Abstract
Although the early stages of intracellular infection by Legionella pneumophila are well established at the ultrastructural level, a detailed ultrastructural analysis of late stages of intracellular replication has never been done. Here we show that the membrane of the L. pneumophila-containing phagosome (LCP) is intact for up to 8 h postinfection of macrophages and Acanthamoeba polyphaga. At 12 h, 71 and 74% of the LCPs are disrupted within macrophages and A. polyphaga, respectively, while the plasma membrane remains intact. At 18 and 24 h postinfection, cytoplasmic elements such as mitochondria, lysosomes, vesicles, and amorphous material are dispersed among the bacteria and these bacteria are considered cytoplasmic. At 18 h, 77% of infected macrophages and 32% of infected A. polyphaga amoebae harbor cytoplasmic bacteria. At 24 h, 99 and 78% of infected macrophages and amoebae, respectively, contain cytoplasmic bacteria. On the basis of lysosomal acid phosphatase staining of infected macrophages and A. polyphaga, the lysosomal enzyme is present among the bacteria when host vesicles are dispersed among bacteria. Our data indicate that bacterial replication proceeds despite physical disruption of the phagosomal membrane. We also show that an lspG mutant that is defective in the type II secretion system and therefore does not secrete the hydrolytic enzymes metalloprotease, p-nitrophenol phosphorylcholine hydrolase, lipase, phospholipase A, and lysophospholipase A is as efficient as the wild-type strain in disruption of the LCP. Therefore, L. pneumophila disrupts the phagosomal membrane and becomes cytoplasmic at the last stages of infection in both macrophages and A. polyphaga. Lysosomal elements, mitochondria, cytoplasmic vesicles, and amorphous material are all dispersed among the bacteria, after phagosomal disruption, within both human macrophages and A. polyphaga. The disruption of the LCP is independent of the hydrolytic enzymes exported by the type II secretion system.
The hallmark of Legionnaires' disease is intracellular replication of Legionella pneumophila in alveolar macrophages (10, 11, 41). L. pneumophila also infects protozoa, which are considered the environmental hosts for this intracellular pathogen (41, 42). The intracellular fates of L. pneumophila in both of these evolutionarily distant host cells are similar, where the bacteria evade fusion of the phagosome to lysosomes (2, 13, 30-32). In addition, the L. pneumophila-containing phagosome (LCP) recruits the endoplasmic reticulum (ER), vesicles, and mitochondria within the first 15 min of infection (2, 13, 30, 45). Formation of the rough-ER-surrounded LCP is independent of autophagy (39, 45). After a lag phase of 4 h, bacterial replication is initiated within both host cells (30, 41). Upon entry into the postexponential growth phase, L. pneumophila exhibits several virulence traits, including a pore-forming activity that may allow the bacteria to lyse the host cell (6, 15, 21, 36).
We have previously shown that upon termination of the intracellular infection, the pore-forming activity of L. pneumophila facilitates lysis of the host cell and bacterial egress (6, 34-36). It has been proposed that bacterial egress may occur in two stages upon termination of intracellular bacterial replication. First, the phagosomal membrane is disrupted allowing the bacteria to egress to the cytoplasm. The second stage is manifested by lysis of the plasma membrane and bacterial release into the extracellular environment (34).
Although numerous studies have examined the early stages of this intracellular infection at the ultrastructural level by electron microscopy, there have been no studies to examine the late stages of this intracellular infection at the ultrastructural level. Therefore, we examined the integrity of the phagosomal membrane throughout intracellular infection of U937 macrophages and Acanthamoeba polyphaga at the ultrastructural level. In addition, we examined the presence of the lysosomal enzyme acid phosphatase in the phagosome. Our data show that the phagosomal membrane remains intact for up to 8 h postinfection. While the plasma membrane remains intact, the LCP membrane is disrupted by 12 h. By 18 to 24 h, almost all of the infected cells harbored bacteria free in the cytoplasm, which coincided with the presence of host cell organelles dispersed between the bacteria, as well as the lysosomal enzyme acid phosphatase. We show that the type II secretion system, which is essential for secretion of many hydrolytic enzymes that can potentially disrupt membranes, is not involved in the disruption of LCPs within macrophages.
MATERIALS AND METHODS
Bacterial and cell cultures.
L. pneumophila serogroup I strain AA100 is a clinical isolate that has been described previously (4). L. pneumophila strain AA100, the isogenic lspG mutant NU259 (40), and the icmT null mutant AA100kmT (40) were grown on buffered charcoal-yeast extract plates or in buffered yeast extract broth. U937 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Gibco). U937 cells were differentiated with phorbol 12-myristate 13-acetate (Sigma) for 48 h before use, as previously described (25). A. polyphaga cells were maintained in peptone-yeast extract-glucose medium.
Electron microscopy.
For examination of infected amoebae or macrophages by transmission electron microscopy, monolayers in six-well plates were infected with L. pneumophila strains at a multiplicity of infection of 10 for 1 h, followed by three washes. At 8, 12, 18, and 24 h postinfection, the infected monolayers were washed with 0.1 M Sorenson's phosphate buffer and incubated for 45 min in 0.1 M Sorenson's phosphate buffer containing 3.5% glutaraldehyde, pH 7.4, at 4°C. After four washes in Sorenson's phosphate buffer for 5 min each time, infected cells were postfixed with 1% OsO4 in the same buffer for 45 min. Samples were dehydrated and processed as we described previously (23). Sections were stained with uranyl acetate and lead citrate and examined with a Hitachi H-7000/STEM electron microscope (Hitachi, Ltd., Tokyo, Japan) at 80 kV as described previously (23).
Fusion between lysosomes of the LCP were determined by examination for the presence of the lysosomal enzyme acid phosphatase as described previously (13, 35). The monolayers were incubated with the acid phosphatase-specific substrate β-glycerophosphate (0.1 M acetate buffer, 2 mM β-glycerol phosphate [as the substrate], 1.2% lead nitrate [as the capture metal]) for 1 h at 37°C prior to fixation by osmium tetroxide as described above.
RESULTS
Egress of L. pneumophila into the cytoplasm during the late stages of intracellular infection of macrophages.
