Abstract
FSH plays an important role in ovarian follicular development, and it functions via the G-protein coupled FSH receptor. The objectives of the present study were to determine if full-length FSHR mRNA and corresponding protein were expressed in fetal through postnatal hamster ovaries to explain the FSH-induced primordial follicle formation, and if FSH or estrogen (E) would affect the expression. A full-length and two alternately spliced FSHR transcripts were expressed from E14 through P20. The level of the full-length FSHR mRNA increased markedly through P7 before stabilizing at a lower level with the formation and activation of primordial follicles. A predicted 87kDa FSHR protein band was detected in fetal through P4 ovaries, but additional bands appeared as ovary developed. FSHR immunosignal was present in undifferentiated somatic cells and oocytes in early postnatal ovaries, but was granulosa cells specific after follicles formed. Both eCG and E significantly up-regulated full-length FSHR mRNA levels. Therefore, FSHR is expressed in the hamster ovary from the fetal life to account for FSH-induced primordial follicle formation and cAMP production. Further, FSH or E regulates the receptor expression.
Keywords: FSH-receptor, ovary, primordial follicle, FSH
Introduction
The follicle-stimulating hormone, a pituitary glycoprotein, plays a critical role in ovarian follicular development (Greenwald and Roy, 1994, Ulloa-Aguirre, Midgley, Beitins et al., 1995). Null mutation of FSHβ or FSHR gene in mice results in arrest in follicular development at the preantral stage (Dierich, Sairam, Monaco et al., 1998, Kumar, Wang, Lu et al., 1997). Numerous studies have shown that FSH regulates follicular development (Greenwald and Roy, 1994, Richards, 1980, Roy, 1999) and follicular estrogen biosynthesis (Erickson and Hsueh, 1978, Gore-Langton and Armstrong, 1994). Evidence suggests that development of preantral follicles requires FSH action (McGee, Spears, Minami et al., 1997, Roy and Greenwald, 1986, Roy and Greenwald, 1989, Yang and Roy, 2004). Further, the formation of primordial follicles in the golden hamster has been shown to require FSH action (Roy and Albee, 2000, Wang and Roy, 2004). FSH acts via FSH receptor (FSHR), a G-protein coupled, seven transmembrane domain receptor, which is coupled to membrane adenylate cyclase, and its gonadal function is mediated by cyclic adenosine- 3′, 5′ monophosphate (cAMP) (Camp, Rahal and Mayo, 1991, Simoni, Gromoll and Nieschlag, 1997, Uilenbroek and Richards, 1979). FSHR is expressed exclusively in the granulosa cells of the adult ovary (Camp et al., 1991, Rannikki, Zhang and Huhtaniemi, 1995, Uilenbroek and van der Linden, 1983), and its expression has been shown to occur as early as primary stage follicles in the hamster (Roy, Wang and Greenwald, 1987). Direct evidence supporting the expression of FSHR in the granulosa cells of primordial follicles is not available primarily because of the limitation in the detection resolution and in enzymatic isolation of structurally intact primordial follicles, which are composed of loosely assembled granulosa cells (Roy and Greenwald, 1985). However, low levels of FSHR transcripts encoding all domains of the full length FSH receptor have been detected in the newborn mouse (O’Shaughnessy, Marsh and Dudley, 1994, O’Shaughnessy, McLelland and McBride, 1997) and rat (Rannikki et al., 1995) ovaries.
In the ovary of CD-1 or C57BL mice, anatomically distinct primordial follicles appear by the morning of embryonic age 19 (E19) and newborn ovaries contain not only primordial but also primary follicles. Remarkable high levels of FSH have been detected in the mouse plasma around the time of birth (Stiff, Bronson and Stetson, 1974). Sokka et al (Sokka and Huhtaniemi, 1990) have shown that while consistent binding of [125]FSH occurs in the rat ovary from postnatal day 7 (P7), cAMP production can be detected in the fetal ovary indicating that a functional adenylate cyclase system develops in the ovary during fetal development. In contrast, the formation of primordial follicles in the hamster ovary begins from postnatal day 8 both in vivo (Roy and Albee, 2000) and in vitro (Mukherjee and Roy, 2013, Wang and Roy, 2007, Wang and Roy, 2004, Yu and Roy, 1999), and plasma FSH is detectable at birth and increases during the formation of primordial follicles (Roy and Hughes, 1994, Vomachka and Greenwald, 1979). Neutralization of plasma FSH with an anti-FSH serum by E12 suppresses primordial follicle formation in postnatal life (Roy and Albee, 2000). Further, FSH stimulates in vitro cAMP production by E13 hamster ovaries (Roy and Albee, 2000) and primordial follicle formation in E15 hamster ovaries in vitro (Wang and Roy, 2004). These lines of evidence suggest that functional FSHR system is present in the hamster ovary from the fetal life. However, molecular evidence supporting the presence of FSHR transcripts or protein during perinatal ovary development and its regulation by FSH in the hamster is lacking. Therefore, the objectives of the present study were to determine the ontogeny of FSHR mRNA and protein expression in the hamster ovary from fetal through early postnatal development, and to examine whether FSH or E would affect receptor expression.
Materials and methods
Animals
Female (90–100 g) and male (100–120 g) golden hamsters were purchased from Harlan Sprague Dawley Laboratories (Indianapolis, IN) and housed in a climate-controlled environment with 14h light and 10h dark cycle, and fed ad libitum according to the UNMC Institutional Animal Care and Use Committee and United States Department of Agriculture guidelines. The use of hamsters in the research was approved by UNMC IACAC. Females with three consecutive estrous cycles were used in the experiments.
Chemicals
The antibody to FSH-receptor (catalogue No. ab103874, Abcam, Cambridge, MA) was generated in rabbits against a peptide sequence corresponding to the extracellular domain (250–350 amino acids) of human FSH-receptor. The anti-FSH antiserum was raised in rabbits and thoroughly characterized in the laboratory (Roy and Albee, 2000), quantitative RT-PCR primers and fluorescence probes were synthesized by Sigma Chemical Company (St. Louis, MO), equine chorionic gonadotropin (eCG) was from Sigma Chemical Company, RNeasy mini kit and Taq DNA polymerase were from Qiagen, Inc. (Valencia, CA), chemiluminescence Western blot detection kit, and donkey anti-rabbit IgG-peroxidase conjugated second antibody were from GE Healthcare (Pittsburgh, PA), and Alexa 594-conjugated donkey anti-rabbit IgG second antibody was from Life Technologies. Other molecular biology grade chemicals were obtained from Sigma Chemical Company (St. Louis, MO), United States Biochemical (Cleveland, OH) or Fisher Scientific Corporation (Pittsburgh, PA).
