Abstract
Background:
The aim was to investigate the relationships among lean mass (LM), fat mass (FM), and bone mineral density (BMD) in women stratified by body mass index (BMI) (BMI – normal-weight, overweight, obese) and to determine threshold at which body fat assumes negative relationship with BMD.
Methods:
This was a cross-sectional study in 471 healthy Caucasian women, aged 18-67 years. BMD, LM, and FM were measured using dual-energy X-ray absorptiometry. Analysis of variance with Bonferroni corrections was used to test the BMI group differences. Linear regression was used to examine independent contributions of LM and FM on BMD of various skeletal sites (controlling for age and height). In overweight/obese women PROC LOESS plots were used to determine the inflection points at which either LM or FM relationship with BMD changes direction. Separate analyses in pre- and post-menopausal women were conducted as well.
Results:
Spine and femoral neck BMD were not different among three BMI groups while total body, femur and radius BMD were statistically different (the highest in the obese group). Linear regression revealed that LM had significant positive association with BMD of various skeletal sites in all groups. FM showed a negative association with BMD of femoral neck and femur in normal-weight and spine in overweight women, but a positive association with radius in obese women. Inflection points showed that body fat between 33% and 38% assumed negative relationship with BMD for most skeletal sites in overweight and obese women.
Conclusions:
Although LM has strong positive relationship with BMD, FM above 33% in overweight/obese women is negatively related to BMD of most skeletal sites. Therefore, overweight/obesity after certain amount of FM, may not be a protective factor against osteoporosis in females. For clinical practice in women, it is important to maintain LM and keep FM accrual below ~30% body fat to maintain good skeletal health.
Keywords: Body mass index, bone mineral density, fat mass, lean mass, menopause
INTRODUCTION
Osteoporosis, a disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, is an important public health challenge worldwide. It results in skeletal fragility, fracture susceptibility and is a significant risk factor for morbidity and mortality among the elderly.[1] Overweight and/or obesity are other clinical and public health challenges worldwide with numerous adverse health consequences. For many years, overweight/obesity and osteoporosis were thought to be mutually exclusive since results from many studies have shown that bone mass is positively associated with body weight and/or fat.[2,3,4,5] This was largely explained by the effect of gravitational loading and mechanical stimulation of bones by higher weight. In addition, obesity is associated with insulin resistance, which may result in androgen and estrogen overproduction in the ovaries and reduced production of sex hormone binding globulin by the liver in premenopausal women.[6] Higher estrogen and leptin levels from fat tissue may lead to increased bone mass due to reduced osteoclast and possibly increased osteoblast activity.[7] In postmenopausal women, adipose tissue is the site of androgen metabolism and thus the only source of extraglandular estrogens.[6] Furthermore, leptin, an adipocyte-derived hormone, is involved in the regulation of bone remodeling on both cellular and systemic level, the former one leading to higher bone mass.[8,9] Therefore, a generally accepted concept was that the excess weight (comprising fat and muscle tissue) protects against osteoporosis.
Although, both bone mineral density (BMD) and bone mineral content are influenced by lean and fat mass (FM),[10,11,12,13,14,15,16,17] it is not clear which component, lean mass (LM) or FM has greater influence on bone and under which circumstances. Some researchers have shown that both LM and FM are equally associated with BMD,[12,16] others showed that LM is a stronger predictor of BMD than FM,[13,15,17] and yet another group suggested that total FM is a better predictor of BMD.[10,11,14] According to Wang et al.,[18] these conflicting results could be attributed to the changes in body composition with age, gonadal stage, and weight. In general, bone and LM decrease and weight and FM increase with aging, especially after menopause.[19] Furthermore, the associations between body composition and BMD in different ethnicities are inconsistent. Reid et al.,[10,11] reported that FM is positively associated with BMD in Caucasian women, but in a recent study of a large cohort of Chinese women of different ages, it was found that the risks of osteoporosis, osteopenia, and non-spine fractures were significantly higher in those with higher percentage of body fat.[20] These conflicting results suggest a complex relationship among FM, LM, and bone mass indicating that other factors need to be taken into consideration, including comparison among different skeletal sites, different body mass index (BMI) groups and menopausal status.
Recently, this idea that a higher body weight, usually reflecting a higher body fat, offers protection against osteoporosis has been questioned,[21] as new data suggest that rising FM is negatively correlated with bone mass, even when adjusted for mechanical loading effects.[22] Adipose tissue, particularly visceral fat, generates various adipokines and other molecules that could provoke detrimental signaling to bone microenvironment,[23] possibly as a consequence of maintaining the low-grade chronic inflammation.[9,24] Therefore, it is necessary to closer examine these relationships and possibly determine the threshold at which FM starts inflicting some unfavorable effects on bone.
