Abstract
Well-differentiated cultures established from airway epithelia of patients with cystic fibrosis (CF cultures) exhibited goblet cell hyperplasia, increased secretion of mucus, and higher basal levels of interleukin-8 than similarly cultured cells from healthy donors. Upon apical infection with low doses (104 to 105 CFU) of Burkholderia cenocepacia isolate BC7, the two cultures gave different responses. While normal cultures trapped the added bacteria in the mucus layer, killed and/or inhibited bacterial replication, and prevented bacterial invasion of the cells, CF cultures failed to kill and/or supported the growth of bacteria, leading to invasion of underlying epithelial cells, compromised transepithelial permeability, and cell damage. Depletion of the surface mucus layer prior to bacterial infection rendered the normal cultures susceptible to bacterial invasion, but the invading bacteria were mainly confined to vacuoles within the cells and appeared to be nonviable. In contrast, bacteria that invaded cells in CF cultures were found free in the cytoplasm surrounded by intermediate filaments and also between cells. Cultured CF airway epithelium was therefore more susceptible to infection than normal epithelium. This mimics CF tissue in vivo and illustrates differences in the way epithelia in CF patients and normal subjects handle bacterial infection. In addition, we found that the CF and normal cell cultures responded differently not only to isolate BC7 but also to isolates of other B. cepacia complex species. We therefore conclude that this cell culture model is suitable for investigation of B. cepacia complex pathogenesis in CF patients.
Cystic fibrosis (CF) is a life-shortening disease in Caucasians and is caused by mutations in the cystic fibrosis transmembrane regulator (cftr), which encodes a chloride channel, CFTR. A major cause of morbidity and mortality in these patients is chronic lung infection by opportunistic pathogens. Although significant advances have been made since the discovery of the cftr gene, it is still not clear how CFTR dysfunction relates to lung disease in CF patients. Several hypotheses have been proposed to explain the vulnerability of CF patients' lungs to opportunistic pathogens. These include impaired mucociliary clearance due to altered fluid and ionic fluxes, excessive mucus production, hypertonic airway surface fluid which inactivates major antibacterial factors, and increased expression of bacterial receptors such as asialo glycoconjugates and cytokeratin 13 (30, 37, 42, 45). There is accumulating evidence that low-grade inflammation may exist in CF airways even without infection, and this may trigger excessive inflammatory responses to infectious stimuli (3, 13, 15, 27, 46).
Although the major opportunistic respiratory pathogen in CF patients is Pseudomonas aeruginosa, the Burkholderia cepacia complex has been recognized as an important pathogen (19, 22, 26). B. cenocepacia (originally B. cepacia genomovar III) (47) has been linked to most of the cepacia syndrome deaths in CF patients (5, 18, 19). We have shown that some isolates of B. cenocepacia express cable pili and an associated 22-kDa adhesin (37, 38), which mediate bacterial binding to the epithelial receptor cytokeratin 13 (40) and potentiate bacterial invasion into squamous epithelium (33). Cytokeratin 13 is expressed abundantly in CF airway epithelium undergoing repair after repeated injury (37). In the lungs of CF patients, B. cenocepacia was found in the mucociliary epithelium in addition to regenerating epithelium rich in cytokeratin 13 and peribronchial, peribronchiolar, and perivascular areas (34). Further, by an in vitro binding assay with fixed lung sections, we showed that many B. cenocepacia isolates bind to mucociliary epithelia in addition to remodeling airway epithelium of CF patient but not normal lungs (37), suggesting heightened affinity of B. cenocepacia for the airway epithelium in CF patients. However it was not possible to study the dynamics of infection in this system.
Previously, members of the B. cepacia complex have been shown to invade undifferentiated cell monolayers of pulmonary pneumocytes (2, 4, 23). To observe invasion of normal well-differentiated airway epithelial cells, however, required a much higher infection dose of B. cepacia complex (41). Chronically infected and inflamed CF airway epithelium in vivo differs from normal epithelium in showing goblet cell hyperplasia, dehydrated and highly viscoelastic mucus which is adherent to the apical surface, impaired mucociliary function, and inactivated or absent antimicrobial factors in the apical mucus layer (1, 3, 8, 16, 29, 43, 48). Some of these changes, such as goblet cell hyperplasia, viscous mucus plugs obstructing the airways, and impaired mucociliary function, have also been reported in the lungs of uninfected fetuses with CF, indicating that pathological changes can occur prior to infection (8).
Although controversial, there is evidence that constitutive activation of NF-κB is the cause of primary inflammatory disorder in CF airways, which may render the CF airways more vulnerable to infection (3, 15, 27, 28). We have hypothesized that one or more of these differences may render CF airway epithelium more susceptible than normal to infection, even with low doses of B. cenocepacia and other B. cepacia complex species. As a partial test of this hypothesis, we compared the responses of mucociliary-differentiated cultures established from CF and normal tracheobronchial epithelial cells to infection with a well-characterized epidemic strain of B. cenocepacia which causes acute and chronic infection, particularly in CFTR knockout mice (17, 36). We also tested representative strains of other B. cepacia complex species. We show that cultures of airway epithelium from CF patients (CF cultures) resemble the in vivo situation closely in exhibiting mucus cell hyperplasia and increased secretion of mucus and interleukin-8 (IL-8) under basal conditions and are more vulnerable than normal cultures to infection with selected strains of the B. cepacia complex. We also demonstrate that the apical mucus layer is less protective in CF than in normal cultures.
MATERIALS AND METHODS
Cell culture.
Airway segments from non-CF donor and CF patients homozygous for the ΔF508 mutation and without a history of infection with B. cepacia complex were collected at the time of double lung transplantation and used to isolate epithelial cells as described previously (12, 31). The Human Ethics Committee at the Toronto General Hospital, Toronto, Canada, approved the collection and use of these normally discarded tissue samples.
Briefly, tracheal or bronchial segments were rinsed with calcium- and magnesium-free 0.1 M phosphate buffer, pH 7.2, containing 0.8% sodium chloride (PBS), cut into small pieces (approximately 4 by 4 mm2), and incubated overnight at 4°C with PBS containing 0.1% trypsin, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (1.25 μg/ml). The mucosa was lightly scraped, the released epithelial cells were passed through a 70-μm filter, the filtrate was centrifuged at 500 × g, and the cells were collected. To remove contaminating microorganisms from cells isolated from CF airway epithelia, cells were incubated for 30 min in RPMI medium (Invitrogen Life Technologies, Burlington, Canada) containing penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), amphotericin B (1.25 μg/ml), fluconazole (2 μg/ml), ceftazidime (77 μg/ml), and primaxin (sodium cilastatin plus imipenem) (12.5 μg/ml) and centrifuged. Cell pellets were suspended in bronchial epithelial cell growth medium (BEGM) (Clonetics Corp., San Diego, Calif.) containing the above-mentioned antimicrobial agents plus 10−8 M retinoic acid and 5 ml of bovine pituitary extract equivalent to 0.13 mg of total protein/ml and 25 ng of epithelial growth factor per ml. Cells were then seeded on collagen (BD Biosciences, Bedford, Mass.)-coated tissue culture dishes at a density of 200 cells/cm2 and grown in a tissue culture incubator. The cells were harvested after they reached 70 to 80% confluence and cryopreserved at passage 1 as described previously (33).