Experimental designs
Female hamsters with three consecutive estrous cycles were mated overnight with males and the presence of sperm in vaginal lavage was considered as day 1 of pregnancy. Pregnant females were either euthanized on gestation day 14 or 15, or allowed to deliver on 16th day of gestation. Ovaries were retrieved from female pups from postnatal day 1 (P1) through P20, and were either frozen in Optimum Cutting Temperature medium (OCT) on liquid N2 or frozen in liquid N2 for protein or RNA extraction.
In the second design, pregnant females were injected sc with 100 μl anti-FSH serum on gestation day 12, and allowed to deliver on 16th day of gestation. Pups were either injected sc with 20 μl saline or 20IU eCG in saline on P1. Ovaries were retrieved on P8, and frozen in liquid N2 for RNA extraction.
In the third design, pups were injected sc with 1 μg estradiol-17β cipionate on P1 and P4. Ovaries were retrieved on P8 and frozen in liquid N2 for RNA extraction.
RT-PCR and Southern blotting of FSHR cDNA
As the first step, we wanted to know if FSHR transcripts were present in perinatal hamster ovaries and if there were spliced variants as reported for the mouse (O’Shaughnessy et al., 1994) and human (Conway, Conway, Walker et al., 1999). Primers for the endpoint PCR and probe for Southern hybridization were designed based on the golden hamster FSHR (accession No. AY509907) nucleic acid sequence corresponding to exon 1 and exon 10 (Zhang and Roy, 2004) in order to detect the full length as well as alternately spliced variants. The sequences for primers and Southern probe have been presented in Table 1. Total ovarian RNA was extracted in Trizol (Life Technologies, Carlsbad, CA) and isolated using RNeasy mini kit from Qiagen, and checked for purity in 1 % formaldehyde agarose gels as described previously (Yang, Kriatchko and Roy, 2002). One μg of total ovarian RNA from E13 to P20 hamsters was reverse-transcribed and amplified using Superscript reverse transcription kit with random hexamers (Qiagen), and Taq DNA polymerase cDNA amplification kit (Qiagen) with the standard PCR primer pair for 40 cycles with 55°C annealing temperature in a M J Research DNA engine thermocycler (Bio-Rad, Hercules, CA). Amplified cDNA was fractionated in an 1% agarose gel in TAE (Tris-Acetate-EDTA, pH 8.0) buffer, capillary transferred to Nytran membrane (Schleicher and Schuell, Gmbh, Germany), UV cross-linked and Southern hybridized with [32]P-labeled probe as described previously (Zhang and Roy, 2004). After thorough washing with appropriate buffer, the membrane was wrapped in plastic film, exposed overnight to an X-ray film, developed and digitized using an UVP-gel documentation system (UVP, Upland, CA). The experiment was repeated three times using ovarian RNA from at least three pups.
Table 1.
End point and quantitative PCR primers and probe for amplifying hamster FSHR cDNA. The end point PCR primers were designed from the hamster FSHR cDNA corresponding to exon 1 and exon 10 sequences while the qPCR primers and fluorescence-labeled probe to detect specifically the full-length FSHR mRNA were designed from the selected area of the full-length hamster FSHR cDNA.
| Primer | sequence |
|---|---|
| FSHR-F1 (end point PCR) | 5′-TGTCATCACTGGCTGTGTCAT-3′ |
| FSHR-R1 (end point PCR) | 5′-ATTTGGATGAAGTTCAGAGGTT-3′ |
| FSHR-Southern probe (end point PCR) | 5′-CCTGATGTGTAACCTCGCCT-3′ |
| FSHR-qPCR-F1-full-length specific | 5′-TAATATCCAACACGGGCATCAA-3′ |
| FSHR-qPCR-R1-full-length specific | 5′-TGGTGTGGAGGTTTATGTTATCTTG-3′ |
| FSHR-qPCR-5′-Fam/3′-Blackhole probe-full-length specific | 5′-TTGCCAGTGGTTCATAAGATTCAGTCTCTTCAA-3′ |
| Actin-qPCR-F1 | 5′-TGACCGAGCGTGGCTACAG-3′ |
| Actin-qPCR-R1 | 5′-CTTCTCTTTGATGTCACGCACAAT-3′ |
| Actin-qPCR-5′-Fam/3′-Blackhole probe | 5′-TCACCACCACAGCCGAGAGGGA-3′ |
Quantitative RT-PCR detection of ovarian FSHR during development
Total ovarian RNA from E14 through P20 hamsters or from hamsters treated with the FSH-antiserum with or without eCG, or E were reverse transcribed using Superscript reverse transcription kit (Qiagen) with random hexamers as previously described (Wang and Roy, 2009). The cDNA was amplified using high fidelity cDNA amplification kit (Invitrogen) along with real-time PCR primer pair and probe (Table 1), which was labeled with 5′-FAM and 3′-Blackhole in the Opticon 2 thermocycler (Bio-Rad). PCR primers and probe were designed based on nucleic acid sequence encompassing exon 5 and a part of exon 6 of the hamster FSHR cDNA to ensure that the amplicon would represent only the full-length FSHR mRNA. Exons 5 and 6 were absent in hamster FSHR splice variants. The amplification was carried out along with a set of gene specific cDNA standards developed from in vitro synthesized FSHR mRNA. Levels of FSHR mRNA were determined from the standard curve and normalized against the levels of β-actin mRNA. The ratios from at least three separate quantifications of FSHR and β-actin mRNA from ovaries of three hamsters were used for determining the mean levels of FSHR mRNA during ovary development.