The purpose of this study was to investigate the interaction of LM and FM with BMD of various skeletal sites in women stratified by BMI into normal-weight, overweight, and obese groups. In overweight and obese women, the inflection points between each skeletal site and LM and/or FM, adjusted for age, were determined as well. We hypothesized that overweight and obese women will have higher BMD values in all measured skeletal sites and that it will be positively associated with LM. We also hypothesized that at certain point, obesity, expressed as FM (kg or %), will be unfavorably associated with bone outcomes. The significance of this study is that it provides a comprehensive analysis of the relationship between body composition (both lean and fat mass) and various skeletal sites in women with different BMIs and menopausal status and shows the changing modulation of FM on bone outcomes. To our knowledge, no study so far has identified the threshold points at which body fat becomes unfavorably related to the BMD of various measured skeletal sites in overweight and obese women of a wide age range.
METHODS
Participants
This was a cross-sectional study in 471 healthy Caucasian women, aged 18 - 67 years, who were recruited from the North Florida and South Georgia communities by various advertisements and word-of-mouth. Participants were excluded for uncontrolled hypertension, diabetes, cardiovascular disease, gastrointestinal disease, thyroid disease, severe osteoporosis, osteoarthritis, deformity of bones, cancers, eating disorders, liver and kidney diseases, smoking over one pack of cigarettes per day, taking calcium and/or Vitamin D supplements, hormones or medications that affect bone metabolism or weight, any type of estrogen formula, bone antiresorptive (e.g. bisphosphonates), or anabolic (e.g. teriparatide) drugs. Participants were also excluded if they were currently involved in any weight loss programs or consuming special diet. Interested women were prescreened via telephone for age, weight, height, medical history, and smoking status. Those who qualified at the prescreening interview were invited for their first visit at which time they reviewed and signed an informed consent approved by the Institutional Review Board at the Florida State University. Participants were assigned code numbers for data recording to ensure anonymity.
Anthropometry
Height without shoes was recorded to the nearest 0.1 cm and was measured using a wall-mounted stadiometer (Medart, St. Louis, MO, USA) with heels, buttocks, upper back, and head touching the vertical plane. Weight was recorded to the nearest 0.1 kg and was measured on a digital scale (Seca Corp., Model 707, Columbia, MD, USA). When measurements were taken, the participants were in normal indoor clothes (t-shirt and shorts or scrub pants) without shoes and jewelries. BMI (kg/m2) was calculated from height and weight.
Body composition measurements
Body composition measurements were performed by dual energy X-ray absorptiometry using the iDXA instrument with Encore software (version 13.11.016) (GE Medical Systems, Madison, WI, USA). The whole body scan was utilized to measure total body LM, FM, and BMD. The iDXA has a wider scan field and higher precision that can accommodate individuals up to 181 kg, avoiding the errors typically encountered when measuring overweight/obese individuals, and therefore providing a more accurate assessment of both bone and body composition.[25] The LM component refers to the total LM (derived from the whole body scan), after excluding bone and FM. BMD of regional skeletal sites, including spine (L2–L4), both left and right femurs (neck and total), and radius (1/3 distal) were measured and analyzed by specialized software for each. The axial skeletal sites, including femoral neck, total femur, and lumbar spine are the sites where fractures usually occur and the ones used to diagnose osteoporosis (based on their T-scores). Therefore, those sites were the ones that we focused our analyses on. The quality analysis for the instrument was conducted on a weekly basis using a standard aluminum spine block (phantom) provided by the manufacturer. Measurements of the phantom were within the manufacturer's precision standard of < 0.05% coefficient of variation.
Statistical analysis
The statistical analyses were performed using the statistical program SPSS (version 20 for Windows; SPSS, Inc., Chicago, IL, USA) and SAS (SAS Institute Inc., Cary, NC, USA) version 9.3, the latter one to conduct the inflection points analyses. The variables were checked for normality and presented as mean ± standard deviation, unless noted otherwise. Frequency and percentages were reported for categorical variables. One-way analysis of variance (ANOVA) was used to determine significant differences among three BMI groups (normal-weight, overweight, and obese) for anthropometrics, body composition, and BMD. When significant differences were found with ANOVA, the post-hoc Bonferroni correction was applied to correct for use of multiple comparisons. Linear regression models were developed to further evaluate the relationship between BMD of the skeletal sites described above and other predictors, including FM and LM within BMI groups and controlled for age and height. FM and LM were examined to predict the association between body composition and BMD of various regional sites (described above). PROC LOESS plots were used to study the relationship between BMD and LM and FM in overweight and obese women, only, and determine the inflection points. The analyses were performed separately on premenopausal and postmenopausal women, as well as on the total sample of overweight/obese women. Based on the plots, the inflection points were determined for nonlinearly distributed data and used to divide the data into linear-linear, linear-quadratic or quadratic-linear models to describe the relationship. PROC NLIN procedure was used to validate the inflection points, and to obtain the parameter estimates and their 95% confidence interval. P < 0.05 was considered as significant for all tests.