We examined the integrity of the phagosomal membrane within U937 macrophages throughout the intracellular life cycle by transmission electronic microscopy. The phagosomal membrane was considered intact when the membrane had no visible disruptions of its integrity and no host cell elements, such as mitochondria, cytoplasmic vesicles, or amorphous material, were present within the phagosome. The phagosomal membrane was considered disrupted when apparent physical disruptions in the integrity of the phagosomal membrane were present and amorphous material was within the phagosome. The bacteria were considered cytoplasmic when the phagosomal membrane was not continuous and host cell cytoplasmic elements such mitochondria and cytoplasmic vesicles, in addition to amorphous material, were dispersed among the bacteria. During the infection process, lysis of infected cell is initiated by 12 to 18 h and the released bacteria initiate secondary infections. To exclude secondary infection from our analysis at 12 h and thereafter, we excluded cells with fewer than 5, 10, and 30 bacteria at 12, 18, and 24 h, respectively. In order to rule out the possibility of deterioration of the membranes of the host cells by the fixation and staining procedures used, we observed the plasma membrane at 12 and 18 h postinfection of cells containing disrupted LCPs.
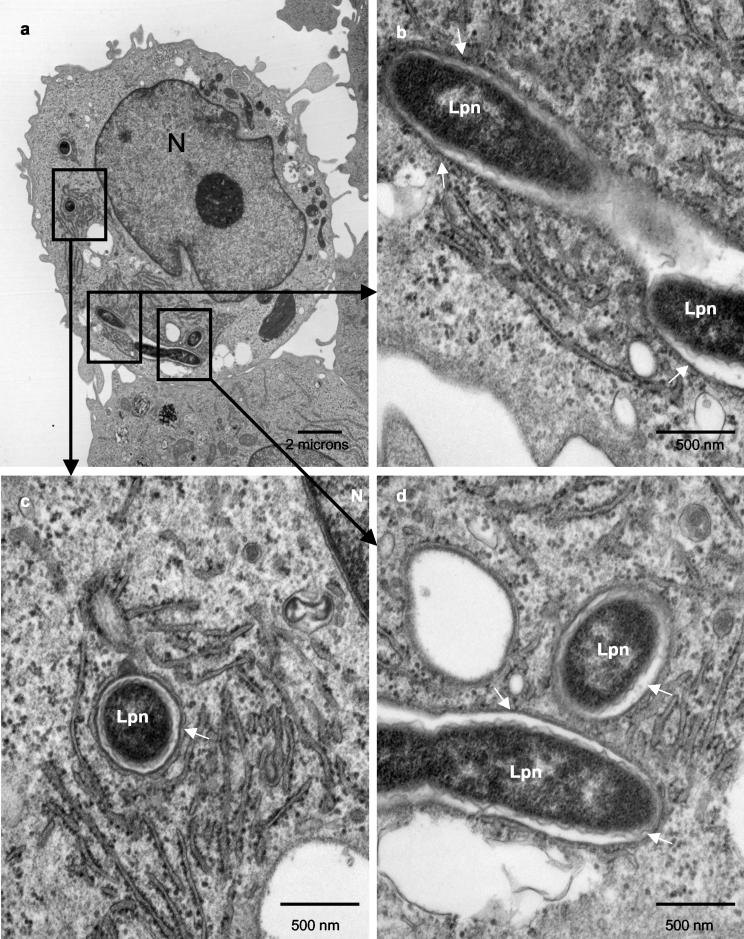
At 8 h postinfection, all of the phagosomal membranes were thick and did not show any sign of disruption of integrity (Fig. 1 and data not shown) and the phagosomal membranes were tightly closed around the bacteria, similar to what has been shown by numerous studies at 2 to 6 h. The lumen of the phagosomes did not contain any host cell cytoplasmic elements. The phagosomal membranes were therefore considered intact at 8 h postinfection. ER was also observed around most phagosomes at 8 h (Fig. 1b and c).
FIG. 1.
L. pneumophila bacteria are contained within intact phagosomes at 8 h postinfection. Representative electron micrographs of L. pneumophila-infected macrophages at 8 h postinfection are shown. The membrane of the LCP is intact, as indicated by the thin arrows. Abbreviations: Lpn, L. pneumophila; N, nucleus.
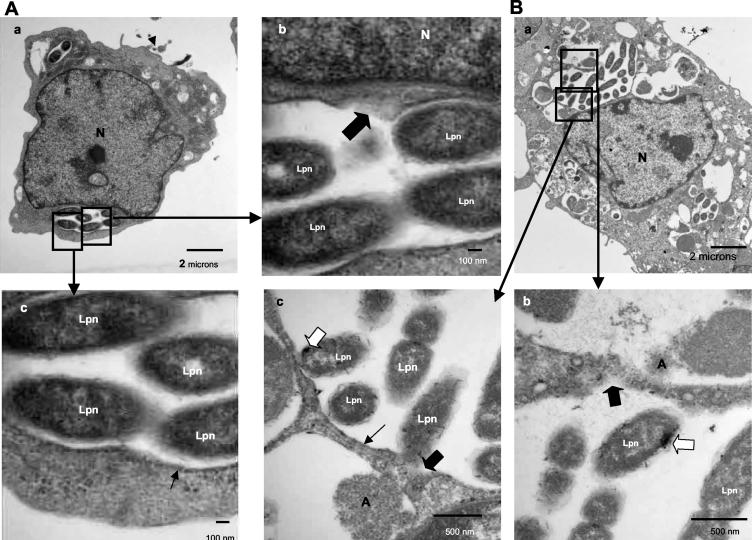
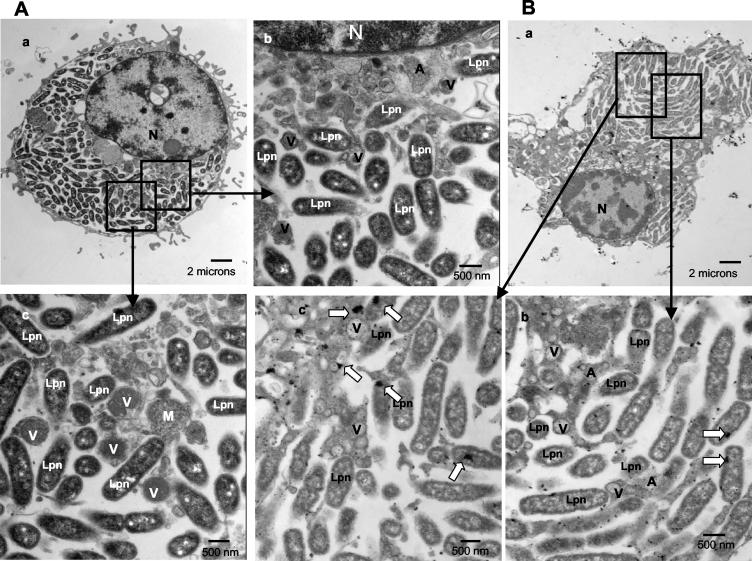
A small portion of the cells had already been lysed by 12 h postinfection. Only ∼2% of the phagosomes had intact membranes with no signs of disruption of integrity in this time period (Fig. 2A; see also Fig. 8A). The lumen of the phagosomes was distended compared to that of 8-h-old phagosomes. Seventy percent of the LCP membranes were disrupted at 12 h (Fig. 2A, part b; see also Fig. 8A). These phagosomes were still free of host cell cytoplasmic elements such as mitochondria and cytoplasmic vesicles. The rough ER was not visible any more around some phagosomes, but few mitochondria were within the vicinity of the phagosomes (data not shown). Twenty-seven percent of the infected cells harbored cytoplasmic bacteria at 12 h (Fig. 8A). Importantly, the plasma membrane was completely intact in 95% of infected cells harboring disrupted LCPs or cytoplasmic bacteria.