Western blot detection of FSHR protein expression during hamster perinatal ovary development
Ovaries from E14 through P10 hamsters were homogenized in RIPA buffer as described previously (Yang, Wang, Shen et al., 2004), and 80 μg protein was denatured in SDS sample buffer, fractionated in 10% polyacrylamide gel and electrotransferred to Optitran nitrocellulose membrane. The membrane was blocked with 5% milk in Tris buffered saline, pH 7.5 and probed overnight with the rabbit polyclonal 1:80 FSHR antibody. The chemiluminescence signal was developed using peroxidase-conjugated donkey anti rabbit IgG and ECL Advance kit as suggested by the manufacturers. To validate the specificity of the FSH-receptor antibody, granulosa cells from antral follicles, KGN cells (human granulosa cell line, which express FSH-receptor), D4:1600h ovaries, leg muscle, liver and uterus were retrieved from hamsters, and processed for immunoblotting or immunofluorescence detection of FSHR as described previously.
Immunofluorescence localization of FSHR in perinatal hamster ovaries
To determine if the FSHR antibody was suitable for specific detection of FSHR, 6 μm frozen sections of ovaries, liver and muscle from proestrus hamsters at 1600h were fixed for 10 min in freshly made 4% paraformaldehyde exactly at 4°C. Sections were rinsed with phosphate-buffered saline (PBS), pH 7.5, permeablized with 0.2% Triton X100 in PBS, treated with the Image-IT FX blocking solution (Life technologies) as suggested by the manufacturer, rinsed, blocked with 10% donkey serum/0.1% Triton X100 in PBS, and incubated with 1:80 dilution of the rabbit polyclonal FSHR antibody in blocking buffer overnight at 4°C. After thorough rinsing, sections were incubated with 10 μg/ml Alexa 594-conjugated donkey anti-rabbit IgG/300 nM 4′,6-diamino-2-phenylindole (DAPI) for 30 min at room temperature, rinsed and mounted with Fluromount G. Similar protocol was used for ovaries obtained from E14 through P15 hamsters. Immunofluorescence images were viewed in a Leica DMR fluorescence microscope under PalnApo 40X or 65X oil immersion lense and captured by a Qimaging Retiga digital camera using the Openlab image analysis software (Improvision). Because of extremely low fluorescence signal intensity, lower magnification imaging was not possible. The exposure time was set to subtract any background fluorescence based on sections incubated with non-immune rabbit IgG. The images were formatted in Adobe Photoshop without altering the original contrast, and finally composed in Microsoft Power Point for presentation.
Statistical analysis
All quantitative values were analyzed by 1-way ANOVA with Tukey’s post-hoc test. The P<0.05 was considered as statistically significant. There were at least three hamsters for each experiment. Each experiment was repeated at least twice for reproducibility.
Results
Southern blot data revealed that three FSHR transcript variants were present in fetal and early postnatal hamster ovaries (Fig. 1). For the sake of clarity, blots up to P4 were furnished. Sequence analysis revealed that whereas the 810bp band represented all 10 exons of the hamster FSHR, 322bp and 280bp bands corresponded to exons 7 through 10, and exon 1 through 4, respectively. Apparently, all three FSHR transcript variants were expressed from fetal day 13; however, the level of expression of full-length FSHR transcript increased appreciably on E15, and the expression level increased many fold by birth (P1) (Fig. 1). Concurrent with the full-length transcript, the levels of shorter transcripts also increased remarkably from E15 to reach very high level by P4 (Fig. 1). FSHR mRNA expression decreased notably by P2 followed by a remarkable increase by P3 through P4. Thereafter, full-length FSHR expression remained at the levels of P4 with prominent expression of the full-length mRNA along with high expression of transcript variants. It was also apparent that despite marked increase in full-length transcript expression; the levels of spliced variants remain very high suggesting that a major part of FSHR mRNA undergo alternate splicing during ovary development in the hamster.
Fig. 1.
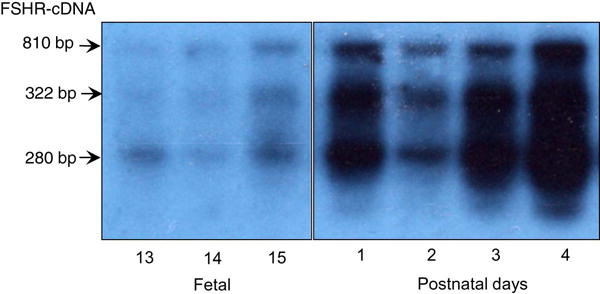
Southern blot hybridization of the FSHR cDNA amplified from the total RNA of fetal and postnatal hamster ovaries. The blots up to P4 were presented for clarity because the pattern remained almost unchanged beyond P4. The arrows highlight the transcript variants of indicated size.
Quantitative PCR (qPCR) revealed that the expression of full-length FSHR mRNA was not only consistent with the Southern blot data, but it also reflected a unique pattern of FSHR expression concurrent with ovarian follicular development (Fig. 2). FSHR mRNA expression increased markedly on P1 and peaked by P4; thereafter, the expression level gradually declined by P10 and remained stable through P20 (Fig. 2).
Fig. 2.
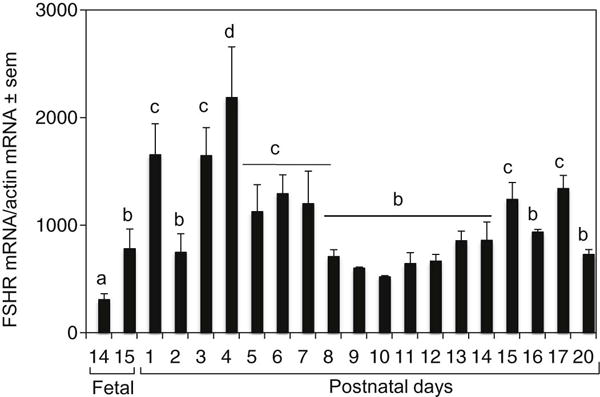
Quantitative RT-PCR analysis of FSHR mRNA levels in perinatal hamster ovaries. Total ovarian RNA was amplified using primers to detect specifically the full-length FSHR mRNA. Each bar represents the ratio of FSHR and β-actin ± standard error of mean (sem). P<0.05, bars with different letter.
