Abstract
The tendency to approach or avoid novel people is a fundamental human behavior and is a core dimension of social anxiety. Resting state fMRI was used to test for an association between social inhibition and intrinsic connectivity in 40 young adults ranging from low to high in social inhibition. Higher levels of social inhibition were associated with specific patterns of reduced amygdala-cingulate cortex connectivity. Connectivity was reduced between the superficial amygdala and the rostral cingulate cortex and between the centromedial amygdala and the dorsal anterior cingulate cortex. Social inhibition also modulated connectivity in several well-established intrinsic networks; higher social inhibition correlated with reduced connectivity with default mode and dorsal attention networks and enhanced connectivity in salience and executive control networks. These findings provide important preliminary evidence that social inhibition reflects differences in the underlying intrinsic connectivity of the brain in the absence of social stimuli or stressors.
Keywords: Resting state, fMRI, Inhibited temperament, Anxiety, Prefrontal cortex, Social anxiety disorder
1. Introduction
Social anxiety disorder is common (Kessler, Chiu, Demler, & Walters, 2005b; Kessler et al., 2005a), follows a chronic course (Bittner et al, 2007; Pine, Cohen, Gurley, Brook, & Ma, 1998; Stein & Stein, 2008), and causes substantial disability (Comer et al., 2011; Katzelnick et al, 2001; Stein, 2006). The disorder is especially detrimental because it typically begins in childhood, causing disruptions in development with pervasive long-term effects on education (Katzelnick et al., 2001; Schneier, Johnson, Hornig, Liebowitz, & Weissman, 1992), employment (Katzelnick et al., 2001; Moitra, Beard, Weisberg, & Keller, 2011; Schneier et al., 1994), relationships (Katzelnick et al, 2001; Schneier et al., 1994), and later psychiatric illness (Beesdo et al., 2007; Cougle, Keough, Riccardi, & Sachs-Ericsson, 2009; Rush et al., 2005; Schneier et al., 2010). Specific dimensional biological markers for social anxiety disorder are essential for the early identification of risk, the development of neuroscientifically-based treatments, and the prediction and assessment of treatment response; however, clinically useful dimensional biological markers are currently unavailable. Prior studies of social anxiety disorder have overwhelmingly used case-control designs, which include heterogeneous patient groups with multiple symptoms. While research using case-control designs has made important contributions to broadly defining which brain regions are involved in social anxiety, the heterogeneity of patient groups may limit the discovery of specific underlying neurobiological mechanisms. A promising alternative is to identify the neurobiology of dimensional measures of core symptoms or traits associated with social anxiety disorder.
High social inhibition—the tendency to withdraw from new people and to avoid social situations—is a core feature of social anxiety (American Psychiatric Association, 2000) and one of the most impairing symptoms of social anxiety disorder. Social inhibition also reflects an underlying trait that exists along a continuum, ranging from low to high social inhibition (Schneier, Blanco, Antia, & Liebowitz, 2002; Stein, Walker, & Forde, 1994). Social inhibition is a fundamental behavior that is heritable (Eley et al., 2003; Emde et al, 1992; Robinson, Reznick, Kagan, & Corley, 1992; Schwartz et al., 2003b), present early in development (Kagan, Snidman, & Arcus, 1998b), and observable across species (Clinton, Stead, Miller, Watson, & Akil, 2011; Fox, Shelton, Oakes, Davidson, &Kalin, 2008; Gosling, 2001; Qi et al., 2010). Social inhibition is assumed to have a biological basis; inhibited children display a pattern of physiological hyperarousal including a high and stable heart rate (Kagan, Reznick, & Snidman, 1998a) and elevated cortisol levels (Kagan et al., 1998a). Consistent with the amygdala's influence on the sympathetic nervous system (Davis, 1992), studies in rodents, non-human primates, and humans point to the amygdala as a key brain region mediating individual differences in social inhibition (Blackford, Avery, Shelton, &Zald, 2009; Kalin, Shelton, & Davidson, 2004; Qi et al., 2010; Schwartz, Wright, Shin, Kagan, & Rauch, 2003a). For example, in adults who are inhibited, or were inhibited as children, the amygdala shows heightened responsivity to novel (Beaton et al., 2008; Schwartz et al., 2003a) or threatening (Pérez-Edgar et al., 2007) faces and fails to show the normal habituation to repeated presentations of faces (Blackford, Avery, Cowan, Shelton, & Zald, 2011; Schwartz et al., 2012).
The overwhelming majority of human neuroimaging studies of social inhibition have relied on tasks designed to elicit brain responses to salient stimuli, such as novel or threatening human faces (Beaton et al., 2008; Blackford et al., 2011, 2009; Pérez-Edgar et al., 2007; Schwartz et al, 2003b; Schwartz et al., 2012). In contrast, neuroimaging studies of non-human primates using FDG-PET have demonstrated that inhibition is associated with differences in brain responses to a novel human experimenter (Fox et al, 2008), paralleling human studies, but have also shown differences in brain activity in the home cage (Fox et al., 2008). Findings from these studies suggest that social inhibition may stem from differences in intrinsic (i.e., non-task related) amygdala activity (Fox et al, 2008). Whether social inhibition in humans is due to intrinsic differences in amygdala activity has yet to be studied.
Functional magnetic resonance imaging (fMRI) can be used to measure intrinsic brain activity and identify underlying functional brain networks (Fox & Raichle, 2007). The utility of resting state fMRI (rsfMRI) has been established based on evidence that intrinsic connectivity patterns replicate across time and individuals (Biswal et al., 2010; De Luca, Beckmann, De Stefano, Matthews, & Smith, 2006; Dijk et al, 2010) and are evident across species (Lu et al., 2012; Pawela et al., 2008; Vincent et al, 2007). Critically, intrinsic connectivity patterns can reflect underlying structural connectivity (Greicius, Supekar, Menon, & Dougherty, 2009; Hagmann et al., 2008; Honey et al, 2009; Van den Heuvel, Mandl, Kahn, & Hulshoff Pol, 2009) and predict patterns of brain activation during tasks (De Luca, Smith, De Stefano, Federico, & Matthews, 2005; Fox, Snyder, Vincent, & Raichle, 2007; Fox, Snyder, Zacks, & Raichle, 2006b; Mennes et al, 2011), suggesting that rsfMRI can be used to assess neuronal networks, independent of specific tasks. For these reasons, alterations in intrinsic connectivity show promise as biomarkers for psychiatric disease (Zhang & Raichle, 2010).
In the present study, we used rsfMRI to determine whether individual differences in social inhibition manifest as differences in intrinsic connectivity. We focused on amygdala networks given previous findings, and hypothesized that social inhibition would be associated with strength of intrinsic connectivity in amygdala networks. We examined intrinsic connectivity for three different amygdala subnuclei based on evidence that these subnuclei have distinct anatomical connections (Amaral, Price, Pitkanen, & Carmichael, 1992), distinct patterns of intrinsic connectivity (Mishra, Rogers, Chen, & Gore, 2013; Roy et al, 2009), and show distinct patterns of alterations in anxiety disorders (Etkin, Prater, Schatzberg, Menon, & Greicius, 2009; Roy et al, 2013).To determine whether social inhibition is associated with differences in other intrinsic networks, we also examined well-established networks, including the default mode, dorsal attention, executive control, and salience networks.
