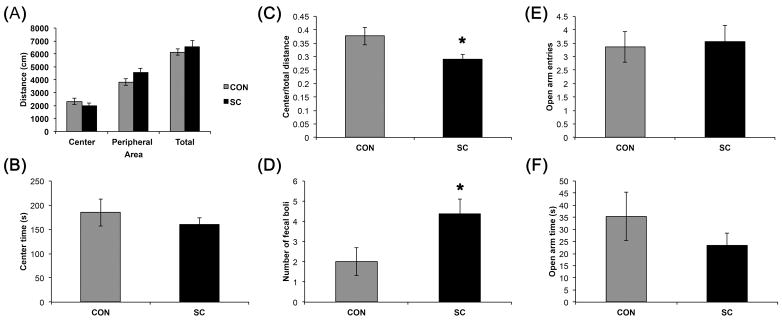
SC mice exhibited a modest increase in anxiety-like behaviors in the open field test but not in the elevated T-maze. (A) Exploration as assessed by total distance traveled in the open field was comparable between the two groups. Distances traveled in the center or the periphery were not significantly different between the groups. (B) The time spent in the center of the open field was similar between SC and control groups. (C) SC mice had a reduced center/total distance ratio and (D) an increased defecation compared to the control group. No significant differences in open arm entries (E) or open arm times (F) were observed in the SC mice. Data shown are mean ± S. E. M. (n = 11 for control, n = 16 for SC). * P < 0.05.
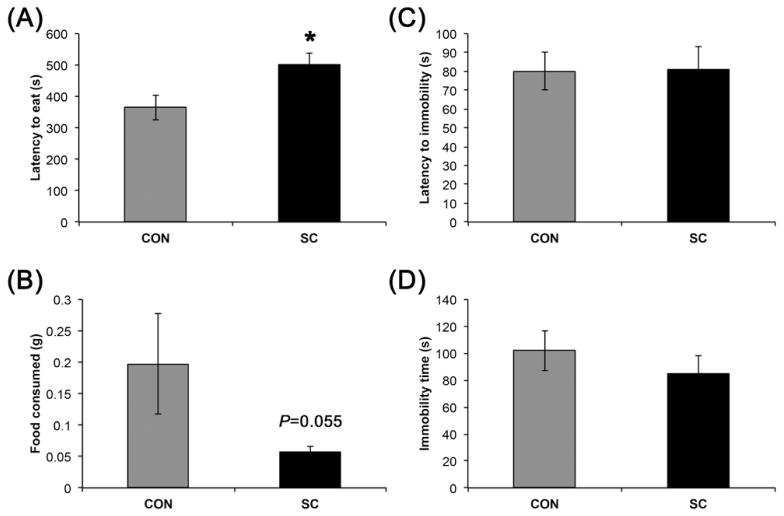
In the NSF, latency to eat was significantly increased in the SC group (P < 0.05, Fig. 2), suggesting an increased anxiety. Interestingly, the increase in latency might in part be due to a reduced appetitive drive, since the SC group showed a trend for reduced total amount of food consumed in 5 min (P = 0.055).
Figure 2.
SC mice exhibited anxiety-like behavior in the novelty suppressed feeding test but not depressive-like behavior in the forced swim test. (A) SC mice had increased latency to eat in a novel environment after overnight fasting. (B) SC mice ate less in 5 minutes after the start of food consumption in the NSF. (C and D) Depressive-like behavior as indicated by immobility in the FST was not affected by SC. Data shown are mean ± S. E. M. (n = 12 for control, n = 16 for SC). * P < 0.05.
Effects of social crowding on depressive-like behaviors
Immobility behavior in the FST is commonly used as an indicator of depressive-like behavior (Cryan and Mombereau, 2004). We did not observe any significant difference in the time mice remained immobile between the control and SC groups (Fig. 2).
Effects of social crowding on body weight and adiposity
Two months of SC significantly increased adiposity without increase body weight (Fig. 3). Basal body weight was comparable between the groups (control, 26.5 ± 0.29 g vs. SC, 26.4 ± 0.35 g, P = 0.88). Body weights were comparable between the groups throughout the experiment (Fig. 3A). Brown adipose tissue (BAT) weight was increased by 1.44 fold (P < 0.01). WATi, WATe, and WATr weights showed increases of 1.74 fold (P < 0.01), 1.62 fold (P < 0.05), and 2.30 fold (P < 0.05), respectively.
Figure 3.