Western blot data confirmed that the full-length FSHR mRNA was translated into an approximately 87kDa FSHR protein in hamster ovarian cells as early as E14, although the expression varied (Fig. 3A–D). Whereas a single 87kDa band was evident till P4 (Fig. 3A and B), a smaller size band appeared by P5 and its intensity increased through P7 (Fig. 3C). The intensity of the 87kDa band declined by P8; however, one additional band at higher molecular weight was visible from P8 onwards (Fig. 3D). The pattern and intensity of ovarian FSHR protein expression remained unaltered beyond P10 (data not shown). Despite the appearance of additional bands, the overall levels of FSHR protein during perinatal development more or less followed the mRNA pattern, although the degree of change was modest (Fig. 3E).
Fig. 3.
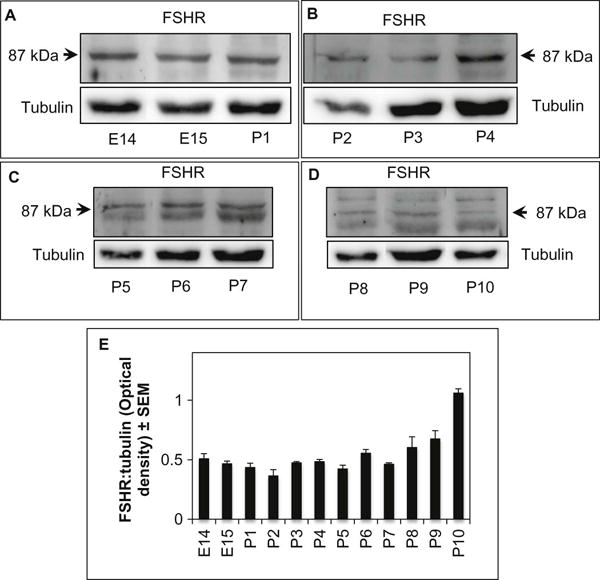
Western blot detection of FSHR and tubulin proteins in ovaries obtained from fetal age 14 (E14) through postnatal age 10 (P10) (A–D). The antibody detected an 87kDa band from E14 through P4 (A–B); however, additional bands appeared concurrent with postnatal development (C–D). (E) Semi-quantitative presentation of the optical density (O. D.) of overall FSHR protein in the immunoblots. Each bar represents digitized values of FSHR proteins normalized to tubulin from three Western blots. The data reflect mean ± SEM.
The specificity of the FSHR antibody was evident from its binding to the granulosa cells of antral follicles at D4:1600 (Figs. 4A, 4B). No FSHR fluorescence was visible when sections of D4:1600h or P10 ovary was incubated with the non-immune rabbit serum (Figs. 4C and 4D). Further, no FSHR immunofluorescence could be localized in sections of the hamster liver (Fig. 4E) or gastrocnemius muscle (Fig. 4F) incubated with the FSHR antibody dilution used on the D4:1600h ovary sections (Fig. 4B). Western blot analysis revealed one distinct band around 87kDa for KGN and hamster antral granulosa cells (D4); however, a very high and two low molecular weight bands were also barely visible (Fig. 4G). A faint band was also visible immediately lower to the prominent band for the hamster (Fig. 2G). No similar size band was visible for hamster muscle (M), liver (L) or uterus (U) (Fig. 2G). The intensity of the band suggested that FSHR existed in the granulosa cells at very low level.
Fig. 4.
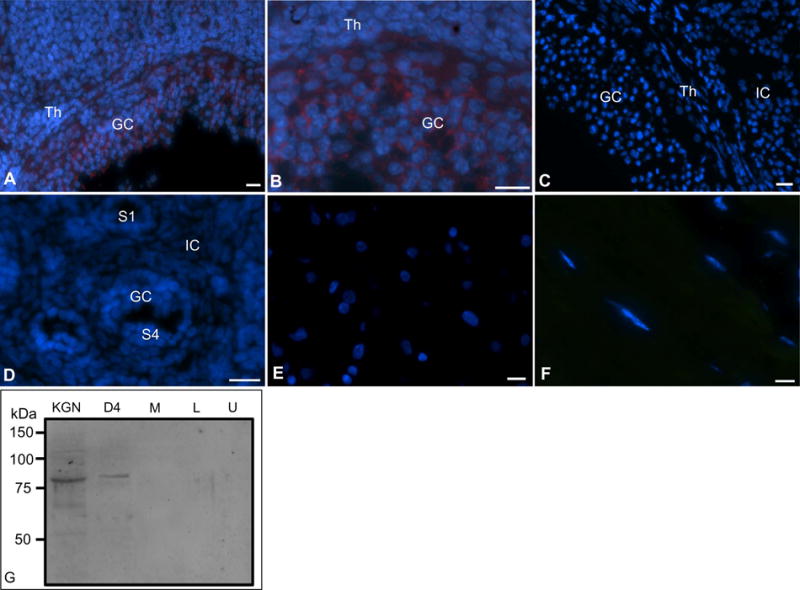
Verification of the specificity of the FSHR antibody using immunofluorescence localization of FSHR protein in hamster ovaries. (A) An antral follicle in proestrus ovary after the gonadotropin surge (D4:1600h), (B) a higher magnification view of the same antral follicle. Note granulosa membrane oriented immunostaining. (C) Section of an ovary showing a part of an antral follicle and adjacent area, which was incubated with the non-immune rabbit serum as negative control, (D) Section of a postnatal day 10 (P10) ovary incubated with the non-immune rabbit serum, (E) section of the hamster liver incubated with the FSHR antibody, (F) section of the hamster gastrocnemius muscle incubated with the FSHR antibody. GC, granulosa cells, Th, thecal cells, IC, interstitial cells, S1, primary follicles, S4, early secondary follicles. FSHR = Red, nuclei = Blue. Bar = 10 μm. Fig. 4G, Western blot analysis of FSHR protein in the homogenates of KGN cells (human granulosa cell line), granulosa cells of hamster antral follicles (D4), hamster leg muscle (M), hamster liver (L) and hamster uterus (U).