To obtain well-differentiated mucociliary cultures, the cells from passage 1 were seeded into 12-mm Transwell-clear inserts (Costar, Cambridge, Mass.) coated with collagen. Cells were grown under submerged conditions in BEGM with antimicrobial agents until they became confluent and then shifted to a 1:1 mixture of BEGM and Dulbecco's modified Eagle's medium. An air-liquid interface was created to promote mucociliary differentiation (33). To detect the carryover of microbial infection from the donor (particularly CF donor), differentiated cultures were maintained in the antibiotic-free medium for 1 week, and spent medium and cell homogenates were plated on blood, Pseudomonas isolation, or inhibitory mold agar plates (Becton Dickinson, Cockeysville, Md.). No bacterial or fungal growth was observed in any of the cultures, indicating that culturing cells from passage 1 prevents carryover of bacterial and fungal infection.
Morphology of cultures.
Cultures were embedded in 2% agar, fixed in 10% buffered formalin overnight at 4°C, dehydrated, and embedded in paraffin. Five-micrometer-thick sections were stained with either hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) reagent and examined by light microscopy (Dialux 22; Leica, Willowdale, Canada).
Antibodies.
Polyclonal and monoclonal antibodies to zona occludens (ZO-1) and a monoclonal antibody to occludin were purchased from Zymed Laboratories Inc., San Francisco, Calif. Monoclonal antibodies to cytokeratin 13 and the C-terminal domain of CFTR were from Vector Laboratories Inc. (Burlington, Canada) and R & D Systems (Minneapolis, Minn.), respectively. A monoclonal antibody to β-tubulin conjugated with the fluorophore indocarbocyanine was obtained from Sigma Chemical Co. (Burlington, Canada). Polyclonal antibodies to B. cenocepacia (designated R418) and tracheobronchial mucin have been described previously (14, 33).
Immunodetection of ZO-1 in intact cultures.
The apical surface of mucociliary-differentiated cultures was incubated with 3% xylitol for 30 min at 22°C to remove surface mucus, washed with PBS, and processed for detection of ZO-1 as described previously (6).
Immunofluorescent detection of CFTR, mucin, and B. cenocepacia.
Five-micrometer-thick paraffin sections were incubated with anti-CFTR (1:100), anti-mucin (1:10,000), or anti-B. cenocepacia (R418) (1:800) antibody as described previously. After the unbound antibody was washed off, bound antibody was detected either by using a second antibody conjugated with indocarbocyanine (for detection of mucin) or indodicarbocyanine (for detection of bacteria) or by using a second antibody conjugated with biotin followed by streptavidin-Texas Red (for detection of CFTR). Sections were then examined under a fluorescence microscope.
Bacteria and growth conditions.
B. cenocepacia, strain ET12, isolate BC7, was originally isolated from the sputum of a of CF patient and has been described previously (35, 38). ATCC 25416, genomovar I, originally isolated from onion rot, was purchased from the American Type Culture Collection, Manassas, Va. B. cenocepacia K56-2 was kindly provided by P. Sokol (University of Calgary, Calgary, Alberta, Canada). All other isolates listed in Table 2 are from the B. cepacia research panel and were kindly provided by E. Mahenthiralingam (University of Wales, Cardiff, United Kingdom). All bacteria were maintained as glycerol stocks at −80°C. For infection of cell cultures, bacteria were subcultured on brain heart infusion agar plates (Becton Dickinson), and a single colony was transferred into 10 ml of tryptic soy broth (Difco Labs, Detroit, Mich.) and grown for 16 h on an orbital shaker at 37°C. Bacteria were harvested by centrifugation at 2,000 × g, washed three times with sterile PBS, and suspended in antibiotic-free BEGM to the required concentration.
TABLE 2.
Persistence of IL-8 release caused by B. cepacia complex isolates in normal and CF culturesa
| Isolate | Species | Mean bacteria associated with cells (CFU/culture) ± SEM
|
Mean IL-8 concn (ng/culture) ± SEM
|
||
|---|---|---|---|---|---|
| Normal cultures | CF cultures | Normal cultures | CF cultures | ||
| None (control) | 0 | 0 | 28.3 ± 1.67 | 146.2 ± 17.07 | |
| ATCC 25416 | Genomovar I | 0 | 0 | 39.8 ± 5.35 | 189.5 ± 16.87 |
| C5393 | B. multivorans | 0 | 1.8 × 102 ± 75 × 102* | 38.4 ± 5.37 | 298.7 ± 12.55* |
| K56-2 | B. cenocepacia | 0 | 6.8 × 104 ± 3,100 × 104* | 51.6 ± 8.43 | 455.6 ± 46.75* |
| FC473 | B. stabilis | 0 | 0 | 41.5 ± 6.98 | 213.9 ± 25.31 |
| CEP40 | B. vietnamiensis | 0 | 3.4 × 102 ± 147 × 102* | 43.8 ± 5.21 | 335.2 ± 42.71* |
Cultures were incubated with B. cepacia (104 CFU/culture) for 24 h. Apical surfaces were washed, and the washes were pooled. Cells were then dissociated in 0.5% Triton X-100, and the number of bacteria associated with the cells was determined by dilution plating. IL-8 in the washes was quantified by ELISA. Numbers represent the means for six individual cultures from one normal subject or one CF patient. Asterisks represent statistical differences between control and treated cultures (P < 0.001 between control and K56- 2; P < 0.05 between control and C5395 and between control and CEP40 in bacterial persistence column; P < 0.001 between control and K56-2; P < 0.05 between control and C5395; P < 0.01 between control and CEP40 in IL-8 column; Mann-Whitney U test).
Infection of cell cultures.
Mucociliary-differentiated CF and normal airway epithelial cell cultures were transferred to antibiotic-free medium for at least 48 h (sometimes up to a week) prior to bacterial infection. Both CF and normal cultures were divided into two groups. Xylitol treatment and infection of these groups are shown schematically in Fig. 1. At the end of the experiment, washes and cell homogenates of each group were mixed with complete protease inhibitor cocktail (Roche Biochemicals, Burlington, Canada) and either snap frozen for later use or serially diluted and plated on B. cepacia isolation agar (11) to determine the number of viable bacteria associated with luminal surface washes (mucus layer) or cells.