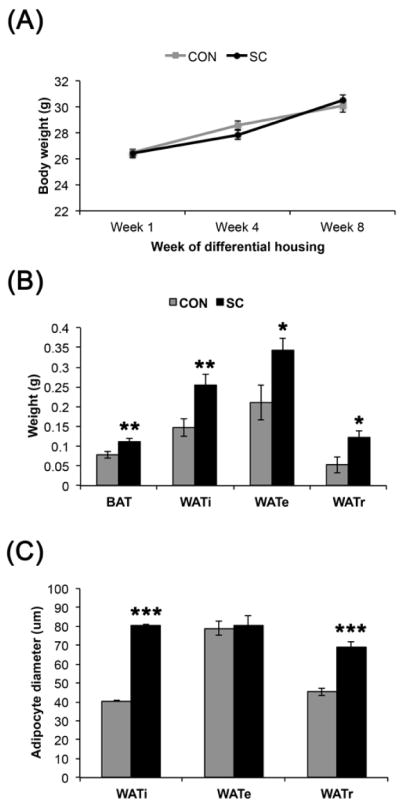
SC increased adiposity without significant impact on body weight. (A) Body weight was comparable between the SC and control groups. (B) All fat depots assessed were markedly larger in the SC mice compared to the controls. (C) The WATi and WATr adipocytes were significantly larger in SC as compared to control group. Data shown are mean ± S. E. M. * P < 0.05, ** P < 0.01. *** P < 0.001. For (A), n = 12 for control, n = 16 for SC. For (B), n = 10 per group. For (C), n =3–4 per group, 2 sections for each WAT depot.
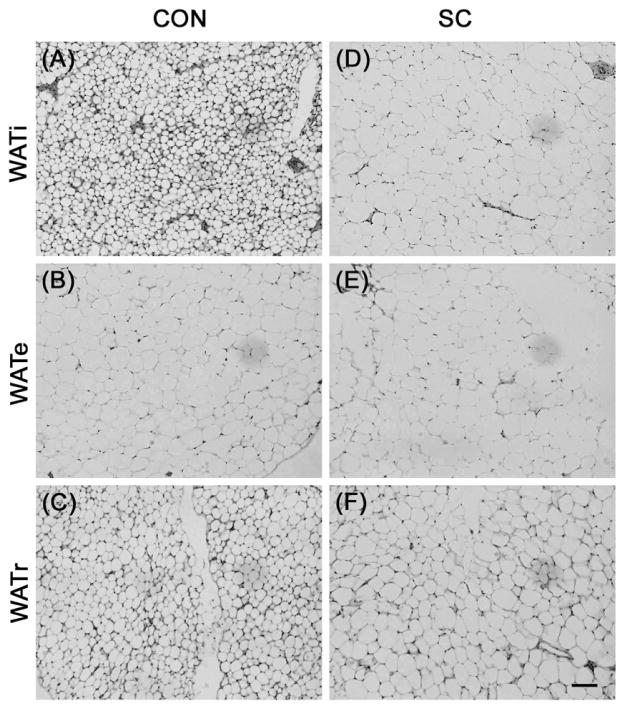
Using Stereo Investigator 7 software to measure the diameters of the adipocytes, we found that the adipocytes in the WATi and WATr were larger in the SC compared to the control group (Fig. 3 and 4, P < 0.001 for both WATi and WATr). In contrast, WATe adipocyte sizes were comparable between the two groups.
Figure 4.
Representative sections of H&E stained adipose tissue showing WATi and WATr adipocytes were enlarged in SC mice (D and F, respectively) compared to control C57BL/6 mice (A and C, respectively). In contrast, WATe adipocytes were comparable in size between the two groups (B and E). Scale bar = 100 μm.
Effects of social crowding on metabolic profile
Activity level as determined when mice were individually placed in the metabolic chamber were comparable between SC and control mice (Fig. 5B, Group effect, P = 0.251). Repeated measure ANOVA revealed a significant effect over time (P < 0.0001), but the interaction effect between time and housing condition was not significant (P = 0.292). Comparing the two groups over time, SC mice exhibited slightly higher activity 4 hours after the onset of dark phase (P < 0.05). Similarly, energy expenditure as determined by heat generated was comparable between groups (Fig. 5A, Group effect, P = 0.399) and there was no significant interaction between time and housing conditions (P = 0.159). A significant effect over time was observed (P < 0.0001), and SC mice exhibited increased energy expenditure at 4 hours after lights off (P < 0.05), concomitant with the slight increase in activity observed. An interaction effect between time and housing condition was detected for RER (P < 0.01), suggesting that SC exerted differential effects depending on the time of day. This was likely due to a lower RER in the SC group during the dark but not the light phase (Fig. 5C). However, the effect was subtle since differences at individual time points did not reach statistical significance. Mean RER did not differ between the two groups either (control, 0.90 ± 0.02 vs. SC, 0.92 ± 0.02; P = 0.516). Daily food intake was not significantly different between the SC and control mice (Fig. 5D, P = 0.591).