Consistent with the Western blot data, distinct FSHR immunosignal was localized in cells of E14 hamster ovary (Fig. 5A). Although punctate FSHR immunofluorescence was associated with undifferentiated somatic cells, low signal was also detected in germ cells located in the oocyte nest (Fig. 5). FSHR immunosignal increased appreciably in somatic cells by E15 (Fig. 5B) and remained distinct throughout development, but the signal was no longer punctate, it was rather more homogenous. However, by P2 strong FSHR immunosignal was associated with somatic cells (S) as well as the oocytes in the egg nest (OC) (Fig. 5D). The expression was most prominent in P4 ovaries in which strong immunosignal was present in the oocytes as well as in somatic cells adjacent to the egg nest (Fig. 5E). FSHR immunosignal was mostly associated with the somatic cells by P6 (Fig. 5F), and then with the granulosa cells of primordial and primary follicles by P9 (Fig. 5G). Notably, granulosa cell surface apposed to the oocyte of the follicles in P9 (Fig. 5G) and P10 (Fig. 5H) ovaries had stronger expression compared the basal site (Figs. 5G and 5H). With the development of preantral follicles by P14, FSHR expression was mostly associated with the granulosa cells and the polarized expression was replaced with more uniform expression throughout the follicles (Fig. 5I). Because follicles developed in close proximity to each other in smaller size ovaries in postnatal hamsters, a broader area of interstitial cells was difficult to identify, especially in fresh, frozen sections. Nevertheless, most of the interstitial cells did not have receptor-specific signal. No oocyte-specific expression was visible with the formation of follicles.
Fig. 5.
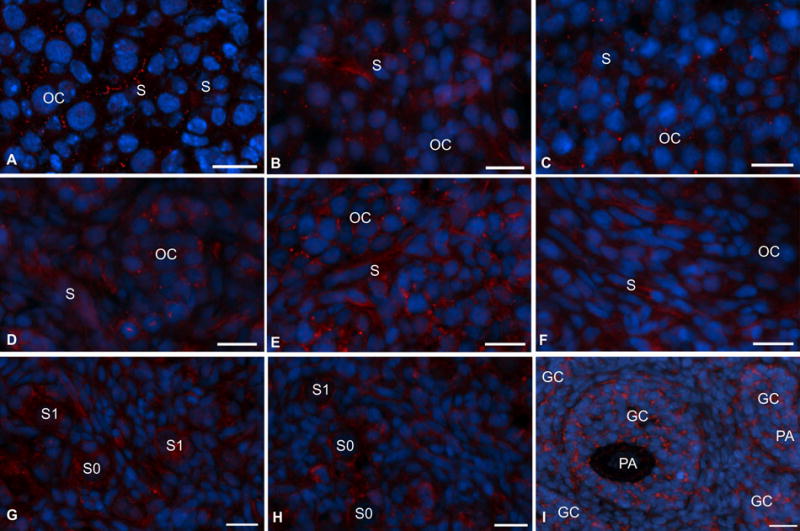
Immunofluorescence localization of FSHR protein in hamster ovaries during perinatal development. (A) embryonic age 14 (E14). (B) E15, (C) postnatal day 1 (P1), (D) P2, (E) P4, (F) P6, (G) P9, (H) P10 and (I) P14. Scant punctate red (FSHR) fluorescence was visible in the oocyte cluster (OC) as well as somatic cells (S) as early as E14. Oc, oocyte clusters, S, somatic cells, S0, primordial follicles, S1, primary follicles, GC, granulosa cells, PA, preantral follicles. FSHR= red, Nuclei= blue. Bar = 10 μm.
To determine if FSH or estrogen (E) upregulates FSHR mRNA levels in developing hamster ovaries, we neutralized serum FSH with an anti-FSH serum (Roy and Albee, 2000) and then added back the FSH-like hormone, equine chorionic gonadotropin (eCG), or treated postnatal pups sc on P1 and P4 with 1 μg estradiol-17β. Neutralization of endogenous FSH did not significantly alter FSHR mRNA levels on P8; however, a 10-fold increase compared to FSH-antiserum treated hamsters and a 24-fold increase compared to normal P8 in FSHR mRNA occurred when pups received eCG on P1 (Fig. 6). E treatment on P1 and P4 also increased FSHR mRNA levels by about 3-fold in P8 hamster ovaries (Fig. 6).
Fig. 6.
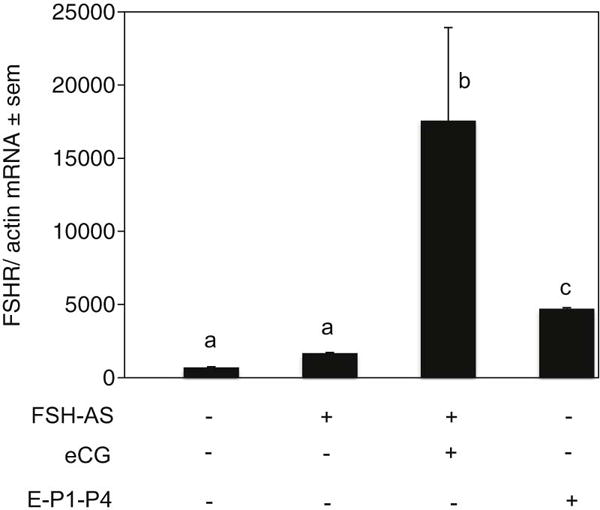
Expression of FSHR mRNA in the ovaries of P8 hamsters exposed in utero with an anti-FSH serum on E12 with or without 20 IU eCG treatment on P1, or with 1 μg estradiol-17β treatment on P1 and P4. Each bar represents the ratio of FSHR and β-actin ± standard error of mean (sem). P<0.05, bars with different letter.