FIG. 1.
Schematic representation of experimental groups and treatments.
Cytokine analysis.
Cytokines IL-1β, IL-8, tumor necrosis factor alpha, gamma interferon, and ICAM-1 in the luminal washes or cell extracts of cultures incubated for 24 h with medium (controls) or B. cenocepacia were determined by enzyme-linked immunosorbent assay (ELISA) with the manufacturer's instructions (R&D Systems, Minneapolis, Minn.).
Transepithelial resistance and permeability.
CF and normal epithelial cell cultures were equilibrated at room temperature for 30 min, and the transepithelial resistance (Rt) of the cultures was measured with a Millicell-ERS (Millipore Canada Ltd.) before and after infection with B. cenocepacia. Values were then corrected for fluid resistance (membrane with no cells), and surface area. Rt was calculated with the mathematical relationship Rt (Ussing chamber) = (0.492 × Rt [Millicell-ERS]) + 158 for comparison with the Rt values obtained with conventional Ussing chambers (12). Transepithelial permeability was determined by measuring the flux of fluorescein isothiocyanate-labeled inulin across the epithelial layer as previously described (6).
Western and slot blot analyses.
Luminal washes or cell extracts (equivalent to 30 μg of total protein) of epithelial cell cultures were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred to Immobilon membranes and blocked with 3% bovine serum albumin. For slot blots, equal volumes of samples were applied to nitrocellulose membranes with a slot blot manifold (Schleicher & Schuell). The blots were incubated with antibody to ZO-1 (4 μg/ml), occludin (1:250), or tracheobronchial mucin (1:10,000) overnight at 4°C, and the bound antibody was detected with a second antibody-alkaline phosphatase conjugate and the color substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP). Slot blots were quantitated by densitometry (Kodak Gel Logic 100 Imaging System). Purified tracheobronchial mucin (35) was used to generate a standard curve.
Electron microscopy.
Cultures were fixed in either universal fixative for 1 h or 4% paraformaldehyde containing 0.1% glutaraldehyde and processed for transmission or immunoelectron microscopy, respectively, as described previously (33). Samples were observed under a Jeol 1200 EXII transmission electron microscope (Jeol USA Inc., Peabody, Mass.).
Statistics.
Data were analyzed with GraphPad Software, the InStat guide to choosing and interpreting statistical tests (GraphPad Software, Inc., San Diego Calif.). Differences between two groups were analyzed by the Mann-Whitney U test. To compare multiple groups, a standard parametric one-way analysis of variance (ANOVA) with Tukey-Kramer posttest was used except for comparisons of bacterial persistence, for which a nonparametric Kruskal-Wallis ANOVA with Dunn's posttest was used because the data did not follow a Gussian distribution.
RESULTS
Characteristics of airway epithelial cell cultures. (i) Morphology.
Both CF and normal cultures showed pseudostratified epithelium with ciliated cells interspersed among mucus secreting cells (Fig. 2a and b), similar to cultures established from primary cells (12, 24). PAS staining indicated the presence of more goblet cells and more luminal glycoproteins in CF cultures (Fig. 2d) than in normal cultures (Fig. 2c). Immunostaining of sections with a specific antibody to tracheobronchial mucin confirmed that the PAS-positive material was mucin (Fig. 2f versus 2e). This difference has not been reported in cultures developed by other investigators. Localization of CFTR with an antibody to the C-terminal domain indicated the presence of CFTR at the apical surface of normal (arrows in Fig. 2g) but not CF (Fig. 2h) epithelium. This is characteristic of CF airway epithelia in vivo (9). Immunostaining of sections with a monoclonal antibody to cytokeratin 13 indicated the presence of cytokeratin 13 only in the basal layer of the epithelium in both CF and normal cultures (data not shown).
FIG. 2.
Morphology of airway epithelial cell cultures. Cultures were fixed in 10% buffered formalin and embedded in paraffin, and 5-μm-thick sections were stained with H&E (panels a and b), PAS reagent (panels c and d), antimucin antibody (panels e and f), or the antibody to CFTR (panels g and h). Arrows in panel g indicate apical CFTR. Panels a, c, e, and g represent normal cultures. Panels b, d, f, and h represent CF cultures. Figures are representative of six individual cultures established from cells obtained from three normal subjects or three CF patients.
(ii) Expression of mucin and cytokines.
Assays were performed on cultures established in triplicate or more from airway epithelial cells of three normal and three CF trachea or bronchi maintained in the antibiotic-free medium for at least 48 h (n = 12 to 16). Total mucin in the cultures (secretions plus goblet cells) was measured by quantitative slot blot immunoassay and was 1,475 ± 105 μg/culture in CF cultures, which was 1.8-fold higher than in normal cultures (831 ± 38 μg/culture) (P < 0.05, Mann-Whitney U test). Cytokines IL-1β, tumor necrosis factor alpha, and ICAM-1 were below the detection limits in both CF and normal cultures. Gamma interferon was marginally increased in CF cultures, but the difference was not statistically significant. IL-8, on the other hand, was 5.2-fold higher in CF than in normal cultures (146 ± 25.7 versus 28 ± 4.6 ng/culture), and the difference was statistically significant (P < 0.0001, Mann-Whitney U test). Therefore even under basal conditions, CF cultures produced larger amounts of mucin and IL-8, in keeping with data obtained from in vivo and in vitro observations (13, 32, 46).
(iii) Transepithelial resistance (Rt).
CF cultures showed somewhat higher Rt than normal cultures (707 ± 42 versus 549 ± 15 Ω-cm2) (P < 0.01, Mann-Whitney U test), in contrast to earlier reports by others (6). To determine if the observed increase was associated with altered expression of tight junction proteins, apical ZO-1 was measured by immunofluorescence, and ZO-1 and occludin were semiquantitated by Western blot analyses. Immunostaining gave a similar ZO-1 pattern in both CF and normal cultures, although occasionally CF cultures showed small areas devoid of signal, suggesting the absence of ZO-1 (data not shown). By Western blot analyses, however, no difference was observed in ZO-1 or occludin concentrations between CF and normal cultures. To account for the higher Rt observed in CF cultures, we tested the possibility that surface mucus might be responsible. Both sets of cultures were washed with 3% xylitol, which is a nonionic osmolyte and has been shown to hydrate mucus, thereby facilitating its removal (39, 50). Xylitol treatment did not alter the Rt of normal cultures but reduced the Rt of CF cultures to 515 ± 31.2 Ω-cm2, which was comparable to the value in normal cultures. Thus, the luminal mucus in CF cultures appears to be responsible for its higher Rt.
Responses of normal and CF cultures to B. cenocepacia infection.