RESULTS
Descriptive characteristics
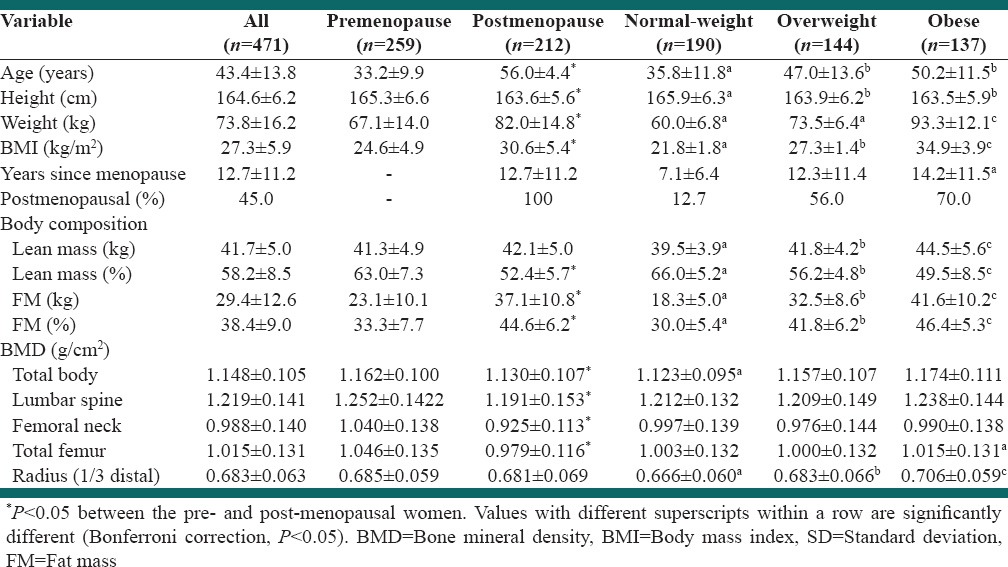
Table 1 presents characteristics of subjects divided by BMI groups. A total of 471 healthy Caucasian women (n = 259 pre and n = 212 postmenopausal) between the ages of 18 and 67 years were evaluated. The range in body weight and BMI from normal to obese was 45.2-127.8 kg and 18.5 - 44.3 kg/m2, respectively, with mean weight of 73.8 ± 16.2 kg and BMI of 27.3 ± 5.9 kg/m2. The correlation coefficient between FM and body weight and between LM and body weight were 0.81 and 0.58, respectively (both P < 0.01). The average proportion of body fat ranged from 30% in the normal-weight to 47.7% in the obese women. There were 12.7%, 56%, 70% postmenopausal women in normal-weight, overweight, and obese groups, respectively. There were significantly more postmenopausal women (P < 0.05) in the overweight and obese groups than in normal-weight group. The age of normal-weight women ranged from 18.1 to 64.1 years, while the age range of the overweight and obese women was from 18.3-66.9 to 19.5-66.6 years, respectively. There was no significant difference in age, height, years since menopause, and BMD of the whole body, spine and femoral neck between overweight and obese women. The obese women had significantly greater amount of LM (kg) but the lower percentage (both P < 0.05). They also had higher BMD of total body, total femur, and radius (all P < 0.05), compared with normal-weight women. No significant difference was observed in the spine and femoral neck BMD between the three groups.
Table 1.
Anthropometrics and BMD variables in women stratified by menopausal status or BMI (mean±SD)
Multiple regression analysis
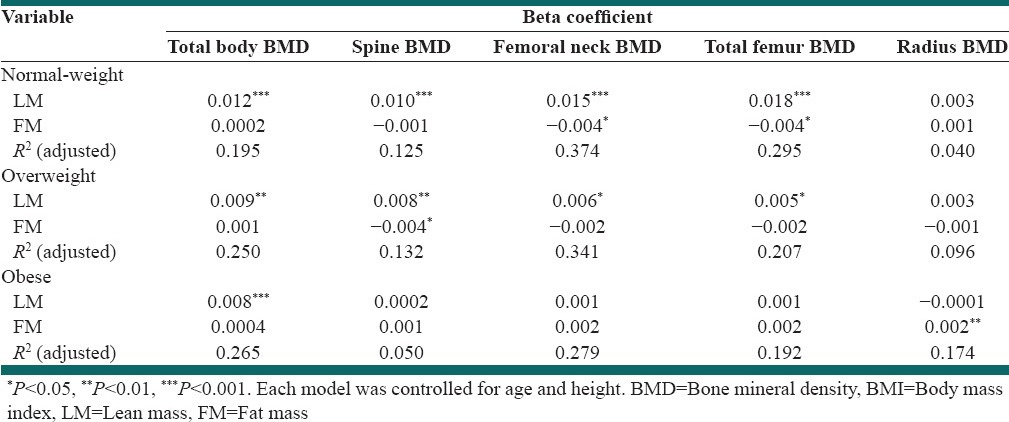
The results of multiple regression analysis with BMD of various skeletal sites as dependent variables in subjects stratified by BMI are shown in Table 2. When adjusted for age and height, a positive association was found between LM and BMD of all skeletal sites except radius in the normal-weight and overweight women. LM lost its significant association with most BMD sites except total body BMD in the obese group. In the normal-weight group, FM was associated with femoral neck and total femur BMD (P < 0.05). FM showed a negative association with spine BMD in the overweight group, but a positive one with radius BMD in the obese group (P < 0.01).