Discussion
The results of the present study show that full-length FSHR mRNA is expressed in fetal through postnatal hamster ovaries, and the mRNA is translated into FSHR protein long before histologically distinct primordial follicles appear in the ovary. The expression levels of FSHR mRNA increase as the ovary develops during the postnatal life, but a large portion of the FSHR mRNA appears to be spliced alternately resulting in a marked increase in the levels of mRNA variants. O’Shaughnessy et al (O’Shaughnessy et al., 1997) have reported that whereas negligible expression of mRNA corresponding to full-length FSHR occurs in postnatal mice at birth, appreciable expression is detected from P5 onwards when ovary contains primordial, primary and early secondary follicles. In contrast, using RT-PCR analysis, Rannikki et al (Rannikki et al., 1995) have detected the presence of mRNA corresponding to the extracellular domain of FSHR in E20.5 rat ovary, while the full-length transcript is detectable from P1.5. Using qPCR primers and probe targeting exons corresponding to only the full-length FSHR, and Western blot using an optimized FSHR antibody, we provide here the first compelling evidence in support of the expression of full-length FSHR mRNA and protein in the hamster ovary from E14. The presence of FSHR immunosignal in undifferentiated somatic cells in fetal and early postnatal ovaries suggests that FSH likely promotes the proliferation and differentiation of these cells into pregranulosa cells. FSH can be detected in the hamster serum as early as P1 (Roy and Hughes, 1994). On the other hand, the exact significance of FSHR protein in the oocytes of fetal and early postnatal ovaries is not apparent at this time, but it is tempting to speculate that FSH may affect the breakdown of the egg nests and the assembly of the oocytes and pregranulosa cells during primordial follicle formation. The shifting of the FSHR immunosignal to granulosa cells after follicle formation lends credence to this conjecture. FSH deprivation with an anti-FSH antiserum during fetal ovary development compromises primordial follicle formation in P8 hamsters (Roy and Albee, 2000). That the FSHR is functional is evident from our previous data, which show increased primordial follicle formation and cAMP production by postnatal hamster ovaries in response to FSH (Roy and Albee, 2000, Roy, Wang, Mukherjee et al., 2012, Wang and Roy, 2004). The distinct FSHR immunosignal in antral granulosa cells of follicles, but not in cells of the theca, liver or muscle, and immunoblot signal in proteins of antral granulosa cells and KGN cells, but not of the muscle, liver or uterus indicates the specificity of the FSHR antibody, although the limited potency presents a limitation for low amount of sample protein. The significance of smaller and higher molecular weight immunoreactive proteins on P5 through P7 and P8 through P10, respectively, is not known at this time. However, these bands may represent post-transnational processing of the FSHR as somatic cells differentiate into pregranulosa cells and as follicles form and develop. Although the overall pattern (sum of all FSHR bands in the Western blots) of FSHR protein and mRNA levels in perinatal ovaries overlap, FSHR protein changes are modest compared to those of the mRNA. However, such a discrepancy in mRNA and protein levels is not uncommon. Because a variety of factors determine the levels of mRNA and corresponding proteins in cells, a 1:1 stoichiometry is often absent. Whereas mRNA quantification by qPCR is relatively straightforward, protein signal in immunoblots depends on the potency and quality of the antibodies. It is important to note that P10 hamster ovary contains primordial through early secondary follicles, which lack a morphologically identifiable thecal layer, and the expression of CYP17a1 does not occur till P13 (Schwartz and Roy, 2000). The results also indicate that full length FSHR gene expression is stimulated on the day before birth and is further upregulated on the day of birth. Because FSH has been shown to increase FSHR gene expression (Findlay and Drummond, 1999, LaPolt, Tilly, Aihara et al., 1992, Tano, Minegishi, Kishi et al., 1999) and appreciable levels of serum FSH are present in hamster pups from P1 (Roy and Albee, 2000) it stands to reason that increased expression of FSHR mRNA in postnatal hamsters is regulated by FSH. This conjecture is supported by remarkable increase in FSHR mRNA following eCG injection.
The marked increase in the level of alternately spliced variants during ovary development suggests a possible molecular mechanism protecting developing follicular cells from FSH over activity. Because FSH stimulates ovarian CYP19a1 expression and activity in postnatal hamsters (Wang and Roy, 2007), a controlled expression of full-length functional FSHR at early stage of ovary development may also regulate the extent of estrogen action on ovarian somatic cells. We have shown that whereas 1 μg estradiol-17β in vivo promotes primordial follicle formation, a 5 or 10μg dose has significant deleterious effect on folliculogenesis (Wang and Roy, 2007). The higher level of FSHR mRNA expression at the early phase of ovary development is particularly noteworthy because the ovary contains fewer somatic cells and large colonies of germ cells in meiosis. Although somatic cell proliferation is expected to contribute to higher FSHR mRNA levels as the ovary grows during the postnatal life, it is logical to speculate that the processing of FSHR mRNA is stabilized as serum FSH levels increase (Roy and Hughes, 1994). The higher levels of FSHR mRNA preceding the day of primordial follicle development (P8) support our hypothesis that FSH affects ovarian somatic cell differentiation into pregranulosa cells (Roy et al., 2012, Wang and Roy, 2009, Wang and Roy, 2004).
The marked increase in FSHR mRNA levels following eCG replacement is consistent with the FSH effect on FSH receptor expression (Findlay and Drummond, 1999, Tano et al., 1999) and suggests that FSH also upregulates its own receptor in the ovaries of developing hamsters. The slight increase in FSHR mRNA in FSH antiserum-treated P8 hamsters reflects a possible compensatory mechanism to restore the FSH signaling in ovarian cells; however, the process appears to have limited progression due to the lack of supporting hormones, such as FSH or estrogen. FSH-antiserum has been shown to markedly reduce serum FSH from postnatal hamsters (Roy and Albee, 2000). On the other hand, retreatment of FSH-treated immature rats with a recombinant human FSH results in significant attenuation of FSHR mRNA levels concurrent with [125]FSH binding (LaPolt et al., 1992). We have previously shown that in adult hamsters, inhibiting the preovulatory gonadotropin surge that also raises serum FSH (Bast and Greenwald, 1974, Roy and Greenwald, 1986) maintains higher levels of FSHR mRNA, but the level decreases markedly within 4h of FSH replacement (Zhang and Roy, 2004). In contrast, treatment of immature rats with eCG has been shown to increase ovarian FSHR mRNA and [125]FSH binding within 24h (LaPolt et al., 1992). Therefore, it appears while FSH may downregulate the FSHR mRNA expression in the short term, a prolong FSH action results in upregulation. Equine chorionic gonadotropin acts as FSH in the hamsters (Greenwald and Roy, 1994) and its prolong action results in follicular development in adult hamsters (Chiras and Greenwald, 1978) and primordial follicle formation in postnatal hamsters (Roy and Albee, 2000). The increase in FSHR mRNA in E-treated postnatal hamsters is consistent with the stimulatory role of E on FSH receptor expression in the ovary (Palter, Tavares, Hourvitz et al., 2001, Richards, Ireland, Rao et al., 1976).