Figure 5.
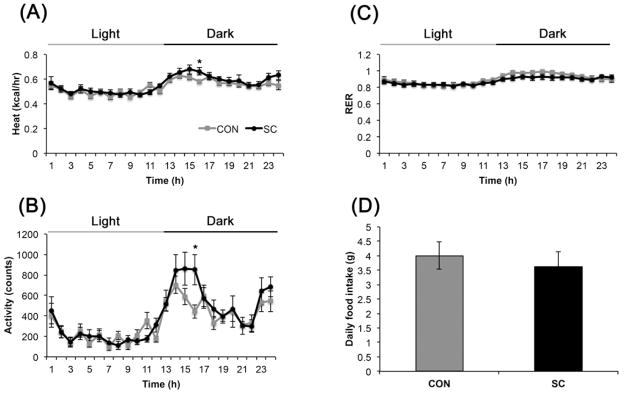
Effect of eight weeks of SC on (A) energy expenditure, (B) physical activity (C) respiratory exchange ratio and (D) food intake (n = 8 per group). Data shown are mean ± S. E. M. * P < 0.05.
Effects of social crowding on serum biomarkers
Consistent with the increase in adipose tissues, SC group showed marked increase in serum leptin level (P < 0.01, Fig. 6A). There were no changes in serum insulin, adiponectin, IGF-1 or corticosterone following 2 months of social crowding. To determine if the lack of change in the corticosterone level in the social crowding paradigm might be due to habituation to a chronic stressor, we analyzed serum from the SC mice at 1 and 3 weeks in the dark and light phase respectively. At both time points, serum corticosterone levels were increased by 2-fold over control animals whether in the light or dark phase (Fig. 6B).
Figure 6.
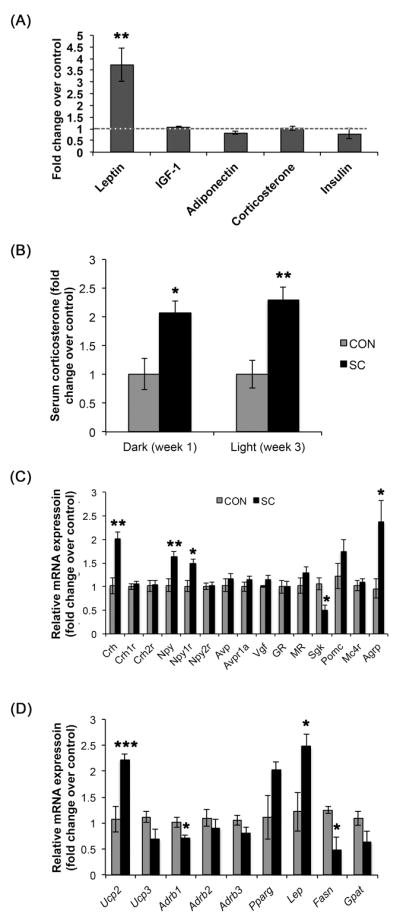
Nine weeks of SC altered (A) serum profile, (C) hypothalamic and (D) WAT gene expression profile. (B) Serum corticosterone was elevated in the SC mice at 1 and 3 weeks post SC initiation during the dark and light phase, respectively. Data shown are mean ± S. E. M. * P < 0.05, ** P < 0.01. *** P < 0.001. For (A), n = 12 for control, n = 16 for SC. For (B), n = 8 per group. For (C), n = 4 – 6 per group, data were derived from averages of duplicate or triplicate reactions. For (D), n = 11 for control and n = 12 for SC.
Effects of social crowding on hypothalamic gene expression
Using quantitative real-time PCR, we compared selected gene expression in microdissected hypothalamus from control and SC mice (Fig. 6C). Expression of Crh mRNA was increased more than twofold in the SC mice (P < 0.01), but its receptors Crh1r and Crh2r were unchanged. SC increased hypothalamic expression of Npy and its receptor Npy1r (P < 0.01, P < 0.05 respectively), but not Npy2r. Concomitantly, Agrp expression was also increased by SC (P < 0.05). In contrast, a twofold reduction in Sgk1 expression was observed in the SC group (P < 0.05). SC did not change expression of Avp, Avpr1a, Vgf, Nr3c1 (glucocorticoid receptor), Nr3c2 (mineralocorticoid receptor), Pomc and Mc4r.