Both group 1 (with an intact apical mucus layer) and group 2 (depleted of apical mucus) cultures of CF and normal cells were shifted to antibiotic-free medium for a minimum of 48 h and then incubated with 20 μl of medium alone (controls) or medium containing 104 CFU of B. cenocepacia isolate BC7 for 24 h at 37°C (33, 35, 36). Transepithelial permeability, cytokine expression, secretion of mucin, and bacterial persistence and invasion of epithelial cells were then determined. The optimal concentration of B. cenocepacia was chosen after preliminary experiments in which 103, 104, 105, and 106 CFU were tested. A bacterial concentration of 104 to 105 CFU/culture was sufficiently low to reveal differences between CF and normal cultures. Concentrations below this had little or no effect on either culture, whereas concentrations greater than 105 CFU abolished the observed differences.
Transepithelial resistance (Rt) and permeability (Kapp).
After incubation with medium or B. cenocepacia for 24 h, cultures were washed with 3% xylitol to remove unbound bacteria and mucus secretions. The Rt of normal group 1 and group 2 cultures was not altered relative to that of medium controls by B. cenocepacia infection (Fig. 3). CF group 1 cultures were also not statistically different from medium controls (Fig. 3a). CF group 2 cultures (Fig. 3b, hatched bars), however, showed a significant decrease in Rt and increase in Kapp (5.5-fold) relative to medium-treated controls (P < 0.05, Mann-Whitney U test). Thus, provided that the cultures were depleted of surface mucus before bacteria were added (group 2), B. cenocepacia effectively decreased the transepithelial resistance and increased the permeability of CF cultures.
FIG. 3.
Effect of B. cenocepacia infection on transepithelial resistance and permeability of well-differentiated airway epithelial cell cultures. Both groups of CF and normal cultures were apically infected with B. cenocepacia (104 CFU/culture) (hatched bars), incubated for 24 h, and washed to remove unbound bacteria, and transepithelial resistance was measured. Control cultures (open bars) were incubated with medium alone. Panel a represents group 1 cultures, and panel b represents group 2 cultures. Numbers represent the mean ± standard error of the mean of 12 to 16 individual cultures. Difference between the two groups was analyzed by the Mann-Whitney U test.
Expression of cytokines.
IL-1β, tumor necrosis factor alpha, IL-8, ICAM-1, and gamma interferon were measured in the luminal washes of groups 1 and 2 of both CF and normal cultures, incubated with medium alone or medium containing B. cenocepacia. The ICAM-1 concentration was also measured in cell extracts. Among the cytokines measured, only IL-8 release was increased after infection (Fig. 4). Groups 1 and 2 of CF cultures treated with medium alone (controls) showed four- and twofold higher expression of IL-8, respectively, than medium-treated normal cultures. In response to infection with B. cenocepacia, group 1 of the normal cultures showed no increase in IL-8 expression, while group 1 of the CF cultures gave a threefold increase over comparable medium controls. In contrast, group 2 cultures of both normal and CF cells showed a four- to fivefold increase in IL-8 expression over that of comparable medium controls. Thus, depletion of mucus before exposure of the cultures to bacteria enhanced the IL-8 response. IL-1β and tumor necrosis factor alpha were under the detection limit, which is likely explained by the fact that these are primary cytokines and are upregulated during the early stages of infection but decrease by 24 h. Release of gamma interferon, soluble ICAM-1, or ICAM-1 associated with cells was not altered by infection of either CF or normal cultures. These are late-phase cytokines and may require a longer incubation time (10, 44).
FIG. 4.
Release of cytokine IL-8 in response to B. cenocepacia infection. Cultures were incubated with B. cenocepacia (solid bars) or medium (open bars) for 24 h as described for Fig. 3. Washes of cultures were collected and mixed with protease inhibitors, and IL-8 was determined by ELISA. Values represent the mean ± standard error of the mean of 12 to 16 individual cultures. Differences between the groups were analyzed by one-way standard ANOVA (overall P < 0.001) with the Tukey-Kramer posttest (P values for corresponding pairwise comparisons are presented).
Secretion of mucus.
CF and normal (both group 1 and group 2) cultures were infected with B. cenocepacia or incubated with medium (control) and washed as before, and mucin present in the apical washes was quantitated. As shown in Table 1, both groups of CF cultures treated with medium alone secreted approximately twofold more mucin than normal cultures. In response to B. cenocepacia infection, however, CF cultures (both groups 1 and 2) did not show significant changes in mucin secretion over medium-treated controls. In contrast, both groups of normal cultures treated with B. cenocepacia showed significantly higher mucin secretion (3.1-fold in group 1 to 5.7-fold in group 2) than medium-treated controls (P < 0.01, ANOVA; P < 0.001 in both the cases, Tukey-Kramer multiple comparisons test). Therefore although the CF cultures had a higher resting or baseline secretion of mucin than normal cultures, they failed to respond to the infectious stimulus by secreting more mucus. Similar to these findings, secretion of mucin and other glycoproteins from submandibular glands in response to β-adrenergic stimulation was found to be defective in CF tissue (20, 25). Later it was shown that CFTR is directly involved in the regulation of secretion of glycoproteins from the submandibular glands (21). It is possible that mucin secretion from airway goblet cells in response to infectious stimuli may depend on a similar CFTR-dependent pathway which is defective in CF.
TABLE 1.
Mucin secretion by normal and CF cultures in response to B. cenocepacia infectiona
| Treatment | Mean mucin secretion (μg/ml) ± SEM
|
|||
|---|---|---|---|---|
| Normal cultures
|
CF cultures
|
|||
| Group 1 | Group 2 | Group 1 | Group 2 | |
| Medium (control) | 133 ± 18.7 | 52.5 ± 6.4 | 301 ± 24 | 105 ± 28.3 |
| B. cenocepacia | 415 ± 68.1* | 298 ± 22.1* | 396 ± 30.1 | 224 ± 65.4 |
Cultures were incubated with medium (control) or B. cenocepacia (104 CFU) before (group 1) or after treatment with xylitol (group 2) to remove surface mucus. The luminal surface of the cultures was washed, and mucin in the washes was quantitated by slot blot immunoassay. Numbers represent means for 12 to 16 individual cultures. Asterisks represent a statistically significant increase over medium controls (P < 0.01 by ANOVA; P < 0.001 between medium- and B. cenocepacia-treated group 1 as well as group 2 normal cultures, Tukey-Kramer posttest).
Persistence of bacteria.