2. Methods
2.1. Participants
Study participants were 40 young adults (24 females), 18–25 years of age (mean = 21.85, standard deviation = 2.01), of various ethnicities (68% Caucasian, 18% African-American, 12% Asian, and 2% other). Participants had been recruited for a larger study of personality and emotion, which included an oversampling at the low and high ends of the social inhibition continuum (see Section 2.2 for details). For the larger study, recruitment methods included fliers, recruitment databases, and word of mouth. Individuals were not eligible for the study if they had any of the following: failure on MRI safety screen; current use of psychoactive medications; major medical illness; history of brain trauma; or past or current psychiatric disorder or substance abuse based on the Structured Clinical Interview for DSM IV Axis I disorders (First, Spitzer, Gibbon, & Williams, 2002), with the exception of social anxiety disorder, generalized anxiety disorder, or specific phobias as these are very common in highly inhibited individuals. Eight participants met criteria for one or more anxiety disorders (three for social anxiety disorder, three for specific phobia, one for social anxiety disorder and specific phobia, and one for social anxiety disorder, generalized anxiety disorder, and specific phobia). This research was conducted in accordance with the Vanderbilt Human Research Protection Program and all participants provided written informed consent. Participants received financial compensation.
2.2. Social inhibition measure
Based on evidence that social inhibition is observed early in life and relatively stable across development, we selected a measure of current inhibition that also has a companion retrospective measure. The Adult Self-Report of Inhibition (ASRI) and Retrospective Self-Report of Inhibition (RSRI) (Reznick, Hegeman, Kaufman, Woods, & Jacobs, 1992) were designed to measure typical responses to social and non-social stimuli and situations. The ASRI and RSRI both use a 1-5 likert scale to measure low to high inhibition and show good reliability and validity (Reznick et al., 1992; Rohrbacher et al., 2008). Previous factor analysis of the scales has revealed two underlying subscales, social and non-social (Reznick et al., 1992; Rohrbacher et al., 2008). For this study we selected the social subscale based on evidence that social inhibition is the stronger predictor of social anxiety disorder (Chronis-Tuscano et al., 2009; Schofield, Coles, & Gibb, 2009). Participants ranged across the full continuum, from very low social inhibition (minimum = 1) to very high inhibition (max = 4.48) with a mean score of 2.66 (SD = 1.1). In this sample, internal consistency of the social subscale was excellent for both the ASRI (Cronbach's α = .96) and RSRI (Cronbach's α = .97). Social inhibition scores for each participant were computed by averaging the scores from the ASRI and RSRI social subscales. To ensure that it was valid to average these two scales, we also compute an average score after standardizing the ASRI and RSRI scores (mean = 0 and standard deviation = 1). The two methods were virtually identical (ṟ =.99), therefore we used the original values to facilitate interpretation of the results.
23. rsfMRI data
2.3.1. Data acquisition
Seven minutes of functional MRI “resting state” data were obtained approximately 20 min after entering the scanner, following structural MRI data collection. Participants were instructed to relax and close their eyes, but not to fall asleep. Structural and EPI images were acquired on a Philips 3T scanner. High resolution T1-weighted structural images were collected using the following parameters: 256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap. Functional images were acquired using the following parameters: 2000 ms TR/35 ms TE; 79° flip angle; 1.8 SENSE; 240 mm FOV; 3 × 3 mm in plane resolution using an 80 × 80 matrix (reconstructed to 128 × 128). Each volume contained 28 4 mm slices (acquisition voxels = 3 mm × 3 mm × 4 mm) and provided whole brain coverage.
2.3.2. Data processing
Functional data were assessed for acceptable quality across six standard measures: signal-to-noise ratio, percent standard deviation, percent standard deviation histogram, percent drift, percent fluctuation, and spatial correlation variance ratio. Scans were determined to be acceptable based on classification criteria derived from an independent data set of 84 subjects (A. Cao, unpublished). Functional data were preprocessed in SPM8 including slice time correction, motion correction, coregistration to the structural image, normalization to MNI space, resampling (3 × 3 × 3 mm) and smoothing (8 mm). All subjects had data within acceptable motion limits (<2 mm translation and 2 degrees rotation) and degree of motion was not correlated with social inhibition scores nor social inhibition groups (based on tertiles).
2.3.3. Intrinsic connectivity
Amygdala seed regions were used to identify amygdala intrinsic networks. Because the amygdala is comprised of functionally heterogeneous subnuclei, we examined connectivity with three distinct amygdala subnuclei: the centromedial, superficial, and laterobasal. The subnuclei were created using a standard probabilistic atlas (Amunts et al., 2005), consistent with previous studies (Roy et al., 2013; Roy et al., 2009). The three subnuclei regions were created by thresholding the probabilistic mask at 50% and by assigning voxels that belonged to more than one subnucleus to the subnucleus with the largest probability of membership. To provide a comparison with prior studies, we also performed an additional analysis with the whole amygdala as a seed. The results of this analysis are provided in Supplementary Material.
The four well-established intrinsic networks were identified using seed regions used in previous studies (Seeley et al., 2007; Vincent et al., 2006; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008; Woodward, Rogers, & Heckers, 2011) (MNI coordinates, x y z): default mode network (posterior cingulate: 1, −55, 17), dorsal attention network (left and right intraparietal sulcus/superior pariet al lobule: −25, −53, 52/25, −57, 52) executive control network (left and right dorsolateral prefrontal cortex: −42, 34, 20/44, 36, 20), and salience network (left and right fronto-insular cortex: −32, 26,−14/38, 22, 10). Seed regions were created by making a 6 mm diameter sphere around each coordinate. Consistent with previous studies, the time series signals from the seed regions within each intrinsic network were averaged to create a single signal (Woodward et al., 2011).
Functional connectivity was estimated using the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012). For each subject, the blood-oxygenation-level-dependent (BOLD) time series was estimated as the average time series for all voxels in each seed region. To remove potential sources of noise, signal was band pass filtered (.01 to. 1 Hz) and white matter, global and CSF signals were removed. Correlations of the time series were estimated between the average time course for each seed region with every other voxel in the brain, producing beta images for each subject and seed region. The resulting beta images were used for all subsequent analyses.
2.3.4. Statistical analysis
For each of the amygdala and intrinsic connectivity networks, regression analyses were performed in SPM8 to determine whether degree of intrinsic connectivity was associated with degree of social inhibition. Although the left and right amygdala intrinsic networks are generally similar (Roy et al., 2009), there is evidence for amygdala laterality in fMRI studies (Baas, Aleman, & Kahn, 2004) and in a previous study of amygdala intrinsic connectivity in anxious adolescents (Roy et al., 2013). Therefore, we interrogated the left and right amygdala networks separately.