Effects of social crowding on WAT gene expression
We also found changes in WATr gene expression induced by chronic SC (Fig. 6D). These include marked increases in Ucp2 (2.2 fold, P < 0.001) and Lep (2.5 fold, P = 0.01). Adrb1 and Fasn were reduced in the SC group (P < 0.05) and there was a strong trend for reduced Ucp3 (P = 0.06). Expression of Adrb2, Adrb3, Pparg and Gpat were not statistically significantly different between the control and SC mice.
Discussion
In this study, we report a chronic stress model that increased anxiety-like behaviors and adiposity prior to any significant body weight gain. Animal models of chronic stress are important tools for the study of the pathophysiology and molecular mediators of stress-related disorders such as anxiety and metabolic syndromes. These animal models are also needed for the verification of potential treatments. The most often used chronic stress models include chronic mild stress, social defeat, chronic subordinate colony housing and social isolation (Toth and Neumann, 2013). Although rodents in overcrowding conditions were studied since the 1970s (Gartner et al., 1973; Pasley et al., 1978; vom Saal and Howard, 1982), they were mostly focused on the reproductive and immunological consequences of overcrowding. There were only a handful of studies that examined the behavioral and metabolic effects of social crowding in mice. In a study by Reiss and colleagues (2007), mice were housed in groups of seven (crowded condition) from 3 weeks of age for 13 weeks (Reiss et al., 2007). Compared to individually housed controls, these mice displayed increased signs of anxiety-like behaviors in the open field and the elevated plus maze tests, and exaggerated acoustic startle response. The reduced center to total distance ratio in the OF exhibited by the SC mice is a parameter widely used as an indication for increased anxiety (Swanson et al., 1998; Bontekoe et al., 2002; Salas et al., 2003; Walz et al., 2006). Moreover, stress induces colonic transit and an increase in stress-induced defecation in the open field was observed in rodents that exhibited increased anxiety behaviors (O’Malley et al., 2010). While the increased latency to feed in the NSF might not be entirely due to hyponeophagia and could be partly due to altered consummatory drive, the change was consistent with an increase in anxiety. Together, these results suggest an anxiogenic effects of social crowding. It is interesting to note that we used adult male mice in our study. While the potential of fighting may result in the loss of animals if adult mice are used, we did not observe any increase in aggressive behavior or fight wounds in the SC mice compared to controls in our study. The SC mice appeared physically healthy with similar fur coat condition as the control mice. Our study thus expands previous findings (Reiss et al., 2007; Tramullas et al., 2012) and shows that social crowding of adult mice for a month was sufficient to produce a mild anxiety-like phenotype.
More recently, overcrowding has been used as a stressor in the mixed social defeat and overcrowding (SD/OC) chronic psychosocial stress model (Reber et al., 2006; Finger et al., 2012; Slattery et al., 2012). The SD/OC model consisted of 19 days of repeated exposure to social defeat with intermittent 24 hour overcrowding. This paradigm is thus distinct from the overcrowding stress model presented here, with additional stressor, increased stress intensity and the unpredictability factor of the different residents and scheduling of the stressors. Nevertheless, the SD/OC model produced a similar behavioral phenotype to the SC model with an increase in anxiety-like behaviors but no effect on depressive-like behaviors (Slattery et al., 2012).
In the SC mice, serum corticosterone was increased in both the dark and light phases following 1 and 3 weeks of social crowding, respectively, but returned to comparable level to the control group by 2 months. This was likely due to an adaptation to the chronic stress. A return to comparable baseline corticosterone following chronic psychosocial stress has been reported by others using the chronic subordinate colony housing paradigm in mice (Reber et al., 2007; Uschold-Schmidt et al., 2012). The increase in corticosterone levels along with the higher Crh expression in the hypothalamus indicate enhanced stress in the SC mice. In addition, the increase in anxiety-like behaviors and physiological responses that are known to be induced by stress (Nishiyama et al., 2004; Julio-Pieper et al., 2012; Campos et al., 2013; McCormick and Green, 2013), are also consistent with increased stress in the SC mice.