Cultures were apically infected with 104 CFU of B. cenocepacia for 24 h and then washed as described before, and the washes were collected. Cells were dissociated with 0.5% Triton X-100 to determine the number of bacteria associated with cells. As shown in Fig. 5, most of the bacteria were recovered in the luminal washes in both groups of CF and normal cultures, suggesting that bacteria may be trapped in the apical mucus and removed by washing (group 2 versus group 1). The major finding was that CF cultures contained many more bacteria in both washes (Fig. 5a) and cells (Fig. 5b) than comparable normal cultures. Removal of the mucus layer from normal cultures resulted in more bacterial association with cells (Fig. 5b, normal), which suggests that normal apical mucus helps to prevent bacteria from reaching the underlying epithelial cells. However, removal of mucus from CF cultures caused little enhancement in the (already large) number of bacteria associated with CF cells (Fig. 5b, CF). This may indicate a less protective role for mucus in CF patients than in normal subjects. These interpretations were tested in later bacterial localization experiments.
FIG. 5.
Cultures were incubated with medium or B. cenocepacia (104 CFU/culture) before (group 1) or after (group 2) treatment with xylitol to remove surface mucus. Cultures were washed, and the washes were pooled. Cells were then dissociated in 0.5% Triton X-100. The number of bacteria present in the apical washes (panel a) and associated with cells (panel b) was determined by dilution plating. Data represent the median with minimum and maximum values obtained from 12 to 16 individual cultures. Multiple group comparisons were made by using Kruskal-Wallis ANOVA (overall P < 0.01) with Dunn's posttest analysis, and P values for corresponding pairwise comparisons are presented.
The total number of bacteria (washes plus cells) recovered from group 1 normal cultures was almost one log less than the infecting dose (1.6 × 104 CFU). In contrast, one or two logs more bacteria than the infecting dose were recovered from group 2 normal cultures and both groups of CF cultures. These results suggest that the apical mucus of intact normal cultures may contain factors that kill B. cenocepacia and/or prevent its replication. The CF cultures and the normal cultures devoid of a mucus layer (group 2) appeared to be much less efficient in preventing bacterial replication.
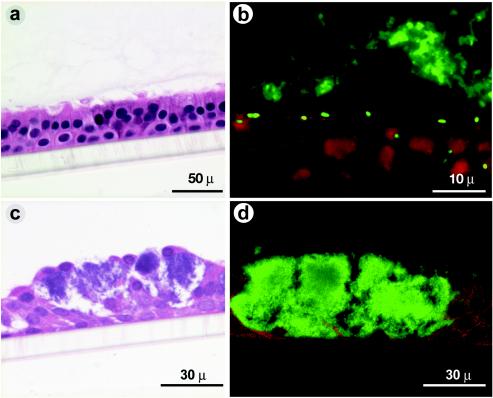
Localization of bacteria.
H&E-stained sections of group 1 CF cultures treated for 24 h with 104 CFU of B. cenocepacia showed no obvious cell damage (Fig. 6a). By immunofluorescence with the antibody to B. cenocepacia, bacteria were readily observed in the form of microcolonies within the mucus layer (Fig. 6b) as well as on the surface of epithelial cells and a few deeper in the cell layer, which appeared to be between and inside the cells, which may indicate some cellular invasion by B. cenocepacia. Similarly treated group 2 CF cultures, on the other hand, showed extensive cell damage (Fig. 6c) in isolated areas of the culture, with bacterial microcolonies prominent within the damaged areas (Fig. 6d). Mild or no damage was observed in areas devoid of bacterial microcolonies. Group 1 normal cultures showed no morphological changes (Fig. 7a), and bacteria were confined to the mucus layer, as determined by immunolocalization (Fig. 7b). Group 2 normal cultures, on the other hand, did not reveal severe cell damage but showed multiple small nuclei in isolated cells (Fig. 7c). Immunofluorescence with B. cenocepacia antibody confirmed that the multiple small nuclei represent microcolonies of bacteria (Fig. 7d).
FIG. 6.
Morphology and localization of bacteria in CF cell cultures: Cultures were treated with medium or B. cenocepacia (104 CFU) before (group 1) or after (group 2) treatment with xylitol. Cultures were fixed in 10% formalin and embedded in paraffin. Five-micrometer-thick sections were either stained with H&E or incubated with antibody R418 to detect B. cenocepacia. Panels a and c represent H&E-stained sections of CF group 1 and group 2 cultures, respectively. Panels b and d represent sections of CF group 1 and group 2 cultures, respectively, treated with anti-B. cepacia antibody R418 and detected by indodicarbocyanine-labeled secondary antibody. Green represents bacteria and red represents cells in the culture.
FIG. 7.
Morphology and localization of bacteria in normal cell cultures. Group 1 (panels a and c) and group 2 (panels b and d) of normal cultures were incubated with B. cenocepacia and processed as described for Fig. 5. Panels a and c represent H&E-stained sections, and panels b and d represent sections treated with antibody R418. Green represents bacteria and red represents cells in the culture.
To further evaluate the location of bacteria and their route of invasion, we performed transmission electron microscopy (Fig. 8). We used 105 CFU of B. cenocepacia per culture to increase the chances of finding bacteria in a relatively small sample size. We chose to use only group 2 CF and normal cultures because more bacteria invaded these cell cultures. In CF cultures (Fig. 8a), bacteria were found on the apical surface in the form of microcolonies, between cells (Fig. 8b) and within cells (Fig. 8c). Many intracellular bacteria were not bound by a vesicular membrane but were partly surrounded by intermediate filaments (Fig. 8d, arrowheads). In group 2 normal cultures, on the other hand, some cells were vacuolated (Fig. 8e) and resembled mucus-producing cells. The vacuoles contained black dense particles (Fig. 8f) that were smaller than intact bacteria but reacted with the antibody to B. cenocepacia (inset in Fig. 8f), and thus we presume that they represent dead bacteria. These studies indicate that CF and normal cultures handle invading bacteria in different ways. Normal epithelial cells enclose invading bacteria with a vacuolar or vesicular membrane and likely kill them. CF cells, on the other hand, fail to enclose invading bacteria but partially surround them with intermediate filaments, which may not effectively prevent bacterial replication or epithelial cell damage.
FIG.8.
Transmission electron microscopy of group 2 CF and normal cultures incubated with B. cenocepacia Panels a to d refer to CF cultures showing bacteria on the surface and between and inside the cells. The arrowhead in panel d represents intermediate filaments surrounding invaded bacteria. Panels e and f refer to normal cultures; asterisks in panel e represent vacuolated cells; the arrow in panel f represents bacteria-like particles. The inset in panel f is a portion of one of the bacteria-like particles showing colloidal gold immunoreactivity with the anti-B. cenocepacia antibody (arrowheads).
Persistence of representative strains from other B. cepacia complex species on CF and normal cultures and their effect on IL-8 production.
