Abstract
MicroRNAs (miRNAs) are a class of recently identified noncoding RNAs that regulate gene expression at posttranscriptional level. Due to the large number of genes regulated by miRNAs, miRNAs play important roles in many cellular processes. Emerging evidence indicates that miRNAs are dysregulated in pituitary adenomas, a class of intracranial neoplasms which account for 10–15% of diagnosed brain tumors. Deregulated miRNAs and their targets contribute to pituitary adenomas progression and are associated with cell cycle control, apoptosis, invasion, and pharmacological treatment of pituitary adenomas. To provide an overview of miRNAs dysregulation and functions of these miRNAs in pituitary adenoma progression, we summarize the deregulated miRNAs and their targets to shed more light on their potential as therapeutic targets and novel biomarkers.
1. MicroRNAs
MicroRNAs (miRNAs) are a class of recently identified noncoding RNAs. Mature miRNAs are short single-stranded RNA molecules, approximately 19–23 nucleotides in length. The miRNA sequence is encoded in a stem-loop structure in the primary transcript that is cleaved in the nucleus by the ribonuclease III enzyme Drosha to form the precursor miRNA (pre-miRNA). The pre-miRNA is subsequently exported to the cytoplasm by the exportin and then is cleaved by another ribonuclease III enzyme Dicer to form mature miRNA [1–3]. Mature miRNAs can regulate the expression of a large number of genes at the posttranscriptional level. miRNA is partially complementary to the sequence of miRNA recognition elements (MRE) in the 3′ untranslated regions (UTRs) of target mRNAs. The seed sequence with seven nucleotides in miRNA determines the specificity of mRNA targeting, whereas the remaining miRNA sequence is supposed to stabilize the miRNA-target complex [4]. miRNA can inhibit translation of target mRNAs by blocking protein translation machinery or by sequestering the mRNA transcript away from ribosomal interaction. miRNA can also induce target mRNA degradation in a similar way like RNA interference [1, 5]. miRNAs have been identified in a wide range of species, and computational analysis shows that nearly 30% of protein-coding genes can be modulated by miRNAs [6]. In general, miRNAs negatively regulate the expression of their targets. However, it is also reported that miR-369-3p can upregulate the expression of its target, tumor necrosis factor-α (TNF-α) [7].
miRNAs have been demonstrated to play important roles in many biological processes, such as cell cycle control, proliferation, apoptosis, differentiation, metabolism, hemopoiesis, and development [8]. A rapidly growing body of evidence shows that miRNAs also have comprehensive functions in tumor progression. Some miRNAs may function as oncogenes (also called oncomirs) while some miRNAs are supposed to be tumor suppressors [9]. The importance of miRNAs in cancer is highlighted by the fact that half of all miRNA genes are located in cancer-associated regions or fragile sites, which are frequently altered or deleted in cancer [10]. Many tumor types show unique miRNA signatures; thus, miRNAs may be of use in cancer diagnosis and prognosis [11, 12].
2. Pituitary Adenomas
Pituitary adenomas are usually benign intracranial neoplasms, accounting for 10–15% of diagnosed brain tumors [13]. Pituitary adenomas can be derived from a single mutant cell of five differentiated cell types within pituitary gland: somatotropes, lactotropes, corticotropes, thyrotropes, and gonadotropes, which, respectively, secrete growth hormone (GH), prolactin (PRL), adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH), and gonadotropins (follicle-stimulating hormone (FSH) and luteinizing hormone (LH)). According to the hormonal activity, pituitary adenomas can be defined as “functioning,” causing endocrine dysfunction such as Cushing's disease in ACTH-secreting pituitary adenomas, acromegaly in GH-secreting pituitary adenomas, galactorrhea and amenorrhea in PRL-secreting pituitary adenomas, and hyperthyroidism in TSH-secreting pituitary adenomas. On the other hand, nonfunctioning pituitary adenomas (NFA) do not give rise to hormone hypersecretion [14].
Pituitary adenomas might be small lesions with slow growth. However, some pituitary adenomas grow rapidly and cause tumor mass effect, the local compressive effect of large pituitary tumors on brain structures and cranial nerves. They can also invade downwards into the paranasal sinuses, laterally into the cavernous sinuses and upwards into the parenchyma of the brain. Occasionally, malignant pituitary carcinomas metastasize to distant locations in the central nervous system, lymph nodes, liver, and other sites throughout the body [13].