Our study shows that chronic social crowding lead to a marked increase in adiposity prior to any change in body weight, indicating a change in body composition with a greater ratio of fat mass. All fat depots collected, including WATi, WATr, WATe and BAT, were significantly larger in the SC mice compared to control mice. Histological examination of the fat depots showed that the adipocytes of WATi and WATr were enlarged. In contrast, WATe adipocyte sizes were comparable between the SC and control mice, suggesting that the enlargement of this particular fat depot might be due to an increase in adipocyte proliferation rather than hypertrophy. The SC model may therefore be useful to study the distinct mechanisms underlying fat mass regulation. The dramatic increase in serum leptin is concomitant with the increase in fat mass. The metabolic phenotype of SC model is thus distinct from other chronic social stress models, such as SD/OC or chronic subordinate colony housing (CSC) paradigms. In both paradigms, body weight was reduced during the presence of stressor (Finger et al., 2012; Slattery et al., 2012). Some studies reported increased body weight gain upon termination of the stressors (Melhorn et al., 2010; Slattery et al., 2012). Closely resembling to the SC model described in this study is the group housed (six per cage) female hamsters, which also exhibited significantly increased adiposity (as determined by percentage of body fat), body weight and body length (Borer et al., 1988; Meisel et al., 1990). However, it should be noted that in these studies, as for the SD/OC studies mentioned above, individual housed animals were used as controls. Considering that social isolation is also often used as a psychosocial stressor (Yamada et al., 2000; Siegfried et al., 2003; Matsumoto et al., 2005), interpretation of data using these animals as controls could be confounded and thus remain to be validated using group housed animals as controls.
In addition to Crh, SC also increased hypothalamic expression of Agrp, Npy and Npy1r, and a reduction in Sgk1. Hypothalamic NPY stimulates appetite and feeding, partly through the Y1 receptors (Kanatani et al., 1996; Lopez-Valpuesta et al., 1996). Both chronic stress and glucocorticoid administration have been shown to upregulate hypothalamic Npy and Npy1r expression (Larsen et al., 1994; Sergeyev et al., 2005). The orexigenic AgRP colocalizes with NPY in the arcuate nucleus and promotes feeding by blocking the melanocortin receptors (Morton and Schwartz, 2001; Koch and Horvath, 2014). The increases in hypothalamic AgRP and NPY signaling likely counteract with the appetite suppressive effect of CRH (Drescher et al., 1994; Pelleymounter et al., 2000) under chronic SC condition, result in the lack of change in food intake in SC mice. The increase in basal expression of Npy and Agrp despite dramatically elevated circulating leptin suggests an induction of leptin resistance in the SC paradigm. The robust reduction in Sgk1 expression is intriguing. Sgk1 is transcriptionally regulated by a number of hormones, mediators and stressors (Lang and Stournaras, 2013). Sgk1 gene expressions in the hippocampus and cortex have been shown to be induced by various stressors such as restraint stress, water-immersion and transient ischemia (Nishida et al., 2004; Murata et al., 2005). However, the role of Sgk1 in the hypothalamus is poorly understood. One study showed an increase in hypothalamic Sgk1 mRNA level following fasting (Nonogaki et al., 2006), although the physiological consequence of this increase was not explored. A follow-up study showed that hypothalamic Sgk1 mRNA is induced by acute isolation stress and likely contributes to the isolation-induced body weight reduction in response to fasting (Kaji and Nonogaki, 2010). The role of hypothalamic Sgk1 in energy homeostasis and metabolism, particularly in response to stress, is an interesting direction for further study.
In the retroperitoneal white adipose tissue, chronic SC led to increases in Ucp2 and Lep, and decreases in Adrb1 and Fasn mRNA expression. Increases in Lep mRNA expression have been reported in animal models of obesity (Lopez et al., 2003; Sato et al., 2010). Our study shows that adipocyte leptin expression is transcriptionally modulated by social crowding stress and contributes to the elevated circulating leptin. Higher leptin level might account for the increase in Ucp2 expression. Unlike Ucp1, Ucp2 is widely expressed in many tissues, including WAT (Lang and Stournaras, 2013). Ucp2 gene expression was highly increased in the epididymal, retroperitoneal, and subcutaneous fat tissue of hyperleptinemic Zucker rats (Zhou et al., 1997). In another study, exogenous leptin administration for 1 week led to an increase in Ucp2 expression in the WATe but not in WATr (Scarpace et al., 1998). Ucp2 is upregulated by free fatty acids and is suggested to be involved in fatty-acid metabolism and may be an adaptive response to protect against oxidative damage caused by excessive fatty acid peroxidation (Reilly and Thompson, 2000; Nedergaard and Cannon, 2003; Brand and Esteves, 2005; Souza et al., 2011). The reduced expressions of both Adrb1 and Fasn in WATr suggest that the enlarged adipose mass was due to the reduced lipolysis through β1 adrenergic receptor suppression rather than an increase in lipogenesis mediated by the lipogenic enzyme fatty acid synthase. β1-adrenergic receptors have been shown to play an important role in catecholamine-induced lipolysis as specific Adrb1 antagonists markedly inhibit this response (Germack et al., 1997; Louis et al., 2000). Moreover, transgenic mice overexpressing Adrb1 in adipose tissue exhibit increased lipolytic rate and are partially resistant to diet-induced obesity (Soloveva et al., 1997). On the other hand, increased expression of Adrb1 mRNA and protein were detected in the WAT of patients with cancer cachexia, a condition exhibiting enhanced lipolysis (Cao et al., 2010a).