In this set of experiments, we chose to use only group 1 cultures because they mimic the in vivo situation more closely, having a protective apical mucus layer. They also showed differential responses to BC7 with respect to bacterial persistence and IL-8 production. CF and normal cultures were infected apically with representative strains of the B. cepacia complex species listed in Table 2 and incubated for 24 h; the apical surface was washed, and the cells were dissociated with 0.5% Triton X-100 as before. The number of bacteria in the cell homogenates (persistence of bacteria on the cells) and the amount of IL-8 in the washes were quantified by dilution plating and ELISA, respectively. Normal cultures did not show bacteria in the cell homogenates irrespective of the B. cepacia complex strain used, suggesting that they were resistant to all of the strains tested. CF cultures showed some bacteria in cell homogenates only when infected with K56-2, C5393, and CEP40. B. cenocepacia isolate K56-2, which is also an ET12 strain, showed the highest number and was comparable to BC7. B. multivorans (isolate C5393) and B. vietnamiensis (CEP40) also persisted on CF cultures, but to a lesser extent (50%) than K56-2 or BC7.
IL-8 production in normal cultures increased minimally over that in controls (noninfected cultures) with all B. cepacia complex species tested, but the increase was not statistically significant. In contrast, CF cultures showed a significant increase over controls in IL-8 release, but only in response to those B. cepacia complex isolates that had the ability to persist on the epithelial cells (i.e., K56-2, C5393, and CEP40), suggesting that bacteria have to bind to and/or invade the underlying epithelial cells to induce IL-8 release.
DISCUSSION
The present study demonstrates for the first time that culturing of CF and normal airway epithelial cells at passage 1 causes them to differentiate into mucociliary epithelia which retain their major in vivo characteristics, including their response to infectious stimuli. Culturing cells at passage 1 is advantageous over using primary cells because it increases the availability of cells for replicate experiments, increases reproducibility, and prevents the carryover of infection that might bias experimental results.
The major features that distinguished CF from normal cultures included the absence of CFTR in the apical cell surface, goblet cell hyperplasia, excessive mucus, and increased levels of secreted mucin and chemokine IL-8, even under basal conditions. The observed increase in mucin secretion and IL-8 in CF cultures may be due to the carryover of residual inflammatory stimuli from the donor (such as lipopolysaccharide, flagella, or other microbial products). However, an increase in IL-8 levels has also been observed in xenografts prepared from naïve airway epithelium from fetuses with CF and in infants with CF who had no detectable infection (13, 46), indicating that a proinflammatory stimulus may be an intrinsic component of CF and may be related to faulty CFTR. It has been shown, for example, that ΔF508-CFTR causes constitutive activation of nuclear factor κB through an endoplasmic reticulum overload response both in vitro and in vivo (15). This may in turn cause upregulation of IL-8 expression and mucin secretion. In addition to showing features similar to those observed in vivo (9, 13, 27-29), CF cultures were also found to be more vulnerable to a low dose of B. cepacia complex infection than normal cultures, suggesting that this cell culture model can be used to study host responses to infection that are specific for airway mucosa from CF patients.
Bacterial dose.
The use of a low infection dose (104 CFU) was very important to obtain the differential responses observed between normal and CF cultures because higher doses damaged both types of cultures. With the low dose, however, CF cultures, unlike their normal counterparts, showed increased secretion of IL-8, compromised tight junctions (which increased the permeability of the epithelium), and epithelial damage as a result of bacterial replication and invasion. Normal cultures mainly trapped the added bacteria in the apical mucus layer, reduced the initial inoculum by one log (which is assumed to represent an intact innate defense function), and did not show compromised tight junctions, excessive production of IL-8, or epithelial damage. Such a dramatic difference between CF and normal epithelium in response to B. cenocepacia has not been described previously.
Role of surface mucus.
The apical mucus layer, which is a first line of defense (7, 8, 16, 32), was found to play an important role in protecting the underlying epithelium from B. cenocepacia and other B. cepacia complex species but was more efficient in normal than in CF cultures. Normal cultures (group 1) trapped, killed, and/or inhibited replication of the bacteria, thus decreasing bacterial density and preventing activation of underlying epithelial cells. Depletion of the apical mucus prior to infecting the cultures (group 2) led to increased surface bacteria and IL-8 and mucin secretion. Thus, in addition to physically entrapping bacteria, mucus may contain a bactericidal and/or bacteriostatic agent(s) that is active against B. cepacia complex strains. Recently, lactoperoxidase, which is present in normal human airway secretions, was shown to be active against members of the B. cepacia complex (49), and we speculate that a similar activity in the secreted mucus of normal cultures may be responsible for reducing bacterial density.
Although CF cultures contained more surface mucus than normal cultures under resting (basal) conditions, it was found to be less protective than normal mucus. The apical mucus CF cultures trapped bacteria but did not reduce bacterial density or bacterium-related epithelial damage. The reduced protective role of CF mucus may be due to its physical properties and/or its composition. It has been shown that there is increased absorption of water and decreased fluid secretion in airways of CF patients, leading to inspissation of secretions, increased adherence of mucus to cells, and ciliary dysfunction (8, 16, 24, 48). As a result, bacteria trapped in the mucus layer may physically be very close to the underlying epithelial cells, increasing the chances of bacterial adherence to and invasion of epithelial cells. In addition, there are reports indicating absence or reduced activity of antibacterial agents in CF airway secretions (1, 43). Preliminary studies in our laboratory have also indicated a lack of antibacterial activity against B. cenocepacia in CF culture secretions relative to those of normal cultures (U. Sajjan and J. Forster, unpublished observations). We speculate, therefore, that, in vivo, bacteria entrapped in dehydrated CF mucus remain in close proximity to underlying epithelial cells for longer periods, replicate, and are not cleared as effectively as in normal mucus, leading to enhanced bacterial invasion of underlying epithelial cells.
Intracellular response to bacterial invasion.
The patterns of B. cenocepacia invasion differed significantly between normal and CF cultures. In normal cultures, invading bacteria were found mainly within vacuoles of cells that resembled mucus-producing goblet cells. The bacteria did not show a distinctive outer membrane around them, indicating that they probably were not viable. In contrast, Schwab et al. (41) reported the presence of viable bacteria within and between cells in normal cultures. The discrepancy in our observations is most likely due to their use of much higher infection doses (5 × 107 CFU/culture). Indeed we saw similar results (i.e., many viable bacteria) in normal cultures when doses of 106 CFU/culture or higher were used (data not presented).
CF cultures showed bacteria within and between cells irrespective of the presence of an intact or previously depleted apical mucus layer. Many bacteria within cells were not held in vacuoles but were free or surrounded by intermediate filaments, indicating that intracellular movement of bacteria in CF cells may involve disruption or rearrangement of these filaments. A similar pattern was observed previously in squamous-differentiated normal airway epithelial cell cultures infected by B. cenocepacia (33). Our findings therefore imply that squamous and CF cells may have an intrinsic impairment in processing and killing of B. cenocepacia.
Responses of CF and normal cultures to other members of the B. cepacia complex.