To restrict our analyses to relevant regions and reduce multiple comparisons, analyses were masked by overall positive or negative networks. These networks were identified using a one-sample analysis with all participants for each of the seeds. A cluster-based threshold of p < .005 and k = 75 provided family wise error correction at α = .05. Cluster size was computed based on simulations performed with AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with the whole brain mask, 10 mm FWHM smoothing (based on actual smoothness), and 5,000 iterations. Restricting the regression analysis to the network connectivity masks ensures that the observed patterns of association are in the context of overall patterns of connectivity. A single connectivity mask was used for each network; for the amygdala networks, the left and right connectivity masks were combined to create the single connectivity mask for each of the three subnuclei. The connectivity masks are shown in Supplementary Figs. 1–3. As expected based on a prior report (Roy et al., 2009), the three amygdala subnuclei showed distinct patterns of connectivity as well as some regions of overlap. The positive amygdala networks were mainly observed in subcortical and prefrontal cortical regions; whereas the negative amygdala networks were predominantly in the occipital and pariet al lobes. The intrinsic connectivity networks were largely distinct with some limited overlap and were consistent with previous descriptions (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006a; Raichle et al., 2001; Seeley et al., 2007).
For each regression analysis, cluster-based thresholding was used to adjust for multiple comparisons within each network tested. Based on simulations performed with AlphaSim with 5,000 iterations and the calculated smoothness of the data (averaged across subnuclei masks, 8 mm FWHM), a family-wise error rate of α ≤ 0.05 is achieved with a voxel threshold of p < .01 and the following cluster sizes: centromedial amygdala (positive k = 35; negative k = 23); laterobasal amygdala (positive k = 38; negative k = 30); superficial amygdala (positive k = 38; negative k = 29); default mode (k = 37), dorsal attention (k = 32), executive control (k = 33), and salience (k = 36). To provide information about the magnitude and direction of the association between social inhibition and connectivity, beta values were extracted from the overall significance map and displayed as a scatterplot. R2 values were computed as an effect size measure. Data were also split into three groups (tertiles of social inhibition scores) and means and standard errors were presented as bar graphs.
For both the amygdala subnuclei and intrinsic network analyses, exploratory cross-network connectivity analyses were performed. Previous studies of patients with anxiety disorders have shown cross-network connectivity alterations in both amygdala subnuclei networks (Etkin et al., 2009; Roy et al., 2013) and intrinsic connectivity networks (Sripada et al., 2012). To test for an association between social inhibition and cross-network connectivity, regression analyses were performed with the seed region of interest (e.g., default mode network seed) and were masked by a separate network (e.g., salience mask). For these analyses, significance was determined by a voxel p-value of .01 and the cluster size for the respective masks (listed above).
2.3.5. Anxiety disorders
Individuals with high social inhibition are at increased risk for developing an anxiety disorder. To provide an accurate representation of the full range of social inhibition, we included participants who met criteria for an anxiety disorder. However, one possible consequence is that the participants with anxiety disorders may significantly influence, or drive, the regression analyses. To explore this possibility we conducted several analyses. First, we compared social inhibition scores between participants with an anxiety disorder and the rest of the high social inhibition participants (using the score tertiles). Next, we tested for differences between the high social inhibition group (tertile) with and without anxiety on each of the significant clusters. Finally, we excluded the participants with an anxiety disorder and calculated the correlation between social inhibition with beta values. These results of these analyses can help clarify the influence of anxiety disorders versus social inhibition on the study findings.
3. Results
3.1. Intrinsic connectivity: Amygdala
3.1.1. Positive connectivity networks
Degree of social inhibition modulated positive intrinsic connectivity with each of the amygdala subnuclei (superficial, centromedial, laterobasal). Within regions that showed overall patterns of positive connectivity, higher social inhibition was associated with reduced connectivity predominantly within limbic, paralimbic, striatal, and prefrontal regions (Fig. 1 and Table 1). For the left superficial amygdala, social inhibition modulated positive connectivity with a large region of the medial prefrontal cortex encompassing the rostral (or pregenual) cingulate cortex, the parahippocampal gyrus, and the cerebellum. Social inhibition also modulated connectivity between the right superficial amygdala and the rostral anterior cingulate, putamen, insula, superior temporal gyrus, and brainstem. For the left centromedial amygdala, higher social inhibition scores were associated with reduced connectivity with the insula, hippocampus, and dorsal anterior cingulate (or the anterior midcingulate cortex (Shackman et al., 2011)). Social inhibition also modulated connectivity between the right centromedial amygdala and the insula, putamen, and superior temporal pole. Finally, for the laterobasal amygdala, social inhibition only modulated connectivity between the right laterobasal amygdala and the putamen.
Fig. 1.
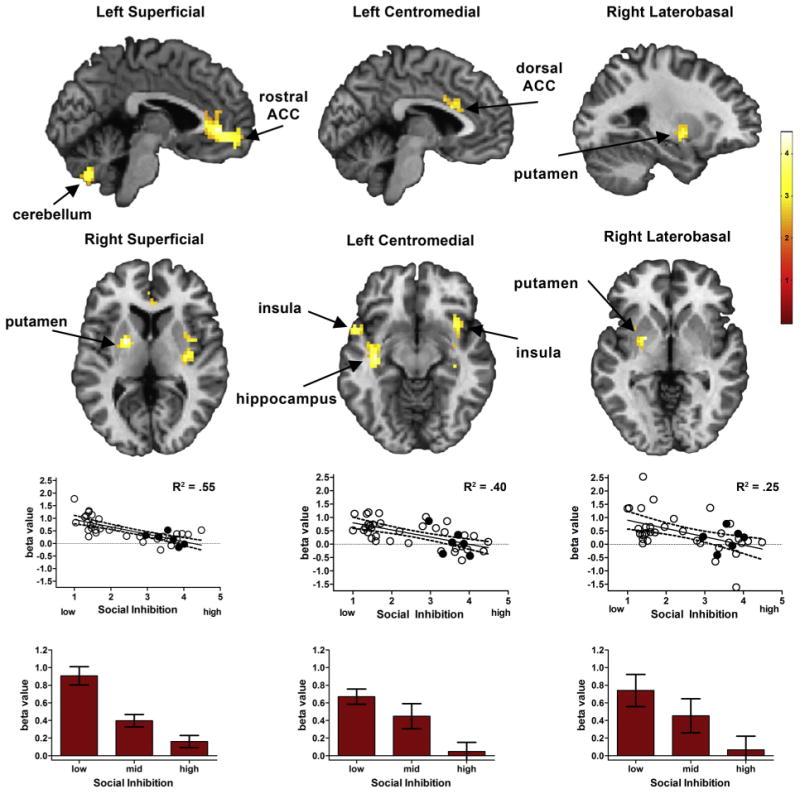
Positive intrinsic connectivity is reduced in high social inhibition. Strength of intrinsic connectivity was negatively associated with social inhibition across each of three amygdala subnuclei. Top row: Significant t-maps are shown on standard template brains (FWE corrected p < .05). Middle row: Scatter plots illustrate the association between social inhibition scores and connectivity extracted from the overall significance map. R2 values are provided as an effect size. Scatterplots show values for right superficial, left centromedial, and right laterobasal amygdala subnuclei, respectively. Filled circles represent participants with social anxiety disorder. Bottom row: Bar graphs show average positive connectivity by social inhibition tertile, with standard error bars, based on data presented in the scatterplots above.
Table 1.
