Abstract
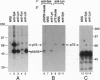
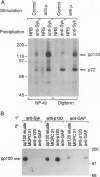
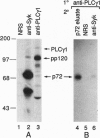
The 72-kDa spleen tyrosine kinase (Syk) and Src-family kinase p53/56Lyn (Lyn) contribute to signaling via the B-cell antigen receptor complex. Here we show that Syk and Lyn from human B lymphocytes can interact directly. Syk and Lyn coimmunoprecipitated from mature and activated B-cell lines, and gel-purified Syk and Lyn reassociated in vitro, demonstrating their direct interaction. This Syk-Lyn interaction may be dependent on the stage of B-cell differentiation, since Syk-Lyn associations were not detected in pre-B and myeloma cell lines and Syk from an immature B-cell line did not reassociate with Lyn in vitro. Serine/threonine kinase activity was also associated with Syk. Crosslinking of cell surface IgM led to rapid activation of both tyrosine and serine/threonine protein kinase activities that resulted in phosphorylation in vitro of proteins coprecipitating with Syk--in particular, a serine/threonine phosphorylated protein 120 kDa in size (pp120). Several phosphoproteins, including one of 72 kDa and one of 120 kDa, coprecipitated with phospholipase C-gamma 1 (PLC gamma 1). Sequential immunoprecipitation identified the 72-kDa protein associated with PLC gamma 1 as Syk. The 120-kDa serine/threonine phosphorylated protein that coprecipitated with PLC gamma 1 resembled the Syk-associated pp120 by several criteria. Thus, pp120 may serve as a link between Syk and PLC gamma 1, coupling the B-cell antigen receptor to the phosphatidylinositol pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Pleiman C. M., Clark M. R. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Shu G. Activation of human B cell proliferation through surface Bp35 (CD20) polypeptides or immunoglobulin receptors. J Immunol. 1987 Feb 1;138(3):720–725. [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Couture C., Baier G., Altman A., Mustelin T. p56lck-independent activation and tyrosine phosphorylation of p72syk by T-cell antigen receptor/CD3 stimulation. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5301–5305. doi: 10.1073/pnas.91.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Chan V. W., Turck C. W., DeFranco A. L. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J Immunol. 1992 Apr 1;148(7):2012–2022. [PubMed] [Google Scholar]
- Gold M. R., Crowley M. T., Martin G. A., McCormick F., DeFranco A. L. Targets of B lymphocyte antigen receptor signal transduction include the p21ras GTPase-activating protein (GAP) and two GAP-associated proteins. J Immunol. 1993 Jan 15;150(2):377–386. [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Geahlen R. L., Deanin G. G., Oliver J. M. Fc epsilon RI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem. 1991 Aug 15;266(23):14846–14849. [PubMed] [Google Scholar]
- Kim K. M., Yoshimura T., Watanabe H., Ishigami T., Nambu M., Hata D., Higaki Y., Sasaki M., Tsutsui T., Mayumi M. Growth regulation of a human mature B cell line, B104, by anti-IgM and anti-IgD antibodies. J Immunol. 1991 Feb 1;146(3):819–825. [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993 Jul 16;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Takata M., Yamanashi Y., Inazu T., Taniguchi T., Yamamoto T., Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994 May 1;179(5):1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. L., Sidorenko S. P., Chandran K. A., Draves K. E., Chan A. C., Weiss A., Edelhoff S., Disteche C. M., Clark E. A. Molecular cloning of human Syk. A B cell protein-tyrosine kinase associated with the surface immunoglobulin M-B cell receptor complex. J Biol Chem. 1994 Apr 22;269(16):12310–12319. [PubMed] [Google Scholar]
- Leprince C., Draves K. E., Geahlen R. L., Ledbetter J. A., Clark E. A. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince C., Draves K. E., Ledbetter J. A., Torres R. M., Clark E. A. Characterization of molecular components associated with surface immunoglobulin M in human B lymphocytes: presence of tyrosine and serine/threonine protein kinases. Eur J Immunol. 1992 Aug;22(8):2093–2099. doi: 10.1002/eji.1830220820. [DOI] [PubMed] [Google Scholar]
- Liao F., Shin H. S., Rhee S. G. In vitro tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2 by src-family protein tyrosine kinases. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1028–1033. doi: 10.1006/bbrc.1993.1320. [DOI] [PubMed] [Google Scholar]
- Malek S. N., Desiderio S. SH2 domains of the protein-tyrosine kinases Blk, Lyn, and Fyn(T) bind distinct sets of phosphoproteins from B lymphocytes. J Biol Chem. 1993 Oct 25;268(30):22557–22565. [PubMed] [Google Scholar]
- Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991 Dec 25;266(36):24249–24252. [PubMed] [Google Scholar]
- Peifer M., Berg S., Reynolds A. B. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994 Mar 11;76(5):789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Pleiman C. M., Clark M. R., Gauen L. K., Winitz S., Coggeshall K. M., Johnson G. L., Shaw A. S., Cambier J. C. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-gamma 2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993 Sep;13(9):5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiman C. M., Hertz W. M., Cambier J. C. Activation of phosphatidylinositol-3' kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994 Mar 18;263(5153):1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- Prasad K. V., Rudd C. E. A Raf-1-related p110 polypeptide associates with the CD4-p56lck complex in T cells. Mol Cell Biol. 1992 Nov;12(11):5260–5267. doi: 10.1128/mcb.12.11.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Herbert L., Cleveland J. L., Berg S. T., Gaut J. R. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992 Dec;7(12):2439–2445. [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. N., June C. H., Yamada H., Rapp U. R., Samelson L. E. Rapid activation of C-Raf-1 after stimulation of the T-cell receptor or the muscarinic receptor type 1 in resting T cells. J Immunol. 1993 Oct 15;151(8):4116–4127. [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Takata M., Sabe H., Hata A., Inazu T., Homma Y., Nukada T., Yamamura H., Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994 Mar 15;13(6):1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Taniguchi T., Yang C., Yasue S., Saito H., Yamamura H. Association with B-cell-antigen receptor with protein-tyrosine kinase p72syk and activation by engagement of membrane IgM. Eur J Biochem. 1993 Apr 1;213(1):455–459. doi: 10.1111/j.1432-1033.1993.tb17781.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yamanashi Y., Toyoshima K. Association of Src-family kinase Lyn with B-cell antigen receptor. Immunol Rev. 1993 Apr;132:187–206. doi: 10.1111/j.1600-065x.1993.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- da Silva A. J., Janssen O., Rudd C. E. T cell receptor zeta/CD3-p59fyn(T)-associated p120/130 binds to the SH2 domain of p59fyn(T). J Exp Med. 1993 Dec 1;178(6):2107–2113. doi: 10.1084/jem.178.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]