FIG. 2.
Disruption of the integrity of the phagosome membrane at 12 h postinfection (A) and presence of lysosomal contents in the LCP in macrophages at 12 h (B). Representative electron micrographs of L. pneumophila-infected macrophages at 12 h postinfection are shown. The membrane of the LCP is mostly intact, as indicated by the thin black arrows in panel A, part c, but partially disrupted in panel A, part b, and panel B, parts b and c, as indicated by the thick black arrows. Lysosomal staining of acid phosphatase is indicated by the large white arrows. Abbreviations: A, amorphous element; M, mitochondria; Lpn, L. pneumophila; N, nucleus.
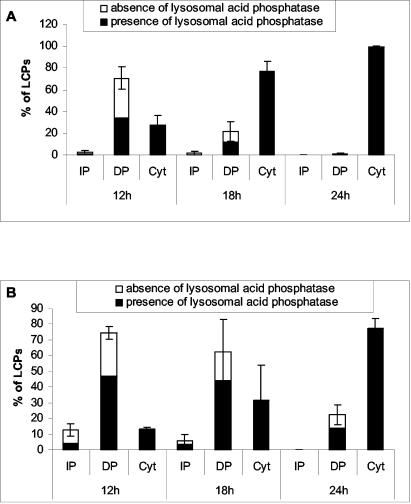
FIG. 8.
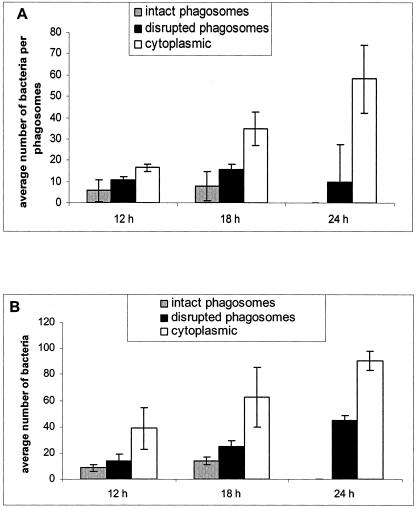
Quantitative analysis of the integrity of LCPs in macrophages (A) and A. polyphaga (B) at 12, 18, and 24 h postinfection. The percentages of LCPs positive for acid phosphatase were evaluated when the bacteria were within an intact phagosome (IP), a disrupted phagosome (DP), and in the cytoplasm (Cyt). Averages of 60, 50, and 10 to 30 LCPs were analyzed for both macrophages and A. polyphaga at 12, 18, and 24 h, respectively. At 24 h, the number of LCPs analyzed was low because most of the cells had been lysed. Error bars represent standard deviations.
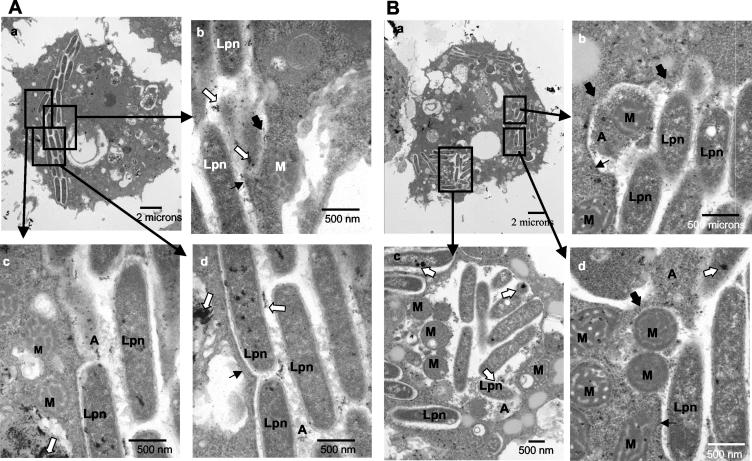
By 18 h, ∼50% of the cells in the monolayers were lysed. Among the remaining infected cells at 18 h, 22% of the phagosomes were disrupted (P < 0.01, Student t test) and 77% of the infected cells harbored cytoplasmic bacteria (P < 0.01, Student t test), which percentages are significantly different from those at the 12-h time point (Fig. 8A). Phagosomal membranes became very thin and were discontinuous in many parts (Fig. 3A, parts b and c). Vesicles and cytoplasmic amorphous elements juxtaposed the bacteria (Fig. 3A, part b). The chromatin in the nucleus was condensed and was located perinuclearly (Fig. 3A and B, parts a), which is a characteristic of apoptosis (22, 29) that has been shown to be triggered during late stages of infection (37). The plasma membrane was intact in 80% of the infected cells harboring disrupted LCPs or cytoplasmic bacteria.
FIG. 3.
Disruption of the phagosome and migration of host cell cytoplasmic contents to the phagosome (A) and presence of lysosomal contents in the disrupted LCP in macrophages at 18 h (B). Representative electron micrographs of L. pneumophila-infected macrophages at 18 h postinfection are shown. L. pneumophila (Lpn) bacteria are within disrupted phagosomes (thick black arrows). The intact portions of the membrane are indicated by thin black arrows. White arrows indicate the presence of lysosomal acid phosphatase. In panel A the bacteria surrounded by few vesicles are cytoplasmic whereas in panel B the LCP is disrupted. Note the perinuclear condensation of the chromatin. Abbreviations: A, amorphous material; M, mitochondria; V, vesicles; N, nucleus.