With a few selected strains belonging to other species of the B. cepacia complex, we found that normal cultures were resistant to all of the isolates tested. This may be due to the efficient trapping of added bacteria in the apical mucus layer, which protects and prevents the activation of underlying epithelial cells. CF cultures, on the other hand, showed differential susceptibility to infecting B. cepacia complex species. One B. cenocepacia ET12 strain and one strain each of B. multivorans and B. vietnamiensis were able to cross the mucus barrier, adhere and/or invade, and activate the underlying epithelial cells. These results suggest that B. cepacia complex strains probably differ in their initial capacity to persist in and activate CF epithelial cells. Mucociliary-differentiated CF cultures can apparently detect such differences between B. cepacia complex species. Hence, this in vitro cell culture model is likely to be able to identify potentially infectious strains of B. cepacia complex in CF patients.
Significance for CF.
The model system developed and described in the present study has revealed significant differences in the susceptibility and intracellular response to infection of normal and CF airway epithelia. In the case of isolates BC7 and K56-2, which are known virulent strains, there was a much greater association of bacteria with CF than with normal cells. Of additional interest are the potential role of normal mucus in bacterial entrapment and viability and the different intracellular fates of ingested or invading B. cenocepacia between CF and normal cultures. Further studies regulating these responses are in progress, which we hope will enhance our knowledge of B. cenocepacia pathogenesis in lung infection in CF patients and lead to the development of new strategies to treat the infection. Based on our results with other species of the B. cepacia complex, this model system should be useful in identifying other potential virulent strains of the B. cepacia complex. Thus, in future studies, more isolates from the B. cepacia research panel will be tested to validate this point.
Acknowledgments
We thank J. Zabner and P. Karp at the University of Iowa for providing airway epithelial cells from CF and non-CF subjects for our initial experiments. We also thank our colleagues Aina Tilups and YewMeng Heng, Department of Pediatric Laboratory Medicine, for help in processing samples for electron microscopy and Danny Aguilar, Graphic Center, Hospital for Sick Children, for help in preparing the figures for publication.
Financial support was obtained from the Canadian Cystic Fibrosis Foundation.
Editor: J. N. Weiser
REFERENCES
- 1.Bals, R., D. J. Weiner, R. L. Meegalla, F. Accurso, and J. M. Wilson. 2001. Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am. J. Respir. Cell Mol. Biol. 25:21-25. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. L., M. Jonas, E. Y. Chi, D. K. Clark, A. Berger, and A. Griffith. 1996. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect. Immun. 64:4054-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmiel, J. F., M. Berger, and M. W. Konstan. 2002. The role of inflammation in the pathophysiology of CF lung disease. Clin. Rev. Allergy Immunol. 23:5-27. [DOI] [PubMed] [Google Scholar]
- 4.Cieri, M. V., N. Mayer-Hamblett, A. Griffith, and J. L. Burns. 2002. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect. Immun. 70:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clode, F. E., M. E. Kaufmann, H. Malnick, and T. L. Pitt. 2000. Distribution of genes encoding putative transmissibility factors among epidemic and nonepidemic strains of Burkholderia cepacia from cystic fibrosis patients in the United Kingdom. J. Clin. Microbiol. 38:1763-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyne, C. B., M. K. Vanhook, T. M. Gambling, J. L. Carson, R. C. Boucher, and L. G. Johnson. 2002. Regulation of airway tight junctions by proinflammatory cytokines. Mol. Biol. Cell 13:3218-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson, S. H., and R. Boucher. 2003. Update on pathogenesis of cystic fibrosis lung disease. Curr. Opin. Pulm. Med. 9:486-491. [DOI] [PubMed] [Google Scholar]
- 9.Dupuit, F., N. Kalin, S. Brezillon, J. HInnarsky, B. Tummler, and E. Puchelle. 1995. CFTR and differentiation markers expression in non-CF and delta F 508 homozygous CF nasal epithelium. J. Clin. Investig. 96:1601-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedges, S., W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266-270. [DOI] [PubMed] [Google Scholar]
- 11.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karp, O. H., T. O. Moninger, S. P. Weber, T. S. Nesselhauf, J. L. Launspach, J. Zabner, and M. J. Welsh. 2002. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures, p. 115-137. In C. Wise (ed.), Methods in molecular biology, vol. 188. Humana Press Inc., Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 13.Khan, T. Z., J. S. Wagnener, T. Bost, J. Martinez, J. Accurso, and D. W. H. Riches. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151:1075-1082. [DOI] [PubMed] [Google Scholar]
- 14.Khatri, I. A., K. R. Bhaskar, J. T. LaMont, U. S. Sajjan, C. K. Y. Ho, and J. F. Forstner. 2003. The effect of chondroitinase ABC on purulent sputum from cystic fibrosis and other patients. J. Pediatr. 53:608-618. [DOI] [PubMed] [Google Scholar]
- 15.Knoree, A., M. Wagner, H. Schaefer, W. Colledge, and H. L. Pahl. 2002. DF508-CFTR causes constitutive NF-kB activation through an ER-overload responses in cystic fibrosis lungs. Biol. Chem. 383:271-282. [DOI] [PubMed] [Google Scholar]
- 16.Knowles, M. R., and R. C. Boucher. 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 109:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler, D. R., U. Sajjan, Y. H. Chow, B. Martin, G. Kent, A. K. Tanswell, C. McKerlie, J. F. Forstner, and J. Hu. 2003. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc. Natl. Acad. Sci. USA 100:15364-15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledson, M. J., M. J. Gallagher, J. E. Corkill, C. A. Hart, and M. J. Walshaw. 1998. Cross infection between cystic fibrosis patients colonised with Burkholderia cepacia. Thorax 53:432-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. I. Campbell, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility factors in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd Mills, C., J. R. Dorin, D. J. Davidson, D. J. Porteus, E. W. F. W. Alton, R. L. Dormer, and M. A. McPherson. 1995. Decreased b-adrenergic stimulation of glycoprotein secretion in CF mice submandibular glands: reversal by the methylxanthine, IBMX. Biochem. Biophys. Res. Commun. 215:674-681. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd Mills, C., M. M. C. Pereira, R. L. Dormer, and M. A. McPherson. 1992. An antibody against a CFTR-derived synthetic peptide, incorporated into living submandibular cells, inhibits b-adrenergic stimulation of mucin secretion. Biochem. Biophys. Res. Commun. 188:1146-1152. [DOI] [PubMed] [Google Scholar]
- 22.Mahenthiralingam, E., A. Baldwin, and P. Vandamme. 2002. Burkholderia cepacia complex infection in patients with cystic fibrosis. J. Med. Microbiol. 51:533-538. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D. W., and C. D. Mohr. 2000. Invasion and intracellular survival of Burkholderia cepacia. Infect. Immun. 68:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui, H., B. R. Grubb, R. Tarran, S. H. Randell, J. T. Gatzy, W. Davis, and R. C. Boucher. 1998. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways diseases. Cell 95:1005-1015. [DOI] [PubMed] [Google Scholar]
- 25.McPherson, M. A., R. L. Dormer, N. A. Bradbury, J. A. Dodge, and M. C. Goodchild. 1986. Defective b-adrenergic secretory responses in submandibular acinar cells from cystic fibrosis patients. Lancet ii:1007-1008. [DOI] [PubMed] [Google Scholar]
- 26.Mohr, C. D., M. Tomich, and C. A. Herfst. 2001. Cellular aspects of Burkholderia cepacia infection. Microbes Infect. 3:425-435. [DOI] [PubMed] [Google Scholar]
- 27.Puchelle, E., S. de Bentzmann, C. Hubeau, J. Jacquot, and D. Gaillard. 2001. Mechanisms involved in cystic fibrosis airway inflammation. Pediatr. Pulmonol. Suppl. 23:143-145. [PubMed] [Google Scholar]
- 28.Puchelle, E., and B. Peault. 2000. Hum. airway xenograft models of epithelial cell regeneration. Respir. Res. 1:125-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puchelle, E., J.-M. Zahm, S. de Bentzmann, and D. Gaillard. 1997. Mucus and airway epithelium alterations in cystic fibrosis, p. 301-326. In D. F. Rogers and M. I. Lethem (ed.), Airway mucus: basic mechanisms and clinical perspectives. Birkhauser Verlag, Basel, Switzerland.