Positivity connectivity results by amygdala subregion.
| Amygdala | Connectivity region | Cluster size | x | y | z | T |
|---|---|---|---|---|---|---|
| Superficial | ||||||
| Left | Rostral anterior cingulate (B) | 256 | 0 | 45 | 0 | 4.84 |
| Cerebellum (L) | 38 | 0 | −72 | −39 | 3.83 | |
| Hypothalamus (B) | 36 | −3 | 6 | −18 | 3.54 | |
| Parahippocampal gyrus (L) | 44 | 36 | −15 | −27 | 3.04 | |
| Right | Putamen (L) | 128 | −21 | −3 | 3 | 5.42 |
| Insula (R) | 51 | 33 | −15 | 6 | 3.72 | |
| Rostral anterior cingulate (B) | 82 | 0 | 27 | 12 | 3.52 | |
| Brainstem (B) | 45 | −6 | −18 | −15 | 3.34 | |
| Superior temporal gyrus (L) | 51 | −42 | 6 | −30 | 3.31 | |
| Centromedial | ||||||
| Left | Insula, Hippocampus (R) | 139 | 42 | −12 | −21 | 4.44 |
| Insula, Hippocampus (L) | 223 | −54 | 6 | −15 | 4.17 | |
| Dorsal anterior cingulate (B) | 39 | 0 | 18 | 24 | 3.17 | |
| Right | Superior temporal pole (L) | 44 | −60 | 6 | −6 | 3.75 |
| Superior temporal pole, Insula (R) | 169 | 36 | −9 | −18 | 3.55 | |
| Insula, Putamen (L) | 55 | −30 | 9 | 6 | 3.59 | |
| Laterobasal | ||||||
| Right | Putamen (L) | 62 | −21 | −3 | −6 | 4.17 |
Note: L = left, R = right, B = bilateral. Cluster Size is number of 3 × 3 × 3 clusters. Coordinates (x,y,z) are in MNI space. All values reported are statistically significant at p < .05, FWE corrected.
To determine whether social inhibition was associated with greater cross-network connectivity—that is, amygdala connectivity within the other amygdala subnuclei networks—we performed cross-network connectivity analysis. There was no evidence for an association between social inhibition and enhanced positive connectivity within the other amygdala subnuclei networks.
3.1.2. Negative connectivity networks
Social inhibition also modulated negative intrinsic connectivity with each of the three amygdala subnuclei, predominantly in visual cortical regions (Fig. 2, Table 2). For the left superficial amygdala, higher social inhibition scores were associated with reduced negative connectivity with the precuneus. For the right superficial amygdala, reduced connectivity was observed in the precuneus, angular gyrus, and superior frontal gyrus. For the centromedial amygdala, social inhibition was associated with reduced connectivity between the left centromedial amygdala and the visual cortex/BA17 and between the right centromedial amygdala and the lingual gyrus. For the left laterobasal amygdala, social inhibition modulated negative connectivity with two posterior regions—the precuneus and lingual gyrus. In contrast, for the right laterobasal amygdala, higher social inhibition was associated with reduced connectivity in the ventromedial prefrontal cortex and dorsolateral prefrontal cortex.
Fig. 2.
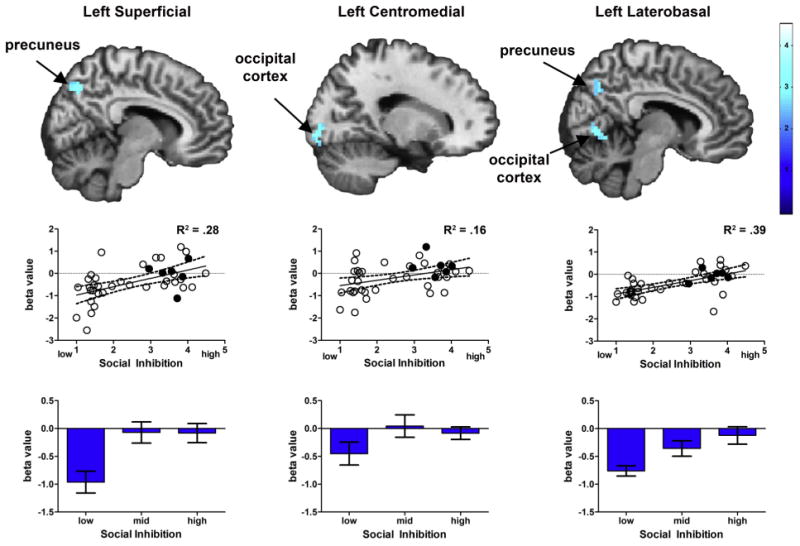
Negative intrinsic connectivity is reduced in high social inhibition. Strength of intrinsic connectivity was negatively associated with social inhibition across each of three amygdala subnuclei. Top row: Significant t-maps are shown on standard template brains (FWE corrected p < .05). Middle row: Scatter plots illustrate the association between social inhibition scores and connectivity extracted from the overall significance map. R2 values are provided as an effect size. Scatterplots show values for left superficial, left centromedial, and left laterobasal amygdala subnuclei, respectively. Filled circles represent participants with social anxiety disorder. Bottom row: Bar graphs show average negative connectivity by social inhibition tertile, with standard error bars, based on data presented in the scatterplots above.
Table 2.
Negative connectivity results by amygdala subregion.
| Amygdala subnucleus | Connectivity region | Cluster size | x | y | Z | t |
|---|---|---|---|---|---|---|
| Superficial | ||||||
| Left | Precuneus (R) | 58 | 3 | −69 | 45 | 3.64 |
| Middle frontal gyrus (R) | 27 | 24 | 15 | 51 | 2.93 | |
| Right | Angular gyrus (R) | 106 | 33 | −51 | 39 | 3.95 |
| Superior frontal gyrus (R) | 45 | 30 | 24 | 54 | 3.21 | |
| Precuneus (R) | 36 | 6 | −63 | 36 | 3.07 | |
| Centromedial | ||||||
| Left | Occipital Cortex/BA17 (R) | 39 | 15 | −93 | −6 | 3.75 |
| Right | Lingual gyrus (R) | 31 | 18 | −48 | −6 | 3.97 |
| Laterobasal | ||||||
| Left | Posterior cingulate/BA31 (R) | 28 | 9 | −48 | 33 | 3.90 |
| Lingual gyrus (R) | 39 | 3 | −72 | 0 | 3.34 | |
| Precuneus (R) | 48 | 12 | −66 | 36 | 3.13 | |
| Right | Ventral prefrontal cortex/BA10 (R) | 67 | 21 | 69 | 0 | 4.54 |
| Dorsolateral prefrontal cortex (R) | 39 | 39 | 15 | 51 | 3.44 |
Note: L=left, R= right, B = bilateral. Cluster size is number of 3 × 3 × 3 clusters. Coordinates (x,y,z) are in MNI space. All values reported are statistically significant at p < .05, FWE corrected.
To determine whether social inhibition was associated with stronger negative cross-network connectivity, we performed regression analyses within the negative connectivity masks. Higher social inhibition was associated with stronger negative cross-network connectivity. Enhanced connectivity from the left and right laterobasal amygdala and the left centromedial amygdala converged in part of the superficial amygdala network in the inferior pariet al lobe (left laterobasal amygdala [−42 −57 −51, k = 51]; right laterobasal amygdala [−39 −51 15, k = 75]; left centromedial amygdala [−54 −51 45, k = 92].