The lack of changes in metabolic parameters measured by the metabolic chamber, including food intake, energy expenditure, RER and activity, together with the specific gene expression changes in WAT suggest that the increase in adiposity may due to a local rather than systemic change in metabolism. This could also account for the increase in adiposity without observable increase in body weight since a significant change in energy intake or expenditure would more likely lead to a more significant change in body weight. The increase in adiposity without body weight gain following 2 months of social crowding stress indicates a body composition change. Future studies using MRI or DEXA can determine if there is a decrease in lean or skeletal mass that accompanies the gain in adipose tissue mass.
In summary, our study described a simple mild chronic stress model that induces a moderate anxiety phenotype and a robust change in body composition with marked increase in adiposity. The social crowding model offers several advantages over other stress models. Compared to social isolation, it is more cost-effective due to reduced housing cost. It is also less labor intensive as no daily application of stressors is required as for the social defeat or chronic mild stress models. Although the social defeat model has been shown to elicit changes within days (Lutter et al., 2008; Chuang et al., 2010; Patterson et al., 2013), the SC paradigm may be a better mild ‘chronic’ stress model since the durations of the other stress models were more subchronic compared to the SC paradigm. The SC model thus represents a good alternative for the study of metabolic process and stress-induced metabolic dysfunctions.
Acknowledgments
We thank the Ohio State University Medical Center core facility for assistance with the WAT section preparation.
Funding sources
Funding for this study was provided by the National Institute of Health (RO1NS044576).
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
Contributors
E.D. Lin designed the study, analyzed the data and wrote the manuscript. E.D. Lin, M. Sun, E. Choi, D. Magee and C. Stets conducted the experiments. M. J. During contributed to the preparation of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. 2009;4:e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170:181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontekoe CJ, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor LA, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, Cheng NC, Merriweather M, Hoogeveen AT, Nelson D, Paylor R, Oostra BA. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet. 2002;11:487–498. doi: 10.1093/hmg/11.5.487. [DOI] [PubMed] [Google Scholar]
- Borer KT, Pryor A, Conn CA, Bonna R, Kielb M. Group housing accelerates growth and induces obesity in adult hamsters. Am J Physiol. 1988;255:R128–133. doi: 10.1152/ajpregu.1988.255.1.R128. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35(Suppl 2):S101–111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- Cao DX, Wu GH, Yang ZA, Zhang B, Jiang Y, Han YS, He GD, Zhuang QL, Wang YF, Huang ZL, Xi QL. Role of beta1-adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci. 2010a;101:1639–1645. doi: 10.1111/j.1349-7006.2010.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–454. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010b;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, Perello M, Elmquist JK, Repa JJ, Zigman JM, Lutter M. Chronic social defeat stress disrupts regulation of lipid synthesis. J Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- De Vriendt T, Moreno LA, De Henauw S. Chronic stress and obesity in adolescents: scientific evidence and methodological issues for epidemiological research. Nutr Metab Cardiovasc Dis. 2009;19:511–519. doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Dodt C, Lonnroth P, Wellhoner JP, Fehm HL, Elam M. Sympathetic control of white adipose tissue in lean and obese humans. Acta Physiol Scand. 2003;177:351–357. doi: 10.1046/j.1365-201X.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- Drescher VS, Chen HL, Romsos DR. Corticotropin-releasing hormone decreases feeding, oxygen consumption and activity of genetically obese (ob/ob) and lean mice. J Nutr. 1994;124:524–530. doi: 10.1093/jn/124.4.524. [DOI] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology. 2012;37:729–741. doi: 10.1016/j.psyneuen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- Gartner K, Reznik-Schuller H, Reznik G. The influence of overcrowding on spermatogenesis, size of Leydig-cell nuclei (histometrical investigation), and the adrenal corticosterone contents in mice. Acta Endocrinol (Copenh) 1973;74:783–791. doi: 10.1530/acta.0.0740783. [DOI] [PubMed] [Google Scholar]
- Germack R, Starzec AB, Vassy R, Perret GY. Beta-adrenoceptor subtype expression and function in rat white adipocytes. Br J Pharmacol. 1997;120:201–210. doi: 10.1038/sj.bjp.0700885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Hunter RG, McEwen BS. Stress and anxiety across the lifespan: structural plasticity and epigenetic regulation. Epigenomics. 2013;5:177–194. doi: 10.2217/epi.13.8. [DOI] [PubMed] [Google Scholar]