- 30.Rajan, S., and L. Saiman. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47-56. [DOI] [PubMed] [Google Scholar]
- 31.Randell, S. H., L. Walstad, U. E. Schwab, B. R. Grubb, and J. R. Yankaskas. 2001. Isolation and culture of airway epithelial cells from chronically infected human lungs. In Vitro Cell Dev. Biol. Anim. 37:480-489. [DOI] [PubMed] [Google Scholar]
- 32.Rogers, D. F. 1994. Airway goblet cell: responsive and adaptable front-line defenders. Eur. Respir. J. 7:1690-1706. [PubMed] [Google Scholar]
- 33.Sajjan, U., C. Ackerley, and J. Forstner. 2002. Interaction of cblA/Adhesin-positive Burkholderia cepacia with squamous epithelium. Cell. Microbiol. 4:73-86. [DOI] [PubMed] [Google Scholar]
- 34.Sajjan, U., M. Corey, A. Humar, E. Tullis, E. Cutz, C. Ackerley, and J. Forstner. 2001a. Immunolocalization of Burkholderia cepacia in the lungs of cystic fibrosis patients. J. Med. Microbiol. 50:535-546. [DOI] [PubMed] [Google Scholar]
- 35.Sajjan, U., S., M. Corey, M. Karmali, and J. F. Forstner. 1991. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J. Clin. Investig. 89:648-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sajjan, U., G. Thanassoulis, V. Cherapanov, A. Lu, C. Sjolin, B. Steer, Y. Wu, O. Rotstein, G. Kent, C. McKerlie, J. Forstner, and P. Downey. 2001. Susceptibility of Cftr−/− mice to pulmonary infection with Burkholderia cepacia. Infect. Immun. 69:5138-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajjan, U., Y. Wu, G. Kent, and J. Forstner. 2000. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J. Med. Microbiol. 49:875-885. [DOI] [PubMed] [Google Scholar]
- 38.Sajjan, U. S., and J. F. Forstner. 1992. Identification of the mucin-binding adhesin of isolated Pseudomonas cepacia from patients with cystic fibrosis. Infect. Immun. 60:1434-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajjan, U. S., J. F. Moreira, M. Liu, A. Humar, C. Charparro, J. Forstner, and S. Keshavjee. A novel model to study bacterial adherence to the transplanted airway: Inhibition of Burkholderia cepacia adherence to human airway by dextran and xylitol. J. Heart Lung Transplant., in press. [DOI] [PubMed]
- 40.Sajjan, U. S., F. A. Sylvester, and J. Forstner. 2000. Cable-piliated Burkholderia cepacia bind to cytokeratin 13 of epithelial cells. Infect. Immun. 68:1787-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwab, U., M. Leigh, C. Ribeiro, J. Yankaskas, K. Burns, P. Gilligan, P. Sokol, and R. Boucher. 2002. Patterns of epithelial cell invasion by different species of Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 70:4547-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwiebert, E. M., D. J. Benos, and C. M. Fuller. 1998. Cystic fibrosis: a multiple exocrinopathy caused by dysfunctions in a multifunctional transport protein. Am. J. Med. 104:576-590. [DOI] [PubMed] [Google Scholar]
- 43.Smith, J. J., S. M. Travis, E. P. Greenberg, and M. J. Welsh. 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85:229-236. [DOI] [PubMed] [Google Scholar]
- 44.Svanborg, C., M. Hedlund, H. Connell, W. Agace, R. D. Duan, A. Nilsson, and B. Wult. 1996. Bacterial adherence and mucosal cytokine responses. Receptors and transmembrane signaling. Ann. N. Y. Acad. Sci. 797:177-190. [DOI] [PubMed] [Google Scholar]
- 45.Tatterson, L. E., J. F. Poschet, A. Firoved, J. Skidmore, and V. Deretic. 2001. CFTR and Pseudomonas infections in cystic fibrosis. Front. Biosci. 6:D890-D897. [DOI] [PubMed] [Google Scholar]
- 46.Tirouvanziam, R., S. de Bentzmann, C. Hubeau, J. Hinnrasky, J. Jacquot, B. Peault, and E. Puchelle. 2000. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 23:121-127. [DOI] [PubMed] [Google Scholar]
- 47.Vandamme, P., B. Holmes, T. Coeyne, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. Govan. 2003. Burkholderia cenocepacia sp. nov.-a new twist to an old story. Res. Microbiol. 154:91-96. [DOI] [PubMed] [Google Scholar]
- 48.Widdicombe, J. H. 2002. Regulation of the depth and composition of airway surface liquid. J. Anat. 201:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wijkstrom-Frei, C., S. El-Chemaly, R. Ali-Rachedi, C. Gerson, M. A. Cobas, R. Forteza, M. Salathe, and G. E. Conner. 2003. Lactoperoxidase and Human Airway Host Defense. Am. J. Respir. Cell Mol. Biol. 29:206-212. [DOI] [PubMed] [Google Scholar]
- 50.Zabner, J., M. P. Seiler, J. L. Launspach, W. R. Kearney, D. C. Look, J. J. Smith, and M. J. Welsh. 2000. The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. Proc. Natl. Acad. Sci. USA 97:11614-11619. [DOI] [PMC free article] [PubMed] [Google Scholar]