Table 2.
Multiple linear regression with BMD of various skeletal sites as dependent variable and lean and FM as independent variables in women stratified by BMI
Inflection points determination
Figure 1 presents the relationship between total body BMD and LM (a) in all overweight/obese women, which was significantly positive and linear, with the equation: Total body BMD = 0.74 + 0.00985* total lean [kg], P < 0.0001. The relationship between total body BMD and FM (b) was not significant (total body BMD = 1.1797–0.001* total fat [kg]), up to the body fat of 40 kg, at which point the relationship became quadratic (total body BMD = 0.8757 + 0.00884* total fat [kg] − 0.00005* total fat2 [kg]), P = 0.0011, with the upward trend, probably due to the excessive amount of weight and its gravitational effect on total body BMD. However, when the percent of total fat was used in the analysis, the relationship (c) was significantly negative (total body BMD = −3.4529 + 0.2885* total fat [%] − 0.0044* total fat2 [%], P = 0.05, with the inflection point at 38%. Thereafter, the relationship continued at a negative trend, but was not significant (total body BMD = 1.9452−0.0344* total fat [%] +0.000375* total fat2 [%]), [Figure 1].
Figure 1.
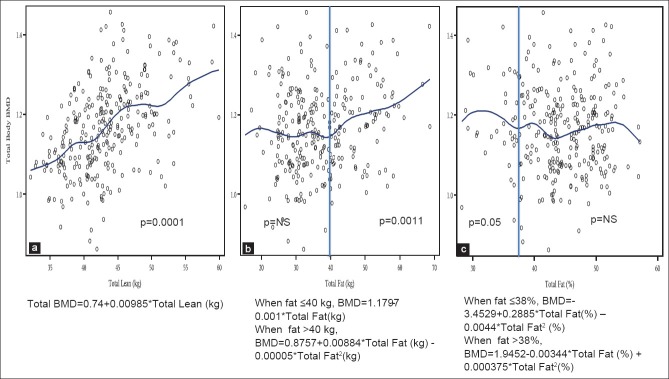
Relationship between total body bone mineral density and total lean mass (a), total body fat (b) and percent body fat (c) in overweight and obese women (n = 279)
Figure 2 presents the relationship between lumbar spine BMD and LM (a) in all overweight/obese women, which was significantly positive and linear, with the equation: L2–L4 BMD = 0.99 + 0.005* total lean (kg), P < 0.0014. The relationship between spine BMD and FM (b) was not significant at any point, with the equation: L2–L4 BMD = 1.37–0.006* total fat (kg), up to the body fat of 32 kg, and: L2–L4 BMD = 1.22 + 0.00012* total fat (kg), above 32 kg. When the percent of total fat was used in the analysis, the relationship (c) was significantly negative (L2–L4 BMD = −10.06 + 0.39* total fat [%]), P = 0.05, with the inflection point at 38%. Thereafter, the relationship was quadratic and continued at a negative trend, but was not significant (L2−L4 BMD = 2.12−0.039* total fat [%] +0.0004* total fat2 [%], [Figure 2].
Figure 2.