By 24 h postinfection, the majority of the cells had been lysed. In 99% of the remaining infected cells, the bacteria were cytoplasmic, which is significantly different from the percentages at 12 and 18 h (P < 0.01, Student t test) (see Fig. 8A). Most of the phagosomal membranes disappeared (Fig. 4A, parts b and c). Cytoplasmic elements such as mitochondria, vesicles, and amorphous material were dispersed among the bacteria (Fig. 4A, parts c and d). The bacteria occupied most of the space within the host cell that did not include the nucleus (Fig. 4A, part a). Perinuclear condensed chromatin was detected in all cells, a clear sign of apoptosis (Fig. 4A, parts a and b).
FIG. 4.
Cytoplasmic L. pneumophila in macrophages at 24 h postinfection (A) and presence of lysosomal contents in the LCP in macrophages at 24 h (B). Representative electron micrographs of L. pneumophila-infected macrophages at 24 h postinfection are shown. L. pneumophila (Lpn) bacteria are cytoplasmic and are surrounded by numerous vesicles (V), lysosomal contents (white arrows), mitochondria (M), and amorphous elements (A). No distinguishable phagosomal membrane surrounds the bacteria. N, nucleus.
Egress of L. pneumophila into the cytoplasm during the late stages of intracellular infection of A. polyphaga.
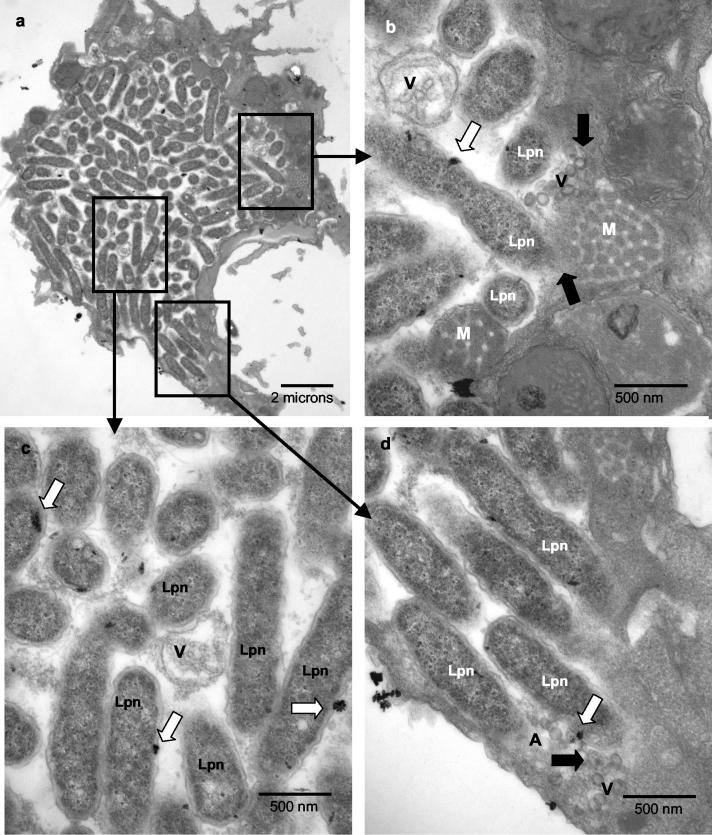
Similar to human macrophages, at 8 h postinfection of A. polyphaga, most of the LCPs were tight and intact with a thick phagosomal membrane around the bacteria (data not shown). A small portion of cells had already been lysed by 12 h postinfection. At this time, 74% of the LCPs were disrupted (Fig. 5A and B; see also Fig. 8B). The lumen of the LCPs was still tight (Fig. 5). Signs of nuclear apoptosis were not visible in A. polyphaga (Fig. 5). Thirteen percent of the infected A. polyphaga cells contained cytoplasmic bacteria at 12 h postinfection, where cytoplasmic elements such as mitochondria and amorphous material were already dispersed among bacteria (Fig. 5B, parts b, c, and d; see also Fig. 8B).
FIG. 5.
Presence of lysosomal contents in the LCP in A. polyphaga at 12 h. Shown are representative electron micrographs of L. pneumophila-infected A. polyphaga at 12 h postinfection where the bacteria are in a disrupted phagosome (A) or cytoplasmic (B). Thick black arrows show sites without any visible phagosomal membrane. Thin black arrows show sites where the phagosomal membrane is still visible. Lysosomal acid phosphatase is indicated by the large white arrows. In panel A, the phagosomal membrane is disrupted (large black arrows) and amorphous material (A) is present within the phagosome. In panel B, although parts of the phagosomal membrane are still visible (thin black arrows), L. pneumophila (Lpn) bacteria are mostly cytoplasmic, as shown by the presence of amorphous material (A) and the presence of mitochondria (M) dispersed among the bacteria.
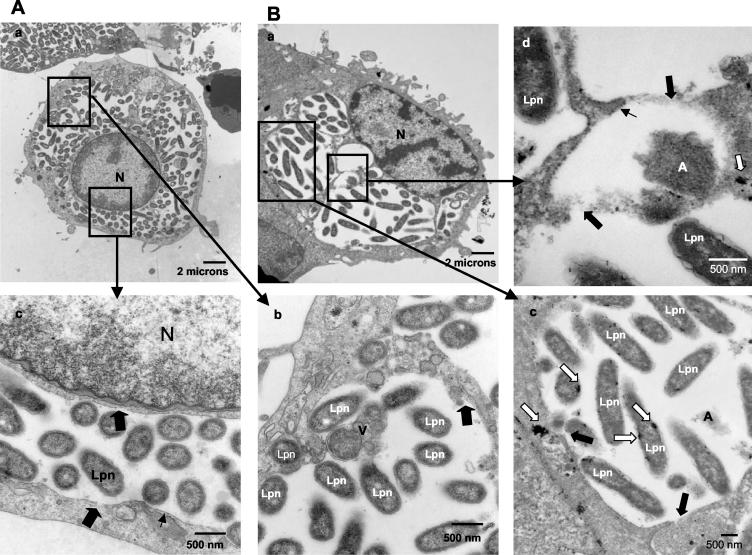
Approximately 50% of A. polyphaga amoebae were lysed by 18 h. At this time, 62% of the LCPs were disrupted and 32% of the infected cells harbored cytoplasmic bacteria (Fig. 6; see also Fig. 8B). In the cells harboring cytoplasmic bacteria, the phagosomal membranes had disappeared almost completely and cytoplasmic host cell elements such as mitochondria, vesicles, and amorphous elements were dispersed among the bacteria (Fig. 6A, parts a, b, and c).