- Julio-Pieper M, O’Mahony CM, Clarke G, Bravo JA, Dinan TG, Cryan JF. Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent. Stress. 2012;15:218–226. doi: 10.3109/10253890.2011.607524. [DOI] [PubMed] [Google Scholar]
- Kaji T, Nonogaki K. Contribution of central SGK-1 to the acute phase responses of mice to social isolation. Front Biosci (Elite Ed) 2010;2:1355–1361. doi: 10.2741/e195. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- Kim H, Whang WW, Kim HT, Pyun KH, Cho SY, Hahm DH, Lee HJ, Shim I. Expression of neuropeptide Y and cholecystokinin in the rat brain by chronic mild stress. Brain Res. 2003;983:201–208. doi: 10.1016/s0006-8993(03)03087-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Horvath TL. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol Psychiatry. 2014;19:752–761. doi: 10.1038/mp.2014.30. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Lang F, Stournaras C. Serum and glucocorticoid inducible kinase, metabolic syndrome, inflammation, and tumor growth. Hormones (Athens) 2013;12:160–171. doi: 10.14310/horm.2002.1401. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Jessop DS, Chowdrey HS, Lightman SL, Mikkelsen JD. Chronic administration of glucocorticoids directly upregulates prepro-neuropeptide Y and Y1-receptor mRNA levels in the arcuate nucleus of the rat. J Neuroendocrinol. 1994;6:153–159. doi: 10.1111/j.1365-2826.1994.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Lin EJ, Choi E, Liu X, Martin A, During MJ. Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behav Brain Res. 2011;216:349–357. doi: 10.1016/j.bbr.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, McMurphy T, Xiao R, Slater A, Huang W, Cao L. Hypothalamic Gene Transfer of BDNF Inhibits Breast Cancer Progression and Metastasis in Middle Age Obese Mice. Mol Ther. 2014 doi: 10.1038/mt.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez IP, Marti A, Milagro FI, de Zulet Md ML, Moreno-Aliaga MJ, Martinez JA, De Miguel C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes Res. 2003;11:188–194. doi: 10.1038/oby.2003.30. [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta FJ, Nyce JW, Griffin-Biggs TA, Ice JC, Myers RD. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypothalamus underlie NPY hypothermia and feeding in rats. Proc Biol Sci. 1996;263:881–886. doi: 10.1098/rspb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Louis SN, Jackman GP, Nero TL, Iakovidis D, Louis WJ. Role of beta-adrenergic receptor subtypes in lipolysis. Cardiovasc Drugs Ther. 2000;14:565–577. doi: 10.1023/a:1007838125152. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, Ronnemaa T. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251:35–43. doi: 10.1046/j.1365-2796.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress. 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurphy T, Xiao R, Magee D, Slater A, Zabeau L, Tavernier J, Cao L. The anti-tumor activity of a neutralizing nanobody targeting leptin receptor in a mouse model of melanoma. PLoS One. 2014;9:e89895. doi: 10.1371/journal.pone.0089895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiol Behav. 1990;47:815–817. doi: 10.1016/0031-9384(90)90002-l. [DOI] [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol. 2010;299:R813–822. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C, Duclos M, Cabanac M, Richard D. Chronic stress reduces body fat content in both obesity-prone and obesity-resistant strains of mice. Horm Behav. 2005;48:172–179. doi: 10.1016/j.yhbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D’Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S56–62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- Murata S, Yoshiara T, Lim CR, Sugino M, Kogure M, Ohnuki T, Komurasaki T, Matsubara K. Psychophysiological stress-regulated gene expression in mice. FEBS Lett. 2005;579:2137–2142. doi: 10.1016/j.febslet.2005.02.069. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Nagata T, Takahashi Y, Sugahara-Kobayashi M, Murata A, Asai S. Alteration of serum/glucocorticoid regulated kinase-1 (sgk-1) gene expression in rat hippocampus after transient global ischemia. Brain Res Mol Brain Res. 2004;123:121–125. doi: 10.1016/j.molbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Mizuta Y, Isomoto H, Takeshima F, Omagari K, Miyahara Y, Murata I, Kohno S. Chronic visceral hypersensitivity renders defecation more susceptible to stress via a serotonergic pathway in rats. Dig Dis Sci. 2004;49:763–769. doi: 10.1023/b:ddas.0000030086.03506.c7. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology. 2007;148:4658–4666. doi: 10.1210/en.2007-0296. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Ohashi-Nozue K, Oka Y. Induction of hypothalamic serum- and glucocorticoid-induced protein kinase-1 gene expression and its relation to plasma des-acyl ghrelin in energy homeostasis in mice. Biochem Biophys Res Commun. 2006;344:696–699. doi: 10.1016/j.bbrc.2006.03.196. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008;27:S20–31. doi: 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Julio-Pieper M, Gibney SM, Dinan TG, Cryan JF. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress. 2010;13:114–122. doi: 10.3109/10253890903067418. [DOI] [PubMed] [Google Scholar]