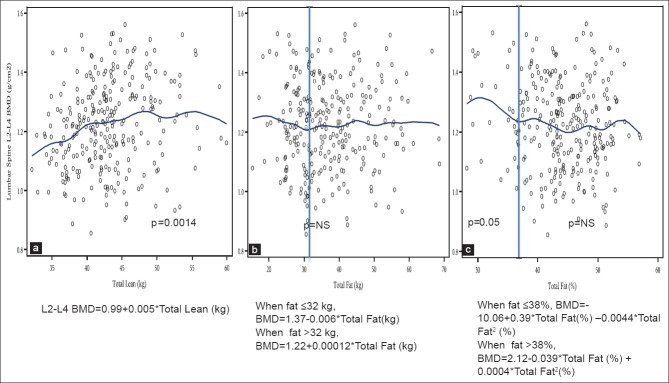
Relationship between lumbar spine (L2–L4) bone mineral density and total lean mass (a), total body fat (b) and percent body fat (c) in overweight and obese women (n = 279)
Figure 3 presents the relationship between femoral neck BMD and LM (a) in all overweight/obese women, which was significantly positive and linear, with the equation: Neck BMD = 0.67 + 0.007* total lean (kg), P < 0.0001. The relationship between neck BMD and FM (b) was not significant at any point, with the equation: Neck BMD = 0.0765 + 0.048* total fat (kg), up to the body fat of 22 kg, and: Neck BMD = 1.048–0.0036* total fat (kg) +0.00005* total fat2 (kg), above 22 kg. When the percent of total fat was used in the analysis, the relationship (c) had a positive trend (neck BMD = 0.403 + 0.047* total fat [%]), P = 0.06, with the inflection point at 33%. Thereafter, the relationship was quadratic and continued at a negative trend, but was not significant (neck BMD = 1.713–0.03* total fat [%] +0.0003* total fat2 [%]), [Figure 3]. The BMD of other femoral sites (Ward's, trochanter, shaft and total femur) and forearm showed similar tendencies; positive relationship with LM and changing relationship with FM, as the FM increases.
Figure 3.
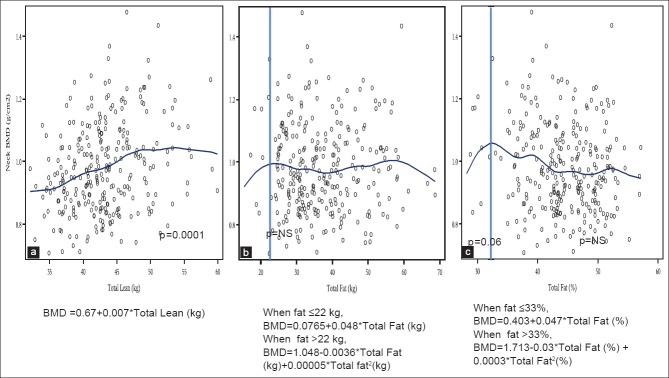
Relationship between femoral neck bone mineral density and total lean mass (a), total body fat (b) and percent body fat (c) in overweight and obese women (n = 279)
Similar results were obtained when the separate analyses for pre- and post-menopausal overweight/obese women were conducted. The relationship between LM and BMD of various skeletal sites was significantly positive and linear, with no inflection points at any site. Regarding the relationship between FM and BMD of various skeletal sites, the trends were very similar as in the whole population of overweight/obese women. Specifically, in premenopausal women the inflection points for a total body, lumbar spine and femoral neck BMD were at 35%, 40% and 40% of FM respectively. In postmenopausal women the inflection points for a total body, lumbar spine and femoral neck BMD were at 38%, 40% and 42% of FM respectively.
DISCUSSION
This study is unique and comprehensive as it provides information about the interaction of body weight, body composition (lean and fat tissue), and BMD of various skeletal sites in women of wide age range and different weight and/or obesity categories. Furthermore, in the overweight and obese cohort, we determined the threshold for the skeletal sites at which FM starts to be unfavorably associated with bone.
As expected, the results showed significant positive relationship between BMD of various skeletal sites and body weight, also reported earlier,[26,27,28] although obese women in this study had similar BMD in spine and femoral neck as normal-weight and overweight women, implying that the higher weight/obesity did not necessarily transfer into higher BMD for all skeletal sites. Additionally, based on multiple regression models in the whole population, there was a significant positive association between LM and BMD of most skeletal sites in normal-weight and overweight women except radius; and with the BMD of total body in obese women. FM showed a positive association with radius BMD in obese women; a negative association with BMD of the femoral neck and total femur in normal-weight and spine BMD in overweight women. When only overweight and obese women were analyzed, the relationship between BMD of each skeletal site and LM was significantly positive and linear. However, both FM and total fat percentage showed nonlinear relationships, with inflection points between 22–40 kg and 33–38% of fat (depending on the skeletal site) after which negative relationships were noted [Figures 1–3]. That was true when pre and postmenopausal women were analyzed separately in which cases; the inflection points in premenopausal women were even lower for the total boy BMD (at 35% vs. 38% of FM). However, for other skeletal sites (including spine and femoral neck BMD), the inflection points for premenopausal women were higher (at 40–42% of FM), compared to postmenopausal women (not presented).
The positive relationship between body weight on bone mass was expected and was in accordance with most of the previous results.[26,27,28] However, Sukumar et al.[29] showed no difference in lumbar spine BMD between heavier (BMI > 25) and normal-weight (BMI < 25) pre- and post-menopausal women, suggesting that other skeletal sites, like spine and femur might not be affected in the same way as total body BMD[30] and implying that when body weight reaches a certain level, its positive association with spine and femoral neck BMD diminishes. This was seen in our overweight/obese cohort where the total body BMD continued to increase with higher weight (reflecting higher body fat), but not with the higher percentage of fat [Figure 1b and c] and not in other skeletal sites [Figures 2, 3b and c]. Based on other studies in older women, greater BMI is generally associated with higher BMD,[4,31,32] therefore, confirming the older assumption that the weight gain in older women may reduce the prevalence of osteoporosis. With the results of this study, it becomes obvious that the weight gain only up to a certain level might be beneficial for bones.