3.2. Intrinsic connectivity: other networks
To determine whether the influence of social inhibition was specific to intrinsic connectivity with the amygdala or reflected a broader pattern of altered connectivity, we also tested for differences with seed regions in the default mode network (DMN), dorsal attention network (DAN), executive control network (ECN) and salience (SAL) network. Social inhibition modulated connectivity with the seed regions that probed each of these networks (Fig. 3, Table 3). As with the amygdala, higher social inhibition was associated with weaker connectivity with the DMN and DAN seeds (Fig. 3, Table 3). However, for the ECN and SAL seeds, higher social inhibition was associated with stronger connectivity within these networks.
Fig. 3.
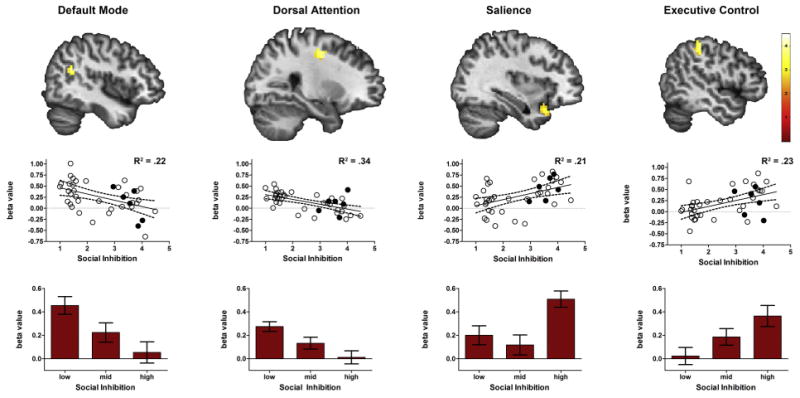
Social inhibition is correlated with intrinsic connectivity in four well-established networks. Significant t-maps maps are shown on standard template brains (FWE corrected p < .05). Scatter plots illustrate the association between social inhibition scores and connectivity in each significant cluster. R2 values are provided as an effect size. Filled circles represent participants with social anxiety disorder. Bar graphs show average positive connectivity by social inhibition tertile, with standard error bars, for each significant cluster.
Table 3.
Connectivity results by intrinsic network.
| Network | Connectivity region | Cluster size | x | y | Z | t |
|---|---|---|---|---|---|---|
| Default mode | Angular gyrus/BA39 (R) | 43 | 45 | −54 | 24 | 3.72 |
| Dorsal attention | Middle frontal gyrus, frontal eye fields (R) | 40 | 27 | −9 | 48 | 3.83 |
| Salience | Superior temporal pole (R) | 39 | 36 | 18 | −27 | 3.44 |
| Executive control | Inferior pariet al lobe/BA40 (L) | 35 | −48 | −33 | 48 | 3.87 |
Note: L= left, R= right, B = bilateral. Cluster Size is number of 3 × 3 × 3 clusters. Coordinates (x,y,z) are in MNI space. All values reported are statistically significant at p < .05, FWE corrected.
To determine whether the reduced connectivity with the DMN and DAN seeds resulted from increased cross-network connectivity, we tested for positive associations between social inhibition and intrinsic connectivity with the DMN and DAN seeds. Individuals with greater social inhibition did not show evidence of heightened cross-network connectivity.
To determine whether the heightened connectivity observed in the salience and executive control networks extended to other intrinsic networks, we performed cross-network analyses. For the SAL seed, degree of social inhibition was positively associated with connectivity within the default mode network (left precuneus: −12 −63 39, k = 65) and dorsal attention network (bilateral fusiform gyrus [36 −60 −15, k = 130; −21 −81 −9, k = 34]). For the ECN seed, degree of social inhibition was positively associated with connectivity within the dorsal attention network (bilateral pariet al lobe [33 39 36, k = 129; 39 −57 −9, k = 67] and bilateral fusiform gyrus [−45 −33 45, k = 79; −42 −75 −18, k = 56]) but not the default mode network.
3.3. The role of anxiety disorders
To determine the influence of highly inhibited individuals with an anxiety disorder on these results, we performed several post-hoc analyses. First, we compared social inhibition scores between participants with an anxiety disorder and participants with high social inhibition but not anxiety. The two groups had comparable social inhibition scores (no anxiety mean = 3.88, anxiety mean = 3.82, t = .38, p = .71) and were equally distributed across the high end of the continuum. Second, for each significant finding, the beta values for those with an anxiety disorder were comparable to the participants in the high social inhibition tertile without an anxiety disorder (all p's > .10). Finally, the regression analyses for each significant cluster remained significant even after removing the participants with anxiety disorders (all p's < .02).
4. Discussion
The goal of the present study was to determine whether individual differences in social inhibition—a stable trait and core feature of social anxiety disorder—reflected underlying differences in amygdala intrinsic connectivity. The main finding was that social inhibition was associated with altered intrinsic connectivity between each of the three amygdala subnuclei and a distributed neural network of cortical and subcortical regions that have putative modulatory influences over the amygdala. In individuals with high levels of social inhibition, amygdala connectivity was diminished relative to individuals with lower levels of social inhibition, suggesting that lack of connectivity with modulatory regions may contribute to the amygdala hyperactivity in response to social stimuli reported in previous studies (Beaton et al., 2008; Blackford et al., 2011; Schwartz et al, 2012). These associations occurred in networks that showed positive connectivity in the overall sample, adding to our ability to suggest that patterns of normal connectivity are diminished in inhibited individuals. Notably, the reductions in amygdala connectivity were observed in a “resting” state in the absence of social stimuli, demonstrating that individual differences in social inhibition reflect fundamental alterations in intrinsic connectivity.
Of particular interest was the reduced connectivity between the centromedial and superficial amygdala subnuclei and two regions of the anterior cingulate cortex, the rostral anterior cingulate cortex (rACC; also known as the pregenual cingulate cortex) and dorsal anterior cingulate cortex (dACC; also known as the anterior midcingulate cortex). Disruptions in prefrontal-amygdala connections figure prominently in anxiety (Kim et al., 2011) and primate tracer studies (Amaral et al., 1992; Carmichael & Price, 1995; Ghashghaei, Hilgetag, & Barbas, 2007) show that these regions of the cingulate cortex have strong bidirectional connections with the amygdala. Evidence from rodent and human studies suggest that these prefrontal cortical regions play a crucial role in regulating amygdala responses (Diekhof, Geier, Falkai, & Gruber, 2011; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Milad & Quirk, 2002; Ochsner et al., 2004; Quirk & Beer, 2006; Urry et al., 2006) and a recent effective connectivity study shows disrupted amygdala-prefrontal connectivity in patients with social anxiety disorder (Sladky et al., 2013). Thus, reduced intrinsic connectivity in prefrontal-amygdala circuits may underlie heightened social inhibition in humans and confer vulnerability for social anxiety.