In summary, we present evidence to suggest that similar to rats and mice, FSHR gene expression occurs in ovary cells from the fetal life. However, in contrast to rats and mice, the results provide the first evidence that full-length FSHR mRNA and FSHR protein are expressed in hamster developing ovaries from the fetal life. Further, FSHR expression increases markedly in early postnatal life concurrent with rising levels of serum FSH, but long before the first cohort of primordial follicles develops in the ovary on P8. Both eCG and estrogen promote FSHR mRNA expression in postnatal hamster ovary, although gonadotropin has the most prominent effect. Although FSHR protein is expressed in undifferentiated ovarian somatic cells, oocytes in the oocyte nest may also contribute to ovarian FSHR expression.
Highlights.
Three FSHR transcript variants were expressed in perinatal hamster ovaries.
Full-length FSH receptor m RNA and protein were expressed from the fetal life.
Besides ovarian somatic cells, FSHR receptor protein was expressed in the oocytes in the fetal and early postnatal ovaries.
FSHR protein expression was granulosa cell-specific after follicles formed.
Both eCG and E in vivo upregulates FSHR mRNA levels in postnatal hamsters.
Acknowledgments
The study was supported by grants from National Institute of Human Health and Child Development (HD38468), UNMC bridge fund and Olson Foundation to SKR. Prabuddha Chakraborty was supported by a UNMC graduate student fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bast JD, Greenwald GS. Serum profiles of follicle-stimulating hormone, luteinizing hormone and prolactin during the estrous cycle of the hamster. Endocrinology. 1974;94:1295–1299. doi: 10.1210/endo-94-5-1295. [DOI] [PubMed] [Google Scholar]
- 2.Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol. 1991;5:1405–1417. doi: 10.1210/mend-5-10-1405. [DOI] [PubMed] [Google Scholar]
- 3.Chiras DD, Greenwald GS. Ovarian follicular development in cyclic hamsters treated with a superovulatory dose of pregnant mare’s serum. Biol Reprod. 1978;19:895–901. doi: 10.1095/biolreprod19.4.895. [DOI] [PubMed] [Google Scholar]
- 4.Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol (Oxf) 1999;51:97–99. doi: 10.1046/j.1365-2265.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 5.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson GF, Hsueh AJW. Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology. 1978;102:1275–1282. doi: 10.1210/endo-102-4-1275. [DOI] [PubMed] [Google Scholar]
- 7.Findlay JK, Drummond AE. Regulation of the FSH receptor in the ovary. TEM. 1999;10:183–188. doi: 10.1016/s1043-2760(98)00144-1. [DOI] [PubMed] [Google Scholar]
- 8.Gore-Langton RE, Armstrong DT. Follicular steroidogenesis and its control. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Raven Press; New York: 1994. pp. 571–627. [Google Scholar]
- 9.Greenwald GS, Roy SK. Follicular development and its control. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 629–724. [Google Scholar]
- 10.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–4. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 11.LaPolt PS, Tilly JL, Aihara T, Nishimori K, Hsueh AJ. Gonadotropin-induced up- and down-regulation of ovarian follicle-stimulating hormone (FSH) receptor gene expression in immature rats: effects of pregnant mare’s serum gonadotropin, human chorionic gonadotropin, and recombinant FSH. Endocrinology. 1992;130:1289–95. doi: 10.1210/endo.130.3.1537292. [DOI] [PubMed] [Google Scholar]
- 12.McGee E, Spears N, Minami S, Hsu SY, Chun SY, Billig H, Hsueh AJ. Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3′,5′-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology. 1997;138:2417–24. doi: 10.1210/endo.138.6.5164. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee A, Roy SK. Expression of ErbB3-Binding Protein-1 (EBP1) during Primordial Follicle Formation: Role of Estradiol-17β. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Shaughnessy PJ, Marsh P, Dudley K. Follicle-stimulating hormone receptor mRNA in the mouse ovary during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse: structure of alternate transcripts. Mol Cell Endocrinol. 1994;101:197–201. doi: 10.1016/0303-7207(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 15.O’Shaughnessy PJ, McLelland D, McBride MW. Regulation of luteinizing hormone-receptor and follicle-stimulating hormone-receptor messenger ribonucleic acid levels during development in the neonatal mouse ovary. Biol Reprod. 1997;57:602–608. doi: 10.1095/biolreprod57.3.602. [DOI] [PubMed] [Google Scholar]
- 16.Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis. Endocr Rev. 2001;22:389–424. doi: 10.1210/edrv.22.3.0433. [DOI] [PubMed] [Google Scholar]
- 17.Rannikki AS, Zhang FP, Huhtaniemi IT. Ontogeny of follicle-stimulating hormone receptor gene expression in the rat testis and ovary. Mol Cell Endocrinol. 1995;107:199–208. doi: 10.1016/0303-7207(94)03444-x. [DOI] [PubMed] [Google Scholar]
- 18.Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- 19.Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley ARJ, Reichert LEJ. Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- 20.Roy SK. Regulation of follicular development: Beyond gonadotropins. In: Jay KP, et al., editors. Comparative Endocrinology and Reproduction. Narosa Publishing House; New Delhi: 1999. pp. 315–330. [Google Scholar]
- 21.Roy SK, Albee L. Requirement for follicle-stimulating hormone action in the formation of primordial follicles during perinatal ovarian development in the hamster. Endocrinology. 2000;141:4449–4456. doi: 10.1210/endo.141.12.7805. [DOI] [PubMed] [Google Scholar]