Our findings are also in accordance with the previous reports of a significant positive association of total body LM and BMD of various sites in women stratified by BMI.[15,33] Ilich and Brownbill[34] showed that LM was the dominant positive predictor of total body, femoral neck, total femur, and spine BMD in normal-weight to slightly overweight postmenopausal women. These studies imply that maintaining LM is critical for maintaining bone mass. In the present study, the positive association of LM with BMD of spine, femoral neck, and total femur in obese women was lost after adjusting for age and height in multiple regression models, although overall significance still persisted when adjusted for age only [Figure 2a]. This might imply that bone mass is primarily determined by the dynamic loads from muscle force, but not necessarily by the static loads, such as muscle mass of the whole body.[35] Furthermore, the increase in FM with weight gain may reduce the impact of LM on BMD.
Some studies showed that total FM positively influences total body BMD in postmenopausal women.[11] In the present study, positive relationship with FM was only observed for the radius BMD in obese women [Table 2] and for total body BMD in overweight/obese women, in a nonlinear analysis with the total amount of fat, but not with the % fat [Figure 1b and c]. Since menopause is accompanied by increased FM and its redistribution, as well as decreased LM and BMD,[16,36] same factors may have a different impact on BMD during premenopausal or postmenopausal period. This concept was supported by the present study, demonstrating that FM was positively related to radius BMD in obese women – the group that included higher percentage of postmenopausal women, with no relation in premenopausal women [Table 2]. Several other studies have suggested that excessive FM may not protect against decreases in bone mass.[6,20,37] Just recently, it was shown that adiposity is negatively associated with BMD in large group of men ranging from 25 to 98 years[38] and adolescent boys and girls.[39] Although FM or % fat were not significantly negatively related with BMD of total body, spine, femoral neck and other femoral sites in the whole population, the clear negative trend was present in our overweight/obese women [Figures 1–3] and inflection points were determined at 38%, 38%, and 33% of FM, respectively. The observed significantly positive relationship in overweight/obese women between FM and total body BMD, after inflection point of 40 kg, was probably due to the effects of high weight, since the effects of percentage of body fat were negative, after the inflection point of 38% [Figure 1b and c]. Based on the recent classification of obesity for men and women being at 30% and 40% of FM, respectively,[40] the negative relation of fat with BMD commences even before the obese state has been reached, according to the present data. We also observed a negative association between FM and femoral neck and total femur BMD in normal-weight women and spine BMD in overweight women but a positive association with radius in obese women, implying that excess body weight may not be beneficial at weight-bearing sites, but might be at nonweight bearing sites, such as the forearm.
The mechanism of the negative relationship between FM and BMD is multifaceted. Current understanding of the skeleton and bone marrow mesenchymal cell differentiation into adipocytes and osteoblasts does support a negative relationship between fat and bone mass. Adipocyte-derived hormones such as leptin and adiponectin may play roles in regulating BMD. Leptin has a dual role on bone and its signaling through sympathetic nervous system may adversely affect bone mass.[8,41] Plasma adiponectin levels are usually lower in obese and diabetic individuals.[42] In vivo and in vitro studies indicated that the adiponectin increases bone mass by suppressing the formation of osteoclast and by activating the formation of osteoblast.[23,43] Based on these studies, decreased adiponectin levels caused by higher amount of adipose tissue could have a negative effect on bone. Furthermore, the visceral adiposity-associated inflammatory markers and adipokines may exert a detrimental influence on BMD.[44,45] Elevated serum levels of interleukin-6 are usually observed in obese children and adults.[46,47] It has been reported that interleukin-6 stimulates osteoclastogenesis in cell culture system[48] and is considered as an osteoresorptive factor.[49] Elevated presence of each of these factors or their combination due to obesity may explain the negative effect of FM on bone, please see the in-depth review on the issue.[9] In the present study, adipocyte-derived hormones and inflammatory markers were not measured. Therefore, the association between FM and BMD could not be explained in terms of molecular functions of FM.