In recent years, some reports demonstrated that pituitary adenomas have altered expression files of miRNAs. Nevertheless, the correlation and function of miRNAs and their target genes in pathogenesis of pituitary adenomas remain largely unknown. Only a small number of miRNAs with their target genes in pituitary adenomas have been validated so far. In this review, we summarize recent advances in the study of miRNAs and their validated or potential targets in pituitary adenomas and discuss the future perspectives.
3. MicroRNAs in Pituitary Adenomas
3.1. Altered miRNA Expression in Pituitary Adenomas
Aberrant expressions of miRNAs have been demonstrated so far (Table 1). miR-15a and miR-16-1 are the first two miRNAs shown to have differential expression in pituitary adenomas. miR-15a and miR-16-1 genes are located at chromosome 13q14, a region which is frequently deleted in pituitary tumors [24]. Previous studies have suggested that the genes in this locus may be responsible for the progression of pituitary adenoma to a more aggressive form [25]. In 2005, miR-15a and miR-16-1 were reported to have lower expression in both GH-secreting and PRL-secreting pituitary adenomas than in normal tissues, and their downregulation was correlated with greater tumor volume and impaired secretion of p43, a potent anticancer cytokine, suggesting that miR-15a and miR-16-1 may function as tumor suppressors and their inactivation may contribute to tumor growth in pituitary adenomas [26]. In another study on ACTH-secreting pituitary tumors, miR-15a and miR-16 were also expressed at a lower level [27], but no association between miRNAs expression and tumor size was observed in this study. This is in accordance with the result of a subsequent report which showed no correlation between downregulation of miR-15a and GH-secreting pituitary tumor size [28]. Mutations in miR-16-1 gene have been reported to be partially responsible for its altered expression in chronic lymphocytic leukemia (CLL) patients [29]. Thus, it is worth exploring whether there are similar mutations in pituitary adenoma patients.
Table 1.
MicroRNAs and their target genes in human pituitary adenomas.
| miRNA | Upregulated or downregulated | Target genes | Tumor type | Reference |
|---|---|---|---|---|
| let-7 | Downregulated | HMGA2 | PRL, ACTH FSH/LH | [15] |
| miR-23b | Downregulated | HMGA2 | GH, NFA FSH/LH |
[16] |
| miR-26a | Upregulated | PRKCD | ACTH | [17] |
| miR-26b | Upregulated | PTEN | GH | [18] |
| miR-34b | Downregulated | HMGA1, HMGA2 | GH | [19] |
| miR-107 | Upregulated | AIP | GH, NFA | [20] |
| miR-128 | Downregulated | BMI1 | GH | [18] |
| miR-128a | Upregulated | Wee1 | NFA | [21] |
| miR-130b | Downregulated | CCNA2 | GH, NFA FSH/LH |
[16] |
| miR-140-5p | Upregulated | Smad3 | NFA | [22] |
| miR-155 | Upregulated | Wee1 | NFA | [21] |
| miR-200c | Upregulated | PTEN | PRL | [23] |
| miR-326 | Downregulated | HMGA2, E2F1 | GH | [19] |
| miR-432 | Downregulated | HMGA2 | GH | [19] |
| miR-516a-3p | Upregulated | Wee1 | NFA | [21] |
| miR-548c-3p | Downregulated | HMGA1, HMGA2 | GH | [19] |
| miR-570 | Downregulated | HMGA2 | GH | [19] |
| miR-603 | Downregulated | E2F1 | GH | [19] |
let-7 is one of the first members in the miRNA family. let-7 family members are located at chromosomal regions that are often altered or deleted in human tumors [10]. Downregulation of let-7 has been reported in breast, lung, colon, and others cancers [30–33] and let-7 is considered a tumor suppressor by targeting RAS oncogene [34]. Recently, some studies revealed that high-mobility group A2 (HMGA2) is negatively regulated by the let-7 miRNAs in vitro [35, 36]. HMGA2 plays diverse roles in many biological processes such as embryogenesis, differentiation, and neoplastic transformation [37]. Overexpression