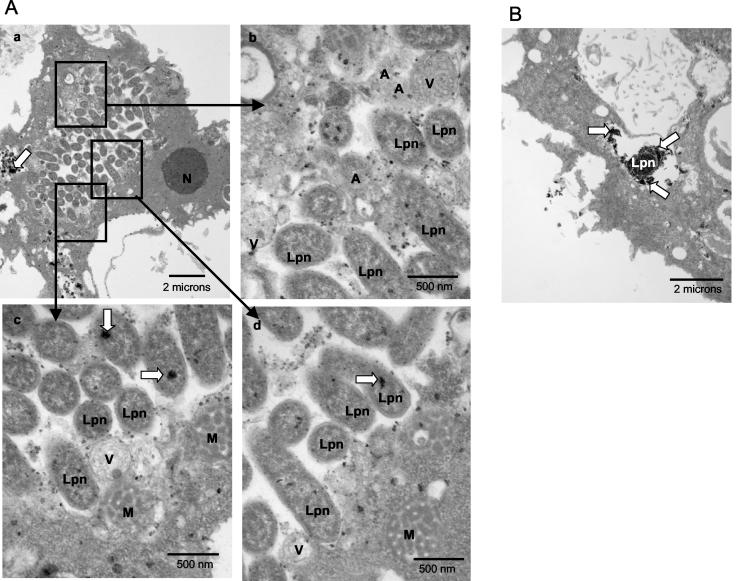
FIG. 6.
Presence of lysosomal contents in the LCP in A. polyphaga at 18 h. (A) Representative electron micrographs of AA100-infected A. polyphaga at 18 h postinfection (A). L. pneumophila (Lpn) bacteria are juxtaposed with mitochondria (M), vesicles (V), and amorphous material (A). Lysosomal acid phosphatase staining (white arrows) is present among the bacteria. N, nucleus. (B) Representative electron micrograph of the positive control for lysosomal acid phosphatase colocalization within the icmT null mutant-containing phagosome. Note the diffuse pattern of the acid phosphatase enzyme in the phagosome that fuses to lysosomes.
At 24 h, similar to infection of macrophages, more than 80% of the cells were lysed. Twenty-three percent of the phagosomes were disrupted in the remaining infected A. polyphaga amoebae (see Fig. 8B). Seventy-eight percent of the infected cells at 24 h harbored cytoplasmic bacteria, which is significantly different from the percentages at the 12- and 18-h time points (P < 0.05 and P < 0.01, respectively), where the phagosomal membrane was not visible and mitochondria, vesicles, and amorphous elements were dispersed among the bacteria (Fig. 7b, c, and d).
FIG. 7.
Presence of lysosomal contents in the LCP in A. polyphaga at 24 h. Representative electron micrographs of L. pneumophila-infected A. polyphaga at 24 h postinfection are shown. L. pneumophila (Lpn) bacteria are cytoplasmic, and mitochondria (M), vesicles (V), and amorphous material (A) are dispersed among the bacteria. Thick black arrows indicate the disrupted areas of the phagosomal membrane. The white arrows show lysosomal staining of acid phosphatase.
We concluded that the phagosomal membrane within infected macrophages and A. polyphaga was intact for up to 8 h postinfection but clear disruption of the integrity of the phagosomal membrane was apparent at 12 h and thereafter. Bacterial egress into the cytoplasm was observed by 12 h, and by 18 to 24 h all infected intact cells harbored bacteria in the cytoplasm. Cytolysis of both host cells was evident by 12 h.
Presence of lysosomal contents between the bacteria at the end of intracellular infection of U937 macrophages.
Since disruption of LCP was accompanied by the presence of host cell organelles such as mitochondria and vesicles that were dispersed between the bacteria after 12 h postinfection, we examined whether some of these vesicles were lysosomes. Since the LCPs were intact and no cytoplasmic materials were found within the phagosomes at 8 h, we tested for the presence of the lysosomal enzyme acid phosphatase within the phagosomes of U937 macrophages at 12, 18, and 24 h postinfection. As mentioned above, to exclude secondary infection from our analysis at 12 h and thereafter, we excluded cells with fewer than 5, 10, and 30 bacteria at 12, 18, and 24 h, respectively. We included a positive control for the presence of acid phosphatase in the LCP with an icmT null mutant (AA100kmT) that has been shown to fuse to lysosomes in U937 cells and A. polyphaga (35, 36) (Fig. 6B).
At 12 h, ∼48% of the disrupted phagosomes in macrophages contained acid phosphatase (Fig. 8A and 2B). All of the infected cells that harbored cytoplasmic bacteria contained lysosomal acid phosphatase concentrated in foci (Fig. 8A), which is most likely within vesicles. However, the membrane of lysosomal vesicles, in the phagosome as well as in the cytoplasm, was masked by the electron-dense substrate. This is in contrast to the diffuse presence of acid phosphatase in the LCP harboring an icmT mutant, which fuses to lysosomes (35, 36) (Fig. 6B).
At 18 and 24 h, cytoplasmic bacteria represented the largest group of intracellular bacteria. In all of these cells containing cytoplasmic bacteria, the lysosomal acid phosphatase was present among bacteria in concentrated foci (Fig. 3B, part c, 4B, parts b and c, and 8A), suggesting its presence within vesicles.
Presence of lysosomal contents between the bacteria at the end of the intracellular infection of A. polyphaga.
Similar to U937 macrophages, at 12 h postinfection of A. polyphaga, lysosomal acid phosphatase was present in concentrated foci among all of the cytoplasmic bacteria, and 63% of the disrupted phagosomes contained lysosomal acid phosphatase in concentrated foci (Fig. 5A and B and 8B).
At 18 h postinfection, the lysosomal enzyme was present among bacteria in all of the infected A. polyphaga amoebae harboring cytoplasmic bacteria (Fig. 6A and 8B). Seventy percent of the disrupted phagosomes also contained the lysosomal enzyme in concentrated foci.