- Pasley JN, Powell EW, Cernosek RM, Cernosek SF. Effects of amygdaloid lesions on reproductive function of grouped caged mice. Neuroendocrinology. 1978;25:77–83. doi: 10.1159/000122731. [DOI] [PubMed] [Google Scholar]
- Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology. 2013;154:1080–1091. doi: 10.1210/en.2012-1834. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148:670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Reilly JM, Thompson MP. Dietary fatty acids Up-regulate the expression of UCP2 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2000;277:541–545. doi: 10.1006/bbrc.2000.3705. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res. 2007;184:167–173. doi: 10.1016/j.bbr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Roberts CJ, Campbell IC, Troop N. Increases in Weight during Chronic Stress are Partially Associated with a Switch in Food Choice towards Increased Carbohydrate and Saturated Fat Intake. Eur Eat Disord Rev. 2013 doi: 10.1002/erv.2264. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Kawakami T, Kondoh M, Takiguchi M, Kadota Y, Himeno S, Suzuki S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J. 2010;24:2375–2384. doi: 10.1096/fj.09-145466. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Nicolson M, Matheny M. UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin. J Endocrinol. 1998;159:349–357. doi: 10.1677/joe.0.1590349. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P, Gaudet L, Moreau JL, Hokfelt T. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178:115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- Serlachius A, Hamer M, Wardle J. Stress and weight change in university students in the United Kingdom. Physiol Behav. 2007;92:548–553. doi: 10.1016/j.physbeh.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiol Behav. 2003;79:39–45. doi: 10.1016/s0031-9384(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73:827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Uschold N, Magoni M, Bar J, Popoli M, Neumann ID, Reber SO. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 2012;37:702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- Soloveva V, Graves RA, Rasenick MM, Spiegelman BM, Ross SR. Transgenic mice overexpressing the beta 1-adrenergic receptor in adipose tissue are resistant to obesity. Mol Endocrinol. 1997;11:27–38. doi: 10.1210/mend.11.1.9870. [DOI] [PubMed] [Google Scholar]
- Souza BM, Assmann TS, Kliemann LM, Gross JL, Canani LH, Crispim D. The role of uncoupling protein 2 (UCP2) on the development of type 2 diabetes mellitus and its chronic complications. Arq Bras Endocrinol Metabol. 2011;55:239–248. doi: 10.1590/s0004-27302011000400001. [DOI] [PubMed] [Google Scholar]
- Stone AA, Brownell KD. The stress-eating paradox: Multiple daily measurements in adult males and females. Psychol Heal. 1994;9:425–436. [Google Scholar]
- Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429–433. [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress. 2012;15:281–292. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- Uschold-Schmidt N, Nyuyki KD, Fuchsl AM, Neumann ID, Reber SO. Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology. 2012;37:1676–1687. doi: 10.1016/j.psyneuen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Howard LS. The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science. 1982;215:1270–1272. doi: 10.1126/science.7058349. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52:355–362. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Walz K, Paylor R, Yan J, Bi W, Lupski JR. Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2) J Clin Invest. 2006;116:3035–3041. doi: 10.1172/JCI28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger SC, Muglia LJ, Jacobson L, Majzoub JA. CRH-deficient mice have a normal anorectic response to chronic stress. Regul Pept. 1999;84:69–74. doi: 10.1016/s0167-0115(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ohki-Hamazaki H, Wada K. Differential effects of social isolation upon body weight, food consumption, and responsiveness to novel and social environment in bombesin receptor subtype-3 (BRS-3) deficient mice. Physiol Behav. 2000;68:555–561. doi: 10.1016/s0031-9384(99)00214-0. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006;87:789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Shimabukuro M, Koyama K, Lee Y, Wang MY, Trieu F, Newgard CB, Unger RH. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc Natl Acad Sci U S A. 1997;94:6386–6390. doi: 10.1073/pnas.94.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]