of HMGA2 is a hallmark of various tumors, including pituitary adenomas, and is associated with highly malignancy [38, 39]. The transgenic mice with overexpressed HMGA2 developed pituitary adenomas, indicating that HMGA2 may be involved in pituitary tumorigenesis [40]. In 2009, Qian et al. reported the clinical significance of HMGA2 overexpression in pituitary adenomas [15]. HMGA2 was frequently upregulated in pituitary adenomas including PRL, ACTH, FSH/LH, or null cell adenomas but relatively rare in GH and mixed GH/PRL adenomas. The authors also reported decreased expression of let-7 in pituitary adenomas. Intriguingly, an inverse correlation between HMGA2 and let-7 was confirmed in this study. HMGA2 overexpression and the decrease of let-7 were significantly correlated with tumor proliferation, growth, invasion, and tumor grade, which lead to a hypothesis that let-7 may also function as a tumor suppressor in pituitary adenomas by targeting HMGA2. Decreased expression of let-7a in pituitary adenomas was also reported in other studies [27, 41], suggesting the general downregulation of let-7 in pituitary adenomas. On the other hand, some other miRNAs such as miR-98 can also regulate HMGA2 expression [42], indicating that HMGA2 may have multiple miRNAs regulators. During pituitary development, let-7b/c was proposed to operate with the RNA-binding protein KSRP in a negative feedback loop, in which KSRP induces the maturation of let-7b/c, and let-7b/c posttranscriptionally downregulates the expression of KSRP itself [43].
As pituitary adenomas can be derived from differentiated cell types within pituitary gland, different subtypes of pituitary adenomas could display distinct miRNA profiles, and these specific profiles might be useful to distinguish pituitary adenoma subtypes. In 2007, a list of thirty miRNAs differentially expressed in pituitary adenomas was generated by microarray [41]. Seven miRNAs were upregulated and twenty-three were downregulated. The most representative ones were miR-212, miR-026a, miR-150, miR-152, miR-191, and miR-192, which were upregulated in pituitary adenomas, while miR-024-1 and miR-098 were downregulated in tumor samples. Twenty-nine miRNAs were identified to be able to predict pituitary adenoma histotype (ACTH-, GH-, PRL-secreting adenomas, and NFA). For the limit of sample numbers, the authors only analyzed the association of deregulated miRNAs and tumor diameter in the NFA group. Five miRNAs were upregulated (miR-140, miR-099a, miR-099b, miR-030b, and miR-030c) and only one (miR-138-2) was downregulated in macroadenomas compared to microadenomas.
In 2009, Amaral et al. investigated the differential expression of some miRNAs in ACTH-secreting pituitary tumors. In addition to the decrease of let-7a, miR-15a, and miR-16, they also found underexpression of miR-21, miR-141, miR-143, miR-145, and miR-150 in ACTH-secreting pituitary adenomas compared with normal pituitary tissues [27]. Among these miRNAs, downregulation of miR-141 has been reported in gastric cancer [44] and renal cell carcinoma [45]. miR-143 expression was decreased in human lung and colorectal cancers [46, 47] and was reported to inhibit KRAS translation in colorectal cancer cell [48]. miR-145 was downregulated in human breast, lung, and colorectal cancers [30, 46, 47, 49]. miR-145 could regulate the expression of various targets in different tumors: FSCN1 in esophageal squamous cell carcinoma [50], OCT4, EGFR, and NUDT1 in lung adenocarcinoma [51, 52], and FLI1 in colon cancer [53]. miR-143/145 cluster is a target of Jagged-1/Notch signaling in vascular smooth muscle cells [54]. miR-150 was overexpressed in hematopoietic progenitor/stem cells [55] and was demonstrated to target NOTCH3 in human T-cell development in a recent study [56].