At 24 h, most of the bacteria within A. polyphaga amoebae were cytoplasmic and 100% of the cells harboring the cytoplasmic bacteria contained the lysosomal enzyme acid phosphatase in concentrated foci among the bacteria (Fig. 7 and 8B). About 61% of the disrupted phagosomes contained the lysosomal enzyme.
We concluded that in both macrophages and A. polyphaga after 12 h, lysosomal acid phosphatase was present in concentrated foci among the cytoplasmic bacteria. This enzyme was present in concentrated foci among the bacteria in macrophages and A. polyphaga similar to its vesicular distribution in the cytoplasm. This is in contrast to its diffuse presence in the icmT mutant phagosome, which fuses to lysosomes (Fig. 6B).
Intracellular multiplication of L. pneumophila in the cytoplasm of both macrophages and A. polyphaga.
Since L. pneumophila exhibits robust replication within the phagosomes, we examined whether disruption of the phagosome and bacterial egress into the cytoplasm resulted in termination of bacterial replication. We performed single-cell analysis to quantitate the number of bacteria per cell at several stages of the intracellular infection of macrophages and A. polyphaga.
Although the number of bacteria within disrupted phagosomes did not show a dramatic increase within macrophages between 12 and 24 h, it was clear that the average number of cytoplasmic bacteria per macrophage increased from ∼17 at 12 h to 35 at 18 h and ∼58 at 24 h. These results indicated that bacterial growth was not arrested by disruption of the integrity of the phagosome (Fig. 9A).
FIG. 9.
Numbers of bacteria per phagosome (or per cell when cytoplasmic) within macrophages (A) and A. polyphaga (B) at 12, 18, and 24 h postinfection. The minimum average number of bacteria per phagosome was obtained by single-cell analysis by electron microscopy. Averages of 60, 50, and 10 to 30 LCPs were analyzed for both macrophages and A. polyphaga at 12, 18, and 24 h, respectively. At 24 h, the number of LCPs analyzed was low because most of the cells had been lysed. Error bars represent standard deviations.
In A. polyphaga, the number of bacteria present per disrupted phagosomes increased from an average of 14 at 12 h postinfection to 25 at 18 h and 45 at 24 h (Fig. 9B). The number of cytoplasmic bacteria per host amoeba increased from an average of 39 at 12 h postinfection to 63 at 18 h and 91 at 24 h (Fig. 9B). Note that the quantitations are based on a single section, and thus the values obtained represent the minimal numbers of organisms.
Taken together, the intracellular growth kinetics and our enumeration of intracellular bacteria in our single-cell analysis of both macrophages and A. polyphaga amoebae indicated that bacterial replication continued despite disruption of the LCP and migration of mitochondria and other host cell organelles that become dispersed among the bacteria.
The type II secretion system is not involved in disruption of the LCP membrane.
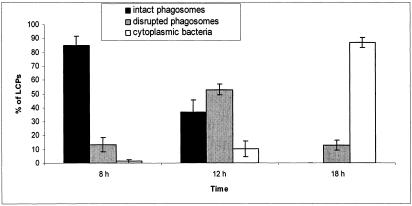
There are many hydrolytic enzymes secreted by the type II secretion system of L. pneumophila, including metalloprotease, p-nitrophenol phosphorylcholine hydrolase, lipase, phospholipase A, and lysophospholipase A (28, 40). Since these hydrolytic enzymes are potential candidates that disrupt the LCP, we used the lspG mutant NU259, which is defective in the type II secretion system owing to a defect in an inner membrane structural protein component of the type II secretion system (40). In order to determine if the secretion of theses exoproteins is responsible for the disruption of the wild-type LCP, U937 macrophage cell lines were infected with the lspG mutant NU259 and examined at 8, 12, 18, and 24 h postinfection by electron microscopy. About 85% of the LCPs were intact at 8 h postinfection (Fig. 10). At 12 h postinfection 53% of the LCPs were disrupted, and at 18 h postinfection 87% of the bacteria were cytoplasmic within U937 macrophages (Fig. 10). At 24 h, most of cells were in a disintegrated state and it was not possible to analyze the data. Thus, the type II secretion system and the hydrolytic enzymes secreted through it are not involved in the disruption of the LCPs.
FIG. 10.
Evaluation of the integrity of the LCPs harboring type II secretion system-deficient lspG mutant strain NU259 within U937 macrophages at 8, 12, and 18 h postinfection. The percentages of bacteria within intact phagosomes, disrupted phagosomes, and the cytoplasm were evaluated among a total of 30 to 50 LCPs. Error bars represent standard deviations.
DISCUSSION
L. pneumophila invades both mammalian and protozoan host cells and resides within a replicative vacuole, evading phagolysosomal fusion. Late stages of the intracellular life cycle and the mechanism of bacterial egress after cessation of intracellular bacterial replication are not well understood. The two-step egress model that we have previously proposed (34) predicts that L. pneumophila first egresses from the phagosome into the cytoplasm, followed by lysis of the host cell plasma membrane. Here we show for the first time in both human-derived macrophages and A. polyphaga amoebae that L. pneumophila is present in an intact phagosomal membrane for up to 8 h postinfection. However, by 12 h and thereafter, physical and structural disruptions of integrity of the phagosomal membrane are apparent and cytoplasmic organelles including mitochondria, vesicles, and lysosomes are dispersed among the bacteria.
While ultrastructural studies of early stages of the LCP are rather numerous, LCP integrity during later stages of intracellular infection has never been examined. Tilney et al. have described an intact phagosomal membrane at 20 h postinfection of U937 macrophages, but the phagosome described contains only two bacteria (45). It is well documented that the bacterial lag phase within U937 macrophages is ∼4 h and the generation time is ∼90 to 120 min (1, 41, 42). Therefore, phagosomes containing two or fewer bacteria at 20 h are most likely secondary infections or bacteria that fail to replicate and are not representative of a 20-h-old LCP. In our study, we have considered that a 12-h LCP may harbor at least 5 bacteria, an 18-h LCP may harbor at least 10 bacteria, and a 24-h LCP may harbor at least 30 bacteria. In that manner, we believe that we have excluded from our analysis most LCPs that are formed as a result of secondary infections.