Higher social inhibition was also associated with reduced intrinsic connectivity between the amygdala and several other regions that have structural connections with the amygdala in primates (Fudge, deCampo, & Becoats, 2012; Heath & Harper, 1974; Stefanacci & Amaral, 2002) and that have been previously implicated in anxiety (Caulfield & Servatius, 2013; Gray, 1982; Paulus & Stein, 2006; Shin & Liberzon, 2010) including the hippocampus, insula, and cerebellum. For example, rodent studies show that infusions of GABA(A) and 5HT1A agonists into the hippocampus reduce anxiety behaviors (Engin & Treit, 2007) and smaller hippocampal volume has been associated with post-traumatic stress disorder (Pitman et al., 2012). Although its precise role in human anxiety remains to be determined, the hippocampus is thought to modulate amygdala activity by engaging memory and pattern discrimination systems that can provide information about the relative threat or safety of a stimulus. The insula has been linked with anxiety sensitivity (Paulus & Stein, 2006; Stein et al, 2007a; Stein, Simmons, Feinstein, & Paulus, 2007b), a marker of sensitivity to the physical effects of anxiety, consistent with the insula's role in interoception (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). Multiple neuroimaging studies have reported increased insula activation in social anxiety (Etkin & Wager, 2007; Paulus & Stein, 2006); however, several studies have found decreased insula activation during tasks probing social anxiety and learning (Sareen et al, 2007; Tillfors et al., 2001). The insula has efferent projections to regions which regulate autonomic responses, including the amygdala and hypothalamus, and a study of effective connectivity found a unidirectional relationship from the insula to the amygdala (Stein et al., 2007a; Stein et al, 2007b). Thus, the reduced connectivity observed in the present study may reflect reduced modulatory input from the insula to the amygdala, resulting in heightened autonomic responses, which are characteristic of anxiety. Although the cerebellum is often not considered a critical part of anxiety neurocircuitry, evidence for a cerebellar role in anxiety is mounting (Caulfield & Servatius, 2013) and links between the cerebellum and anxiety warrant further exploration.
Brain regions with negative correlations of intrinsic activity are also detectable using rsfMRI and appear to have a biological basis (Fox, Zhang, Snyder, & Raichle, 2009). Individuals with higher social inhibition had reduced negative connectivity between the amygdala subnuclei and multiple regions in the pariet al and occipital cortices, including the precuneus, as well as several prefrontal cortical regions. All of these regions showed negative connectivity in the overall sample and are consistent with previous reports of amygdala subnuclei connectivity (Roy et al, 2009). The amygdala has strong bidirectional connections to the occipital cortex (Catani, Jones, Donato, & Ffytche, 2003; Gschwind, Pourtois, Schwartz, Van De Ville, & Vuilleumier, 2012) and in one study, greater occipital cortex activation to angry relative to neutral faces predicted better treatment response in patients with social anxiety disorder (Doehrmann et al., 2013). In healthy adults, the precuneus is negatively connected with the amygdala (Zhang & Li, 2012) and connectivity is reduced in patients with social anxiety disorder (Hahn et al., 2011). Although caution is recommended in interpreting negative connectivity, these findings suggest that the amygdala alterations are observed across both positive and negative connectivity networks and extend to posterior regions of the brain.
The three subnuclei had distinct patterns of overall connectivity and specific associations with social inhibition. For positive connectivity, the superficial amygdala was associated with differences in prefrontal cortical connectivity, whereas the centromedial amygdala was associated with differences in connectivity with the insula and hippocampus. However, there were also some regions that showed similar alterations in connectivity with all three sub-nuclei, for example, the putamen. For negative connectivity, the findings also showed separation across the three subnuclei. The associations with social inhibition were observed between the centromedial amygdala and occipital cortex, the superficial amygdala and the pariet al cortex and a region of the dorsal prefrontal cortex, and the laterobasal amygdala and three regions of the prefrontal cortex. Similar to the positive connectivity findings, there was one region that showed an overlap—the precuneus. Social inhibition was associated with connectivity between the precuneus and both the superficial and laterobasal subnuclei. These patterns of findings suggest that there is utility in examining amygdala subnuclei and that the reduced connectivity observed in individuals with higher social inhibition is not restricted to a particular subnucleus, but instead is widespread across all three amygdala subnuclei.
The lateralization of amygdala function remains a topic of debate. Meta-analyses of amygdala function during tasks point to greater left amygdala activation (Baas et al., 2004; Sergerie, Chochol, & Armony, 2008); however, it remains unknown whether this pattern is also present at rest. Comparing patterns of the left and right amygdala subnuclei in the present study provides some evidence for laterality. Several regions showed an association with social inhibition across both the left and right amygdala subnuclei seeds; for example, the association between social inhibition and connectivity between the superficial amygdala and rostral anterior cingulate was observed for both the left and right amygdala seeds. Similarly, Prater and colleagues report alterations in resting state connectivity between both the left and right amygdala and the rostral anterior cingulate in patients with generalized anxiety disorder (Prater, Hosanagar, Klumpp, Angstadt, & Luan Phan, 2013). However, many of the connectivity findings were observed for either the left or right amygdala, with findings relatively equally balanced across the two. Two previous studies of resting state differences in patients with an anxiety disorder also report lateralization of findings (Rabinak et al, 2011; Roy et al., 2013). Speculations about the importance of this lateralized pattern of findings would be premature and clearly, additional studies are needed in this area.
Importantly, the association between social inhibition and connectivity was not limited to the amygdala but was also observed in each of the four well-established intrinsic connectivity networks that we examined. Individuals with high social inhibition failed to show the typical patterns of connectivity in the default mode and dorsal attention networks and had enhanced connectivity in the salience and executive control networks. Cross-network connectivity analyses suggest that the lack of connectivity in the highly inhibited individuals with the default mode and dorsal attention network seeds do not result from enhanced connectivity in other networks. In contrast, highly inhibited individuals showed enhanced cross-network connectivity with both the salience and executive control network seeds. Both seeds showed greater connectivity with the fusiform gyrus, a key region for the processing of faces (Kanwisher, McDermott, & Chun, 1997), that is strongly connected to the amygdala (Herrington, Taylor, Grupe, Curby, & Schultz, 2011), and has been implicated in the pathophysiology of social anxiety disorder (Etkin & Wager, 2007; Frick, Howner, Fischer, Kristiansson, & Furmark, 2013).
Of interest is the opposing pattern of findings in two “control” networks, the executive control network and the dorsal attention network. In the executive control network, individuals with high social inhibition showed stronger connectivity between the dlPFC seed and a region of the inferior pariet al lobule (IPL; BA40). The IPL plays a role in preparation (Rosano et al., 2005; Ruge, Braver, & Meiran, 2009) and inhibitory control, cognitive functions that are generally considered to be protective for anxiety. However, evidence suggests that for temperamentally inhibited children, heightened inhibitory control predicts increased social withdrawal and social anxiety (McDermott et al, 2009; White, McDermott, Degnan, Henderson, & Fox, 2011). Thus, the results from this study provide initial evidence for a neural substrate linking heightened executive control and social anxiety.