- 22.Roy SK, Greenwald GS. An enzymatic method for dissociation of intact follicles from the hamster ovary: histological and quantitative aspects. Biol Reprod. 1985;32:203–215. doi: 10.1095/biolreprod32.1.203. [DOI] [PubMed] [Google Scholar]
- 23.Roy SK, Greenwald GS. Effects of FSH and LH on incorporation of [3H]thymidine into follicular DNA. J Reprod Fert. 1986;78:201–209. doi: 10.1530/jrf.0.0780201. [DOI] [PubMed] [Google Scholar]
- 24.Roy SK, Greenwald GS. Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. J Reprod Fertil. 1989;87:103–114. doi: 10.1530/jrf.0.0870103. [DOI] [PubMed] [Google Scholar]
- 25.Roy SK, Hughes J. Ontogeny of granulosa cells in the ovary: Lineage-specific expression of transforming growth factor β2 and transforming growth factor β1. Biol Reprod. 1994;51:821–830. doi: 10.1095/biolreprod51.5.821. [DOI] [PubMed] [Google Scholar]
- 26.Roy SK, Wang C, Mukherjee A, Chakraborty P. In vitro culture of fetal ovaries: a model to study factors regulating early follicular development. Methods Mol Biol. 2012;825:151–71. doi: 10.1007/978-1-61779-436-0_12. [DOI] [PubMed] [Google Scholar]
- 27.Roy SK, Wang SC, Greenwald GS. Radioreceptor and autoradiographic analysis of FSH, hCG and prolactin binding sites in primary to antral hamster follicles during the periovulatory period. J Reprod Fertil. 1987;79:307–313. doi: 10.1530/jrf.0.0790307. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JR, Roy SK. Developmental expression of cytochrome P450 side-chain cleavage and cytochrome P450 17 alpha-hydroxylase messenger ribonucleic acid and protein in the neonatal hamster ovary. Biol Reprod. 2000;63:1586–1593. doi: 10.1095/biolreprod63.6.1586. [DOI] [PubMed] [Google Scholar]
- 29.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 30.Sokka T, Huhtaniemi I. Ontogeny of gonadotrophin receptors and gonadotrophin-stimulated cyclic AMP production in the neonatal rat ovary. J Endocrinol. 1990;127:297–303. doi: 10.1677/joe.0.1270297. [DOI] [PubMed] [Google Scholar]
- 31.Stiff ME, Bronson FH, Stetson MH. Plasma gonadotropins in prenatal and prepubertal female mice: disorganization of pubertal cycles in the absence of a male. Endocrinology. 1974;94:492–6. doi: 10.1210/endo-94-2-492. [DOI] [PubMed] [Google Scholar]
- 32.Tano M, Minegishi T, Kishi H, Kameda T, Abe Y, Miyamoto K. The effect of follicle-stimulating hormone (FSH) on the expression of FSH receptor in cultured rat granulosa cells. Life Sci. 1999;64:1063–1069. doi: 10.1016/s0024-3205(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 33.Uilenbroek JT, Richards JS. Ovarian follicular development during the rat estrous cycle: gonadotropin receptors and follicular responsiveness. Biol Reprod. 1979;20:1159–1165. doi: 10.1095/biolreprod20.5.1159. [DOI] [PubMed] [Google Scholar]
- 34.Uilenbroek JTJ, van der Linden R. Changes in gonadotrophin binding to rat ovaries during sexual maturation. Acta Endocrinologica. 1983;103:413–419. doi: 10.1530/acta.0.1030413. [DOI] [PubMed] [Google Scholar]
- 35.Ulloa-Aguirre A, Midgley AR, Jr, Beitins IZ, Padmanabhan V. Follicle-stimulating isohormones: characterization and physiological relevance. Endocr Rev. 1995;16:765–87. doi: 10.1210/edrv-16-6-765. [DOI] [PubMed] [Google Scholar]
- 36.Vomachka AJ, Greenwald GS. The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology. 1979;105:960–966. doi: 10.1210/endo-105-4-960. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Roy SK. Development of Primordial Follicles in the Hamster: Role of Estradiol-17{beta} Endocrinology. 2007;148:1707–16. doi: 10.1210/en.2006-1193. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Roy SK. Expression of bone morphogenetic protein receptor (BMPR) during perinatal ovary development and primordial follicle formation in the hamster: possible regulation by FSH. Endocrinology. 2009;150:1886–96. doi: 10.1210/en.2008-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Roy SK. Growth Differentiation Factor-9 and Stem Cell Factor Promote Primordial Follicle Formation in the Hamster: Modulation by Follicle-Stimulating Hormone. Biol Reprod. 2004;70:577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- 40.Yang P, Kriatchko A, Roy SK. Expression of ERa and ERb in the hamster ovary: differential regulation by gonadotropins and ovarian steroid hormones. Endocrinology. 2002;143:2385–2398. doi: 10.1210/endo.143.6.8858. [DOI] [PubMed] [Google Scholar]
- 41.Yang P, Roy SK. Follicles stimulating hormone-induced DNA synthesis in the granulosa cells of hamster preantral follicles involves activation of cyclin-dependent kinase-4 rather than cyclin D2 synthesis. Biol Reprod. 2004;70:509–517. doi: 10.1095/biolreprod.103.023457. [DOI] [PubMed] [Google Scholar]
- 42.Yang P, Wang J, Shen Y, Roy SK. Developmental expression of estrogen receptor (ER) alpha and ERbeta in the hamster ovary: regulation by follicle-stimulating hormone. Endocrinology. 2004;145:5757–66. doi: 10.1210/en.2004-0779. [DOI] [PubMed] [Google Scholar]
- 43.Yu N, Roy SK. Development of primordial and prenatal follicles from undifferentiated somatic cells and oocytes in the hamster prenatal ovary in vitro: effect of insulin. Biol Reprod. 1999;61:1558–1567. doi: 10.1095/biolreprod61.6.1558. [DOI] [PubMed] [Google Scholar]
- 44.Zhang YM, Roy SK. Downregulation of Follicle-Stimulating Hormone (FSH)-Receptor Messenger RNA Levels in the Hamster Ovary: Effect of the Endogenous and Exogenous FSH. Biol Reprod. 2004;70:1580–8. doi: 10.1095/biolreprod.103.026898. [DOI] [PubMed] [Google Scholar]