Single-cell analysis has shown an increasing number of bacteria per disrupted phagosome in both macrophages and A. polyphaga at 12, 18, and 24 h, which indicates that the bacteria are multiplying within their disrupted phagosomal environment and also when they are cytoplasmic. Goetz et al. have shown that upon microinjection of L. pneumophila into the cytoplasm of epithelial-type Caco-2 cells, the bacteria are unable to replicate in the cytoplasm (27). This can be due to one of two reasons. First, legionellae primarily infect alveolar macrophages in humans and animal models, and in Caco-2 cells, L. pneumophila is likely to behave differently than in macrophages (24, 38). It may therefore be premature to extrapolate data from macrophages to Caco-2 cells. In support of this, it has been shown that the Salmonella sifA mutant, which is released into the cytosol of macrophages, fibroblasts, and epithelial cells, replicates in the cytoplasm of epithelial cells (HeLa cells) but not in that of macrophages and fibroblasts (12). Second, L. pneumophila undergoes phenotypic modulation upon intracellular infection accompanied by alterations in the gene expression and ultrastructural characteristics of the bacteria (3, 5, 26, 43). Thus, microinjection bypasses adjustment of the bacteria to the phagosomal microenvironment, which may be essential for subsequent adjustment to the cytoplasm. In addition, it is clear that the cytoplasm of macrophages is different from that of other types of cells in permissiveness to bacterial replication.
The cytoplasmic microenvironment that the bacteria inhabit during the late stage of infection contains mitochondria, vesicles, and amorphous material. The acid phosphatase enzyme is present at concentrated foci in the cytoplasm before disruption of the phagosome and later in the vicinity of the cytoplasmic bacteria within disrupted phagosomes. The lysosomal membrane is not visible in either the phagosome or the cytoplasm, most likely because of the electron-dense deposits of the substrate that mask the lysosomal membrane. Thus, the bacteria may not be in direct contact with the lysosomal enzyme, which is further supported by the lack of bacterial degradation. In contrast, fusion of the lysosomes with L. pneumophila mutants such as the icmT null mutant results in diffuse distribution of the acid phosphatase throughout the phagosome and bacterial degradation (35, 36). This is further supported by our lack of detection of lysosomal vesicles making contact or fusing with the LCP in thousands of cells that we have examined. In addition, the presence of mitochondria among the bacteria indicates that intact lysosomes, similar to mitochondria and other vesicles, are present among the bacteria. Our data suggest that the lysosomes may migrate to and not fuse with the LCP. Thus, the presence of lysosomal contents among bacteria is unlikely to be due to a direct fusion of lysosomes to the LCP. However, limited direct lysosomal fusion, in addition to passive migration of the lysosomes through the disrupted phagosomal membrane, cannot be excluded at this time. By confocal laser scanning microscopy, it has been shown that during late stages of infection of A/J mouse macrophages, a significant proportion of the LCPs colocalizes with lysosomal proteases (43). It is not known whether lysosomal fusion at late stages also occurs in human cells or whether phagosomal disruption also occurs in A/J mouse macrophages. Sturgill-Koszycki and Swanson have also shown by confocal microscopy that bacterial replication also proceeds in the phagosomes that become colocalized with lysosomal contents, which is consistent with our observations (43). Support for lysosomal fusion to the LCP A/J mice macrophages has been shown by observations that inhibition of the proton pump ATPase activity of infected macrophages with bafilomycin A1 or treatment with ammonium chloride or chloroquine to neutralize the acidic pH reduce intracellular bacterial replication (43). However, although these treatments have no effect on bacterial replication in vitro (43), it is not known how these treatments affect bacterial replication within macrophages. Importantly, elevation of the pH in the LCP is expected to block bacterial replication, since bacterial growth in vitro occurs within a very limited pH range around pH 6.9. Taken together, our data suggest that colocalization of the LCP with lysosomal contents during late stages of infection may be due to migration of lysosome vesicles to the disrupted LCPs. Our data do not exclude direct fusion and release of lysosomal contents to the LCP lumen.
L. pneumophila possesses a type II secretion system that allows the secretion of many hydrolytic enzymes that have the potential to degrade membranes, including a metalloprotease, p-nitrophenylphosphorylcholine hydrolase, lipase, phospholipase A, and lysophospholipase A. In this study, we have used an lspG mutant that is defective in an inner membrane structural protein component of the type II secretion system (40) in order to determine whether the type II secretion system and the enzymes secreted through it are involved in disruption of the LCP membrane. Our data show that the type II secretion system and its secreted enzymes are not required for disruption of the LCPs. Thus, these hydrolytic enzymes do not play any detectable role in disruption of the LCPs.
Necrosis is usually the cause of a severe cellular insult, such as loss of membrane integrity caused by a toxin (33). L. pneumophila has been shown to have a cytopathogenic effect on host cells leading to necrosis, which is thought to be mediated by a pore-forming activity (6, 21, 35, 36). It is likely that the pore-forming activity during late stages of infection of macrophages contributes to cytolysis of the host cell. However, many other factors may be involved. First, apoptosis in mammalian cells is likely to be a contributing factor, since it is triggered during late stages of infection when the LCPs harbor more than 20 bacteria (37). Second, whether other phospholipases secreted by L. pneumophila (7-9, 16-20, 44) also contribute to dismantling of the cell is not known. Third, it is possible that disruption of the phagosomal membrane may be partially mediated by physical pressure from the increasing number of bacteria in the phagosome. Fourth, it is also possible that, similar to S. enterica serovar Typhimurium (14), L. pneumophila is actively involved in maintaining the integrity of the phagosome during early stages of infection but loses that ability later. Therefore, the mechanism of egress of L. pneumophila into the cytoplasm is likely to be complex and multifactorial.
In summary, we have shown that L. pneumophila is within an intact phagosome for up to 8 h in both macrophages and A. polyphaga. The phagosomal membrane is disrupted at 12 h, and the bacteria egress into the cytoplasm between 12 and 24 h, prior to disruption and lysis of the host cell plasma membrane. Cytoplasmic elements such as mitochondria and vesicles are dispersed among the bacteria at the end of intracellular replication, and this phagosomal disruption does not seem to affect bacterial replication. Disruption of the LCP is not mediated by hydrolytic enzymes secreted by the type II secretion system.