In the default mode network, positive correlations between the posterior cingulate cortex and angular gyrus were lacking in individuals with high social inhibition. Qiu and colleagues (2011) reported decreased regional coherence—a measure of local synchronization in resting state BOLD signal—in the angular gyrus in patients with social anxiety, which may account for the decreased connectivity observed in this study. The angular gyrus supports episodic memory (Sestieri, Corbetta, Romani, & Shulman, 2011; Vilberg & Rugg, 2008), suggesting that the deficits in angular gyrus function may contribute to episodic memory dysfunction in social anxiety (Airaksinen, Larsson, & Forsell, 2005). Memory deficits may be especially critical for anxiety given the importance of memory creation and recall for extinguishing conditioned fear. Thus we propose that alterations in the intrinsic attention network may contribute to deficits in directing attention. In the dorsal attention network, intrinsic connectivity between the intrapariet al sulcus and the frontal eye fields was absent in individuals with high social inhibition. Socially anxious and temperamentally inhibited individuals have been shown to have trouble inhibiting reflexive orienting to faces (Perez-Edgar et al., 2011; Wieser, Pauli, & Mühlberger, 2009), consistent with theories that anxiety impairs goal-directed attention (Eysenck, Derakshan, Santos, & Calvo, 2007).
Finally, in the salience network, high social inhibition was associated with greater connectivity between the insula and superior temporal pole, a region involved in social and emotional processing (Olson, Plotzker, & Ezzyat, 2007). Although the salience network has been associated with anxiety (Seeley et al., 2007), the temporal pole finding is novel and may relate specifically to social inhibition.
Although the findings generally showed reduced negative connectivity in individuals with higher social inhibition, these same individuals showed enhanced connectivity in the cross-network connectivity analyses. These results provide some preliminary evidence that individuals with high social inhibition may have less differentiation between networks or may have somewhat different networks. For the amygdala negative connectivity analyses, higher social inhibition was associated with greater negative connectivity between two amygdala subnuclei (laterobasal and centromedial) and a region of the inferior pariet al lobe (BA 40) that was part of the superficial amygdala network. Interestingly, that cluster is similar to the area that showed enhanced connectivity with the ECN seed. In addition, higher social inhibition was associated with enhanced connectivity between the ECN seed and a slightly more anterior region of the inferior pariet al lobe that is part of the dorsal attention network (i.e., enhanced cross-network connectivity). Taken together, these findings point to enhanced positive connectivity between two attention/inhibitory control regions—the dlPFC and the inferior pariet al lobule—and greater decreased connectivity with the amygdala. While this inhibitory pattern could be considered adaptive, especially during a challenging task, the current findings suggest that at rest, this pattern is associated with a core feature of social anxiety. It will be important replicate these findings in an independent sample and to further explore the connectivity between these regions at rest and during social tasks.
Dimensional methods provide a different perspective from case-control approaches, since the trait is measured across a continuum without a definition of “healthy” and “diseased” states. However, a question that may arise is which pattern of brain activity is “normal” and which is altered. Previous studies performed in healthy controls can be useful in this regard. Previous studies of amygdala connectivity in healthy adults found that the centromedial amygdala showed connectivity with dorsal anterior cingulate (dACC) and the superficial amygdala showed connectivity with the rostral anterior cingulate (rACC) (Mishra et al., 2013; Roy et al., 2009). In our study, these same patterns of connectivity were seen at the lower and medium levels of social inhibition suggesting that lack of connectivity, as observed at the higher level of social inhibition, is aberrant. A related question is whether the patterns observed at the high inhibition end of the continuum reflect underlying trait differences that predispose for anxiety or reflect the clinical manifestation—social anxiety disorder. In the present study, we explicitly tested this question and found that the results were not driven specifically by participants who met criteria for an anxiety disorder. However, given that social inhibition is a critical component of social anxiety disorder, it is arguably difficult to tease the two apart. Recently, several studies have examined resting state connectivity in patients with social anxiety disorder (Ding et al, 2011; Gentili et al., 2009; Hahn et al., 2011; Liao et al., 2010a; Liao et al., 2010b; Liao et al., 2011; Prater et al., 2013; Qiu et al., 2011), of which three specifically looked at the amygdala (Hahn et al., 2011; Liao et al., 2010a; Liao et al., 2010b; Prater et al., 2013) and two used similar measures of functional connectivity (Hahn et al., 2011; Prater et al., 2013). Prater and colleagues (2013) found that patients with social anxiety disorder had reduced connectivity between the amygdala and the rACC and Hahn and colleagues (2011) reported reduced connectivity in a similar region of the ventromedial prefrontal cortex. Thus our findings confirm these studies and provide initial evidence that altered amygdala-rACC connectivity reflects the social inhibition trait and is therefore not specific to social anxiety disorder.
Several limitations to this study should be noted. First, social inhibition was assessed via self-report. Although self-report measures are subject to bias, the questionnaires used here were designed to minimize subjective ratings by focusing on objective questions about discrete behaviors. Furthermore, dimensional measures of social anxiety and other psychiatric symptoms often rely on self-report as well. Next, oversampling at the extreme ends provided a full continuum of social inhibition, including individuals with social anxiety disorder. However, this approach may have overemphasized the patterns observed in the extreme groups at each end of the continuum. Finally, social inhibition is only one aspect of social anxiety and different patterns might emerge for other aspects such as social-evaluative concerns.
In conclusion, individual differences in social inhibition reflect underlying differences in intrinsic connectivity with three amygdala subnuclei and across four well-established networks. Taken together, the findings suggest that high levels of social inhibition are predicted by a lack of connectivity with brain regions involved in modulating amygdala hyperactivity and directing attention away from stimuli, combined with enhanced connectivity in regions involved in salience detection and inhibitory control. Critically, these alterations were all observed in the absence of social stimuli, suggesting that differences in social inhibition reflect underlying differences in patterns of intrinsic brain connectivity. Given that social inhibition is a trait observed early in development and social anxiety has an early onset, it is imperative to develop prevention and early intervention strategies. Preventions and treatments that target circuit-level connectivity may be effective in reducing social inhibition, one of the most impairing components of social anxiety.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by funding from the National Institute of Mental Health (MH083052 to J.U.B.; MH097344 to J.A.C.; MH102008 to S.N.A.; MH073800 to R.L.C.; and MH018921), the National Institute of Drug Abuse (DA015137, DA020149 to R.L.C. and DA000357 to American Academy of Child and Adolescent Psychiatry supporting MMB), and the Vanderbilt Institute for Clinical and Translational Research (RR024975; RR024978), the Vanderbilt Medical Scientist Training Program (National Institute of General Medical Studies; GM07347); and the Vanderbilt University Institute of Imaging Science. Within the past 3 years, Dr. Cowan has received publication royalties from Lippincott Williams and Wilkins, consultant income from the Southwest Michigan First Life Science Fund and the University of West Alabama, and research and salary support from Shire Pharmaceuticals and Novo Nordisk for projects not overlapping with this report. The other authors have no financial relationships relevant to this article. Portions of this work were presented at the American College of Neuropsychopharmacology annual meeting, Hollywood, FL, December 2012.
Footnotes
Appendix A. Supplementary data: Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biopsycho.2014.02.003.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York City: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- American. Psychiatric association diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Schulkin J, Antony MM, Swinson RP, Hall GB. Different neural responses to stranger and personally familiar faces in shy and bold adults. Behavioral Neuroscience. 2008;122:704–709. doi: 10.1037/0735-7044.122.3.704. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, Wittchen HU. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, K+tter R. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Costello EJ, Foley DL, Angold A. What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry. 2007;48:1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience. 2011;6:621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Caulfield MD, Servatius RJ. Focusing on the possible role of the cerebellum in anxiety disorders. Durbano F, editor. New Insights Into Anxiety Disorders InTech. 2013 Retrieved from http://www.intechopen.com/books/new-insights-into-anxiety-disorders/focusing-on-the-possible-role-of-the-cerebellum-in-anxiety-disorders.