Studies were conducted with the aim of investigating the aberrant expression of miRNAs in GH-secreting pituitary adenomas. In 2010, Mao et al. identified totally fifty-two miRNAs to be differentially expressed in GH-secreting pituitary adenomas. Nine of these miRNAs had altered expression between macro- and microadenomas. miR-184, miR-524-5p, miR-629, and miR-766 were upregulated, while miR-124, miR-222, miR-32, miR-744, and miR-765 were downregulated [28]. In 2012, another set of miRNAs were identified to be differentially expressed in GH-secreting pituitary adenomas [19]. Eighteen miRNAs, including miR-34b, miR-326, miR-432, miR-548c-3p, miR-570, and miR-603, were drastically and constantly downregulated in GH adenomas, whereas only miR-320 was significantly upregulated. miR-34b and miR-548c-3p were demonstrated to regulate both HMGA1 and HMGA2 expression, whereas miR-326, miR-432, and miR-570 target HMGA2 only. miR-326 and miR-603 could decrease the expression of the E2 transcription factor 1, E2F1. Besides, miR-107 was found to be overexpressed in GH-secreting and nonfunctioning pituitary adenomas and inhibited the expression of pituitary tumor suppressor gene aryl hydrocarbon receptor-interacting protein (AIP) [20]. Recently, Palumbo et al. identified 17 miRNAs which were differentially expressed in GH-secreting pituitary tumors. Specifically, five miRNAs (miR-26b, miR-26a, miR-212, miR-107, and miR-103) were upregulated and twelve miRNAs (miR-125b, miR-141, miR-144, miR-164, miR-145, miR-143, miR-15b, miR-16, miR-186, let-7b, let-7a3, and miR-128) were downregulated. miR-26b and miR-128 controlled pituitary cell properties through regulation of their direct targets, PTEN, and BMI1, respectively [18]. miR-26b also targeted Lef-1 and increased Pit-1 expression in GH3 cells [57].
miRNAs are also dysregulated in nonfunctioning pituitary adenomas (NFA). In 2011, Butz et al. analyzed miRNAs expression in NFA and the signaling pathways altered in these pituitary tumors [22]. Expressions of Smad3, Smad6, Smad9, MEG, and DLK1 were significantly decreased in NFA. Through pathway analysis and in silico target prediction, a specific subset of miRNAs was identified that may potentially downregulate TGF-β signaling pathway in NFA. Five miRNAs predicted to target Smad3 (miR-135a, miR-140-5p, miR-582-3p, miR-582-5p, and miR-938) were overexpressed, of which miR-140-5p has already been validated to target Smad3 directly [58]. In addition, an inverse correlation between tumor size and the expression of eighteen miRNAs was observed. Six miRNAs of them (miR-450b-5p, miR-424, miR-503, miR-542-3p, miR-629, and miR-214) were significantly underexpressed, while one miRNA (miR-592) was significantly overexpressed in NFA compared to normal pituitary tissues. In another study, miR-124a was the most upregulated miRNA, and miR-31 was the most downregulated miRNA in nonfunctioning pituitary adenomas [59].
In gonadotropin-secreting pituitary adenomas, a study demonstrated that miR-10b was upregulated and miR-503 was downregulated [59]. Furthermore, the integration and coordination of hormones and pituitary cells are important for the regulatory function of pituitary tissues. Gonadotropin-Releasing Hormone (GnRH) acts on pituitary gonadotropes to stimulate LH and FSH synthesis and secretion. GnRH induces expressions of miR-132 and miR-212 in LβT2 pituitary gonadotrope cells to regulate cellular morphology and migration. The p250RhoGAP protein is a downstream target of miR132/212 and its downregulation is involved in the morphological change and migration altered by GnRH [60].