There are several limitations to the present study. The dual energy X-ray absorptiometry technology assesses lean tissue that was used as a proxy for the actual muscle mass and strength. Also, we used the amount and/or percentage of total body fat to calculate the inflection points. Abdominal fat might be even more detrimental for bone due to the secretion of various cytokines and their proinflammatory effects.[9,50] However, presenting the data with a total body fat might be more practical, as abdominal fat is not routinely analyzed in clinical settings, when bone measurements are conducted. Because we present data from Caucasian women, the results might not be applicable to individuals from other races/ethnicities or in men, however, the biological uniformity of our sample population makes the data stronger for the interpretation to this particular ethnic/age/gender group – Caucasian women, who are at the highest risk of developing osteoporosis. There were more postmenopausal women in the overweight and obese groups compared to the normal-weight women, possibly causing some skewed results. Since the blood was not drawn in all participants, it was not possible to assess endocrine factors that have been already identified to have an association with BMD and body composition. The physical activity was not assessed, but none of the women were engaged in any strenuous physical activity or regular exercise (as/exclusion criteria), therefore, we can assume it not to be a significant confounder in this population. Due to the cross-sectional nature, the associations presented between body composition and BMD do not necessarily represent causal relationships.
CONCLUSIONS
This study showed interactions of LM and FM with BMD in women stratified by BMI, as well as inflection points for the changed relationship between FM and BMD of several skeletal sites in overweight and obese women. Based on the linear regression analyses, LM had a positive association with BMD of all skeletal sites except with radius BMD in normal-weight and overweight groups and spine, femoral neck, and femur BMD in obese women. FM showed a negative association with BMD of femoral neck and femur in normal-weight women and spine in overweight women after adjusting for age and height. FM also showed a positive association with radius BMD in obese women but had no association with BMD of various skeletal sites in normal-weight and overweight women in multiple regression models. The separate analyses in overweight and obese women showed inflection points and the amount or percentage of FM at which it becomes unfavorable for bone. For most skeletal sites, the turning point was between 30 and 38% of body fat. The LM, however, remained significantly positively related to all skeletal sites [Figures 1a, 2a and 3a]. Similar relationships remained in the analyses of overweight/obese premenopausal women. It is now obvious that the amount/percentage of FM is responsible for the difference in the association of adiposity with BMD of various skeletal sites between different BMI groups, therefore overweight or obesity may not be considered a protective factor against osteoporosis in female population. For clinical practice, it is important to maintain LM and lower FM buildup (below ~30%) to improve/maintain bone health, particularly in postmenopausal women.
ACKNOWLEDGMENTS
This work was supported in part by CSREES/National Research Initiative/USDA, no. 2004-05287 (PI, JZI) and Hazel K. Stiebeling Professorship Award (JZI). The authors are in debt to all women who participated in the study.
Footnotes
Source of Support: Supported in part by CSREES/National Research Initiative/USDA, no. 2004-05287.
Conflict of Interest: None declared.
REFERENCES
- 1.Consensus development conference: Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–10. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 2.Pesonen J, Sirola J, Tuppurainen M, Jurvelin J, Alhava E, Honkanen R, et al. High bone mineral density among perimenopausal women. Osteoporos Int. 2005;16:1899–906. doi: 10.1007/s00198-005-1958-5. [DOI] [PubMed] [Google Scholar]
- 3.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–23. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J Bone Miner Res. 1993;8:567–73. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 5.Albala C, Yáñez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–32. [PubMed] [Google Scholar]
- 6.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–55. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 8.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 9.Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15:51–60. doi: 10.1016/j.arr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–82. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 11.Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women – A key role for fat mass. J Clin Endocrinol Metab. 1992;75:45–51. doi: 10.1210/jcem.75.1.1619030. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., 3rd Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–63. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: Which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12:144–51. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 14.Coin A, Sergi G, Benincà P, Lupoli L, Cinti G, Ferrara L, et al. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos Int. 2000;11:1043–50. doi: 10.1007/s001980070026. [DOI] [PubMed] [Google Scholar]
- 15.Ilich-Ernst J, Brownbill RA, Ludemann MA, Fu R. Critical factors for bone health in women across the age span: How important is muscle mass? Medscape Womens Health. 2002;7:2. [PubMed] [Google Scholar]