Editor: J. T. Barbieri
REFERENCES
- 1.Abu Kwaik, Y. 1998. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect. Immun. 66:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y. 1996. The phagosome containing Legionella pneumophila within the protozoan Hartmanella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 62:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik, Y., B. I. Eisenstein, and N. C. Engleberg. 1993. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect. Immun. 61:1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik, Y., and N. C. Engleberg. 1994. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol. Microbiol. 13:243-251. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik, Y., L.-Y. Gao, O. S. Harb, and B. J. Stone. 1997. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol. Microbiol. 24:629-642. [DOI] [PubMed] [Google Scholar]
- 6.Alli, O. A. T., L.-Y. Gao, L. L. Pedersen, Zink. S., M. Radulic, M. Doric, and Y. Abu Kwaik. 2000. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68:6431-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aragon, V., S. Kurtz, and N. P. Cianciotto. 2001. Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect. Immun. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragon, V., O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. [DOI] [PubMed] [Google Scholar]
- 9.Baine, W. B. 1985. Cytolytic and phospholipase C activity in Legionella species. J. Gen. Microbiol. 131:1383-1391. [DOI] [PubMed] [Google Scholar]
- 10.Baskerville, A., A. B. Dowsett, R. B. Fitzgeorge, P. Hambleton, and M. Broster. 1983. Ultrastructure of pulmonary alveoli and macrophages in experimental Legionnaires' disease. J. Pathol. 140:77-90. [DOI] [PubMed] [Google Scholar]
- 11.Baskerville, A., R. B. Fitzgeorge, M. Broster, P. Hambleton, and P. J. Dennis. 1981. Experimental transmission of Legionnaires' disease by exposure to aerosols of Legionella pneumophila. Lancet ii:1389-1390. [DOI] [PubMed] [Google Scholar]
- 12.Beuzon, C. R., S. P. Salcedo, and D. W. Holden. 2002. Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology 148:2705-2715. [DOI] [PubMed] [Google Scholar]
- 13.Bozue, J. A., and W. Johnson. 1996. Interaction of Legionella pneumophila with Acanthamoeba catellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brumell, J. H., P. Tang, M. L. Zaharik, and B. B. Finlay. 2002. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar Typhimurium in the cytosol of epithelial cells. Infect. Immun. 70:3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flieger, A., S. Gong, M. Faigle, M. Deeg, P. Bartmann, and B. Neumeister. 2000. Novel phospholipase A activity secreted by Legionella species. J. Bacteriol. 182:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flieger, A., S. Gong, M. Faigle, H. Northoff, and B. Neumeister. 2001. In vitro secretion kinetics of proteins from Legionella pneumophila in comparison to proteins from non-pneumophila species. Microbiology 147:3127-3134. [DOI] [PubMed] [Google Scholar]
- 18.Flieger, A., S. Gong, M. Faigle, S. Stevanovic, N. P. Cianciotto, and B. Neumeister. 2001. Novel lysophospholipase A secreted by Legionella pneumophila. J. Bacteriol. 183:2121-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flieger, A., S. Gongab, M. Faigle, H. A. Mayer, U. Kehrer, J. Mussotter, P. Bartmann, and B. Neumeister. 2000. Phospholipase A secreted by Legionella pneumophila destroys alveolar surfactant phospholipids. FEMS Microbiol. Lett. 188:129-133. [DOI] [PubMed] [Google Scholar]
- 20.Flieger, A., B. Neumeister, and N. P. Cianciotto. 2002. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect. Immun. 70:6094-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, L.-Y., and Y. Abu Kwaik. 2000. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ. Microbiol. 2:79-90. [DOI] [PubMed] [Google Scholar]
- 22.Gao, L.-Y., and Y. Abu Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends. Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 23.Gao, L.-Y., O. S. Harb, and Y. Abu Kwaik. 1998. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect. Immun. 66:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao, L.-Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. 1998. Different fates of Legionella pneumophila pmi and mil mutants within human-derived macrophages and alveolar epithelial cells. Microb. Pathog. 25:291-306. [DOI] [PubMed] [Google Scholar]
- 25.Gao, L.-Y., M. Susa, B. Ticac, and Y. Abu Kwaik. 1999. Heterogeneity in intracellular replication and cytopathogenicity of Legionella pneumophila and Legionella micdadei in mammalian and protozoan cells. Microb. Pathog. 27:273-287. [DOI] [PubMed] [Google Scholar]
- 26.Garduno, R. A., E. Garduno, M. Hiltz, and P. S. Hoffman. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70:6273-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz, M., A. Bubert, G. Wang, I. Chico-Calero, J. A. Vazquez-Boland, M. Beck, J. Slaghuis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67:3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilbi, H., A. Zychlinsky, and P. J. Sansonetti. 1997. Macrophage apoptosis in microbial infections. Parasitology 115(Suppl.):S79-S87. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwitz, M. A., and F. R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knodler, L. A., and B. B. Finlay. 2001. Salmonella and apoptosis: to live or let die? Microb. Infect. 3:1321-1326. [DOI] [PubMed] [Google Scholar]
- 34.Molmeret, M., and Y. Abu Kwaik. 2002. How does Legionella pneumophila exit the host cell? Trends Microbiol. 10:258-260. [DOI] [PubMed] [Google Scholar]
- 35.Molmeret, M., O. A. Alli, M. Radulic, M. Susa, M. Doric, and Y. Abu Kwaik. 2002. The C-terminus of IcmT is essential for pore formation and for intracellular trafficking of Legionella pneumophila within Acanthamoeba polyphaga. Mol. Microbiol. 43:1139-1150. [DOI] [PubMed] [Google Scholar]
- 36.Molmeret, M., O. A. Alli, S. Zink, A. Flieger, N. P. Cianciotto, and Y. Abu Kwaik. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molmeret, M., S. D. Zink, L. Han, A. Abu-Zant, R. Asari, D. M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell. Microbiol. 6:33-48. [DOI] [PubMed] [Google Scholar]
- 38.Neumeister, B., M. Faigle, K. Lauber, H. Northoff, and S. Wesselborg. 2002. Legionella pneumophila induces apoptosis via the mitochondrial death pathway. Microbiology 148:3639-3650. [DOI] [PubMed] [Google Scholar]
- 39.Otto, G. P., M. Y. Wu, M. Clarke, H. Lu, O. R. Anderson, H. Hilbi, H. A. Shuman, and R. H. Kessin. 2004. Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum. Mol. Microbiol. 51:63-72. [DOI] [PubMed] [Google Scholar]
- 40.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678-689. [PubMed] [Google Scholar]
- 42.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorpe, T. C., and R. D. Miller. 1981. Extracellular enzymes of Legionella pneumophila. Infect. Immun. 33:632-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilney, L. G., O. S. Harb, P. S. Connelly, C. G. Robinson, and C. R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637-4650. [DOI] [PubMed] [Google Scholar]