3.2. Functional Association of miRNAs in Pituitary Tumorigenesis and Progression
3.2.1. miRNAs and Cell Cycle in Pituitary Adenomas
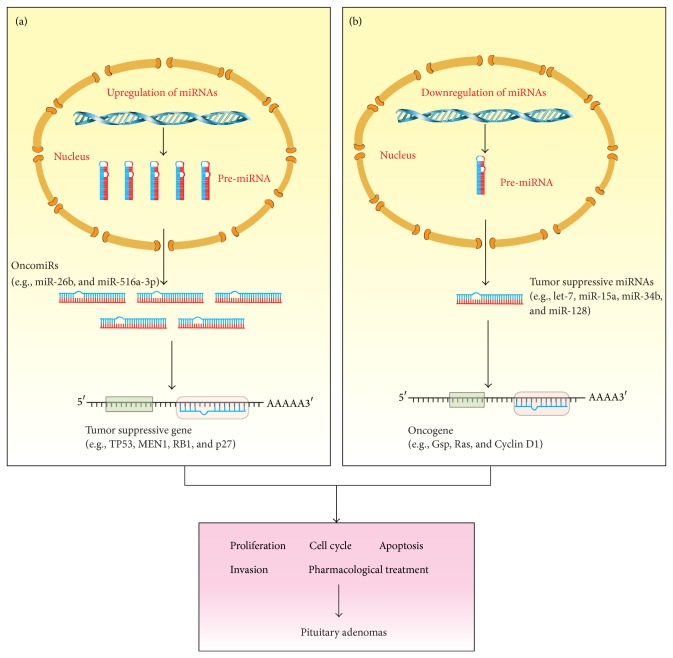
It is well known that the dysfunction of cell cycle control is a critical step in initiation and progression of human cancers. Some oncoproteins or tumor suppressors play important roles in cell cycle control by interacting with critical cell cycle regulators, such as cyclin, cyclin-dependent-kinase (CDK), or cell cycle inhibitors. During tumor progression, the genes involved in cell cycle control often have aberrant expression, resulting in unlimited tumor cell growth [61]. Some reports suggested that the deregulated miRNAs might also regulate cell cycle of pituitary adenomas at the post-transcriptional level (Figure 1).
Figure 1.
Functions of miRNAs as oncogenic and tumor suppressive genes in pituitary adenomas. (a) Upregulation of oncogenic miRNAs (oncomiRs) in pituitary adenomas results in suppression of their target tumor suppressor genes (e.g., TP53, MEN1, and RB1). (b) Downregulation of tumor suppressive miRNAs results in upregulation of their target oncogenes (e.g., Gsp, Ras, and Cyclin D1). The consequence of oncomiRs and tumor suppressive miRNAs regulation in pituitary adenomas might involve aberrant proliferation, cell cycle control, apoptosis, and invasiveness.
Wee1 was described as a tumor suppressor to inhibit cell cycle. Wee1 phosphorylates Cdk1 and inhibits its activity to block cell cycle in G2/M checkpoint [62]. Wee1 was downregulated in GH-secreting and NFA pituitary adenomas. miR-128a, miR-155, and miR-516a-3p target 3′-UTR of Wee1, and exogenous overexpression of these miRNAs inhibited Wee1 expression [21]. miR-128a is a brain-enriched miRNA and was reported to be decreased in pituitary adenomas [41]. Its ectopic overexpression reduced neuroblastoma cell motility and invasiveness [63], suggesting its tumor suppressive role. miR-155 was reported as an oncomir in both hemopoietic malignancies and solid tumors [64]. miR-516a-3p was involved in glioblastoma development [65] and was associated with progression of breast cancer [66]. These miRNAs may take part in the regulation of cell cycle in pituitary adenomas together with other related miRNAs.
HMGA2 is associated with the E1A-regulated transcriptional repressor p120 (E4F), interfering with p120 (E4F) binding to the cyclin A promoter. Ectopic expression of HMGA2 resulted in the activation of cyclin A promoter and induction of endogenous cyclin A expression. Moreover, chromatin immunoprecipitation experiments showed that HMGA2 was associated with cyclin A promoter only when the gene was transcriptionally activated. These data indicate cyclin A as a cellular target of HMGA2 and, for the first time, lead to a mechanism of HMGA2-dependent cell cycle regulation [67]. Thus, let-7, as a regulator of HMGA2, may exert its effects in cell cycle control of pituitary adenomas by targeting HMGA2. miR-23b and miR-130b, which were reduced in GH, gonadotroph, and NFPA adenomas, were demonstrated to target HMGA2 and cyclin A2, respectively. Overexpression of miR-23b and miR-130b arrested the cells in the G1 and G2 phase of the cell cycle [16].