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JDH, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. European Journal of Neuroscience. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer JS, Blanco C, Hasin DS, Liu SM, Grant BF, Turner JB, Olfson M. Health-related quality of life across the anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions (NESARC) The Journal of Clinical Psychiatry. 2011;72:43–50. doi: 10.4088/JCP.09m05094blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Keough ME, Riccardi CJ, Sachs-Ericsson N. Anxiety disorders and suicidality in the National Comorbidity Survey-Replication. Journal of Psychiatric Research. 2009;43:825–829. doi: 10.1016/j.jpsychires.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental Brain Research. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, Van Dijk KRA. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, Gong Q. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magnetic Resonance Imaging. 2011;29:701–711. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Gabrieli JD. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87–97. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Bolton D, O'Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in preschool children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:945–960. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- Emde RN, Plomin R, Robinson JA, Corley R, DeFries J, Fulker DW, Zahn-Waxler C. Temperament, emotion, and cognition at fourteen months: the MacArthur Longitudinal Twin Study. Child Development. 1992;63:1437–1455. [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behavioural Pharmacology. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview For DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature Neuroscience. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Translational Psychiatry. 2013;3:e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, deCampo DM, Becoats KT. Revisiting the hippocampal amygdala pathway in primates: association with immature-appearing neurons. Neuroscience. 2012;212:104–119. doi: 10.1016/j.neuroscience.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Research Bulletin. 2009;79:409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling SD. From mice to men: What can we learn about personality from animal research? Psychological Bulletin. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety- an inquiry into the functions of the septo-hippocampal system. Behavioral and Brain Sciences. 1982;5:469–484. [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cerebral Cortex. 2012;22:1564–1576. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen V, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6:1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Experimental Neurology. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Taylor JM, Grupe DW, Curby KM, Schultz RT. Bidirectional communication between amygdala and fusiform gyrus during facial recognition. NeuroImage. 2011;56:2348–2355. doi: 10.1016/j.neuroimage.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1998;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Development. 1998;69:1483–1493. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick DJ, Kobak KA, DeLeire T, Henk HJ, Greist JH, Davidson JRT, Helstad CP. Impact of generalized social anxiety disorder in managed care. American Journal of Psychiatry. 2001;158:1999–2007. doi: 10.1176/appi.ajp.158.12.1999. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Chen HF, Feng YA, Mantini D, Gentili C, Pan ZY, Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, Chen H. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PloS One. 2010;5:e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JEC, Chen H. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Research. 2011;1388:167–177. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein E, Yang Y. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. NeuroImage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mishra A, Rogers BP, Chen LM, Gore JC. Functional connectivity-based parcellation of amygdala using self-organized mapping: A data driven approach. Human Brain Mapping. 2013 doi: 10.1002/hbm.22249. http://dx.doi.org/10.1002/hbm.22249. [DOI] [PMC free article] [PubMed]
- Moitra E, Beard C, Weisberg RB, Keller MB. Occupational impairment and social anxiety disorder in a sample of primary care patients. Journal of Affective Disorders. 2011;130:209–212. doi: 10.1016/j.jad.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Hyde JS. Resting-state functional connectivity of the rat brain. Magnetic Resonance in Medicine. 2008;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson H, Degnan K, Pérez-Edgar K. Attention biases to threat link behavioral inhibition to social withdrawal overtime in very young children. Journal of Abnormal Child Psychology. 2011;39:1–11. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, Perez-Edgar K. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma YJ. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Luan Phan K. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Roseboom PH, Nanda SA, Lane JC, Speers JM, Kalin NH. Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes Brain and Behavior. 2010;9:974–984. doi: 10.1111/j.1601-183X.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, Gong Q. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Research: Neuroimaging. 2011;194:47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Development and Psychopathology. 1992;4:301–321. [Google Scholar]
- Robinson JL, Reznick JS, Kagan J, Corley R. The heritability of inhibited and uninhibited behavior: a twin study. Developmental Psychology. 1992;28:1030–1037. [Google Scholar]
- Rohrbacher H, Hoyer J, Beesdo K, Höfler M, Bittner A, Lieb R, Wittchen HU. Psychometric properties of the Retrospective Self Report of Inhibition (RSRI) in a representative German sample. International Journal of Methods in Psychiatric Research. 2008;17:80–88. doi: 10.1002/mpr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman aB, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. NeuroImage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele a, Carlisi T, Ernst CM. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2013:1–12. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly MC, Uddin LQ, Gotimer K, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Braver T, Meiran N. Attention, intention, and strategy in preparatory control. Neuropsychologia. 2009;47:1670–1685. doi: 10.1016/j.neuropsychologia.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Trivedi MH. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. Journal of Affective Disorders. 2005;87:43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sareen J, Campbell DW, Leslie WD, Malisza KL, Stein MB, Paulus MP, Reiss JP. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biological Psychiatry. 2007;61:396–404. doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Blanco C, Antia SX, Liebowitz MR. The social anxiety spectrum. Psychiatric Clinics of North America. 2002;25:757–774. doi: 10.1016/s0193-953x(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Foose TE, Hasin DS, Heimberg RG, Liu SM, Grant BF, Blanco C. Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2010;40:977–988. doi: 10.1017/S0033291709991231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, Liebowitz MR. Functional impairment in social phobia. The Journal of Clinical Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia - comorbidity and morbidity in an epidemiologic sample. Archives of General Psychiatry. 1992;49:282–288. doi: 10.1001/archpsyc.1992.01820040034004. [DOI] [PubMed] [Google Scholar]
- Schofield CA, Coles ME, Gibb BE. Retrospective reports of behavioral inhibition and young adults' current symptoms of social anxiety, depression, and anxious arousal. Journal of Anxiety Disorders. 2009;23:884–890. doi: 10.1016/j.janxdis.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry. 2012;17:1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, pariet al cortex, and the default mode network: functional and topographic analyses. The Journal of Neuroscience. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Hö A, Küblböck M, Kraus C, Baldinger P, Moser E, Lanzenberger R. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by Dynamic Causal Modeling for fMRI. Cerebral Cortex, epub ahead. 2013 doi: 10.1093/cercor/bht279. http://dx.doi.org/10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. The Journal of Comparative Neurology. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stein MB. An epidemiologic perspective on social anxiety disorder. The Journal of Clinical Psychiatry. 2006;67(Suppl. 1):3–8. [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Stein MB, Stein DJ. Social anxiety disorder. The Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Setting diagnostic thresholds for social phobia - considerations from a community survey of social anxiety. American Journal of Psychiatry. 1994;151:408–412. doi: 10.1176/ajp.151.3.408. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. The American Journal of Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the pariet al cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan K, Henderson H, Fox N. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Mühlberger A. Probing the attentional control theory in social anxiety: an emotional saccade task. Cognitive, Affective & Behavioral Neuroscience. 2009;9:314–322. doi: 10.3758/CABN.9.3.314. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Rogers BP, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophrenia Research. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nature Reviews Neurology. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CR. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