- 16.Lim S, Joung H, Shin CS, Lee HK, Kim KS, Shin EK, et al. Body composition changes with age have gender-specific impacts on bone mineral density. Bone. 2004;35:792–8. doi: 10.1016/j.bone.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Wagner R, Holm K, Lehotsky J, Zinaman MJ. Relationship between soft tissue body composition and bone mass in perimenopausal women. Maturitas. 2004;47:99–105. doi: 10.1016/s0378-5122(03)00249-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Hassager C, Ravn P, Wang S, Christiansen C. Total and regional body-composition changes in early postmenopausal women: Age-related or menopause-related? Am J Clin Nutr. 1994;60:843–8. doi: 10.1093/ajcn/60.6.843. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 21.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, et al. Correlation of obesity and osteoporosis: Effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl 7):S52–4. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownbill RA, Ilich JZ. Measuring body composition in overweight individuals by dual energy X-ray absorptiometry. BMC Med Imaging. 2005;5:1. doi: 10.1186/1471-2342-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson-Hughes B, Shipp C, Sadowski L, Dallal G. Bone density of the radius, spine, and hip in relation to percent of ideal body weight in postmenopausal women. Calcif Tissue Int. 1987;40:310–4. doi: 10.1007/BF02556691. [DOI] [PubMed] [Google Scholar]
- 27.Rico H, Arribas I, Casanova FJ, Duce AM, Hernández ER, Cortes-Prieto J. Bone mass, bone metabolism, gonadal status and body mass index. Osteoporos Int. 2002;13:379–87. doi: 10.1007/s001980200043. [DOI] [PubMed] [Google Scholar]
- 28.Cifuentes M, Johnson MA, Lewis RD, Heymsfield SB, Chowdhury HA, Modlesky CM, et al. Bone turnover and body weight relationships differ in normal-weight compared with heavier postmenopausal women. Osteoporos Int. 2003;14:116–22. doi: 10.1007/s00198-002-1324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukumar D, Schlussel Y, Riedt CS, Gordon C, Stahl T, Shapses SA. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int. 2011;22:635–45. doi: 10.1007/s00198-010-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. Femoral bone loss progresses with age: A longitudinal study in women over age 65. J Bone Miner Res. 1994;9:1959–65. doi: 10.1002/jbmr.5650091216. [DOI] [PubMed] [Google Scholar]
- 31.Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–95. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsatti FL, Nahas EA, Nahas-Neto J, Orsatti CL, Marocolo M, Barbosa-Neto O, et al. Low appendicular muscle mass is correlated with femoral neck bone mineral density loss in postmenopausal women. BMC Musculoskelet Disord. 2011;12:225. doi: 10.1186/1471-2474-12-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douchi T, Oki T, Nakamura S, Ijuin H, Yamamoto S, Nagata Y. The effect of body composition on bone density in pre- and postmenopausal women. Maturitas. 1997;27:55–60. doi: 10.1016/s0378-5122(97)01112-2. [DOI] [PubMed] [Google Scholar]
- 34.Ilich JZ, Brownbill RA. Habitual and low-impact activities are associated with better bone outcomes and lower body fat in older women. Calcif Tissue Int. 2008;83:260–71. doi: 10.1007/s00223-008-9171-0. [DOI] [PubMed] [Google Scholar]
- 35.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–76. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism. 1995;44:369–73. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Yu Z, Zhu Z, Tang T, Dai K, Qiu S. Effect of body fat stores on total and regional bone mineral density in perimenopausal Chinese women. J Bone Miner Metab. 2009;27:341–6. doi: 10.1007/s00774-009-0036-z. [DOI] [PubMed] [Google Scholar]
- 38.Pasco JA, Gould H, Brennan SL, Nicholson GC, Kotowicz MA. Musculoskeletal deterioration in men accompanies increases in body fat. Obesity (Silver Spring) 2014;22:863–7. doi: 10.1002/oby.20496. [DOI] [PubMed] [Google Scholar]
- 39.Mosca LN, Goldberg TB, da Silva VN, da Silva CC, Kurokawa CS, Bisi Rizzo AC, et al. Excess body fat negatively affects bone mass in adolescents. Nutrition. 2014;30:847–52. doi: 10.1016/j.nut.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The Framingham Study. J Gerontol A Biol Sci Med Sci. 2013;68:168–74. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motyl KJ, Rosen CJ. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie. 2012;94:2089–96. doi: 10.1016/j.biochi.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koroglu BK, Kiris F, Ersoy IH, Sutcu R, Yildiz M, Aksu O, et al. Relation of leptin, adiponectin and insulin resistance to bone mineral density in type 2 diabetic postmenopausal women. Endokrynol Pol. 2011;62:429–35. [PubMed] [Google Scholar]
- 43.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 44.Jürimäe J, Rembel K, Jürimäe T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res. 2005;37:297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- 45.Ozkurt B, Ozkurt ZN, Altay M, Aktekin CN, Caglayan O, Tabak Y. The relationship between serum adiponectin level and anthropometry, bone mass, osteoporotic fracture risk in postmenopausal women. Eklem Hastalik Cerrahisi. 2009;20:78–84. [PubMed] [Google Scholar]
- 46.Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 47.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–77. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. Intercellular adhesion molecule-1 on synovial cells attenuated interleukin-6-induced inhibition of osteoclastogenesis induced by receptor activator for nuclear factor kappaB ligand. Clin Exp Immunol. 2011;163:88–95. doi: 10.1111/j.1365-2249.2010.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franchimont N, Wertz S, Malaise M. Interleukin-6: An osteotropic factor influencing bone formation? Bone. 2005;37:601–6. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Ilich JZ, Kelly OJ, Kim Y, Spicer MT. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh Hig Rada Toksikol. 2014;65:139–48. doi: 10.2478/10004-1254-65-2014-2541. [DOI] [PubMed] [Google Scholar]