Recently, a study revealed that miR-15a and miR-16-1 cluster could modulate prostate cancer by targeting multiple genes, including cyclin D1 [68]. Regarding the deregulation in pituitary adenomas, miR-15a and miR-16-1 may exert their roles as tumor suppressors by regulating cell cycle. Expression of miR-126 and miR-381 was decreased in GH-secreting pituitary adenomas [28]. Previous study has shown that mir-126 could modulate phosphatidylinositol 3-kinase (PI3K) signaling by limiting the PI3K regulatory subunit beta (p85b). Loss of miR-126 would eliminate the check point and increase PI3K signaling, which facilitate tumor growth during colon carcinogenesis [69]. miR-145 was downregulated in GH-secreting pituitary adenomas [28], which is in line with the results in 11 samples of cortitropinomas [27]. The potential targets of miR-145 include myc, kras, fos, yes, fli, cyclin D2, and MAPK transduction proteins [30], indicating that miR-145 might function in cell cycle control by targeting multiple genes. miR-503 was highly expressed in NFA and had a negative correlation with tumor size [22]. miR-503 has been validated to directly target cyclin D1 and is thought to be a tumor suppressor [70]. Furthermore, an important potential target of miR-503 is the cell cycle regulator CDC25.
miR-26b and miR-128 were found to directly regulate PTEN and BMI1, respectively. Moreover, miR-128 regulated PTEN expression and Akt activity in the pituitary tumor cells by interfering with the binding of BMI1 to PTEN promoter [18]. Since PTEN-Akt pathway plays important roles in cell cycle control, miR-26b and miR-128 might regulate cell cycle through PTEN-Akt pathway [71]. Moreover, miR-26a was also overexpressed in ACTH-secreting pituitary adenomas and plays an important role in cell cycle control by modulating protein kinase C delta [17].
3.2.2. miRNAs and Apoptosis in Pituitary Adenomas
Apoptosis, the process of programmed cell death, is an important barrier for tumor cells. During malignant transformation and tumor progression, tumor cells have to escape this regulated cell death to obtain an advantage in growth and expansion. At the early stage of apoptosis, cells receive death signals, and then the “apoptotic trigger” is controlled by pro- or antiapoptotic members of B-cell lymphoma 2 (Bcl-2) family and other regulatory proteins [72]. Accumulating evidence have shown that miRNAs can regulate cancer cell apoptosis by targeting Bcl-2 family or other apoptosis regulators (Figure 1).
miR-15a and miR-16-1 were demonstrated to induce apoptosis by targeting Bcl-2 in CLL [73]. Bcl-2 is a founding member of the Bcl-2 family, a family of antiapoptotic proteins governing mitochondrial death signaling. Bcl-2 is frequently overexpressed in many types of human cancers, including carcinomas, lymphomas, and leukemias [74]. In CLL, some other apoptosis related genes were identified to be targets of miR-15a and miR-16-1 cluster, such as MCL1, which could enhance cell survival by inhibiting apoptosis. Therefore, it is possible that, in pituitary adenomas, miR-15a and miR-16-1 influence apoptosis by targeting multiple antiapoptotic genes. Besides, miR-214 and miR-629, two miRNAs overexpressed in NFA and negatively correlated with tumor size, also potentially target Bcl2 [22].
miR-21 was differentially expressed in ACTH-secreting pituitary adenomas compared with normal pituitary tissues [27]. miR-21 has been identified to be upregulated in human breast, lung, colorectal and other cancers [30, 46, 49, 75]. Suppression of miR-21 by antisense oligonucleotides or miR-21 knockdown was associated with increased apoptotic activity and inhibition of tumor cell growth, probably by downregulating the target tumor suppressor genes [76]. miR-21 may exert its function in apoptosis by targeting tumor suppressor Pdcd4 [77] and PTEN [78]. Overexpression of PDCD4 was able to result in apoptotic death [79], and PTEN can induce apoptosis through phosphoinositol-3-kinase/Akt dependent and independent pathways [80]. miR-21 is upregulated both in vitro and in vivo by oncogenic Ras [81].
miR-212 is strongly upregulated in pituitary adenomas [41]. Putative targets of miR-212 include death effector domain-containing protein (DEDD), a protein involved in apoptotic signaling [82], as well as other proteins participating in apoptosis. miR184 was markedly upregulated in GH-secreting pituitary adenomas and was correlated with tumor diameter [28]. Contrary to that, another study reported that ectopic overexpression of miR-184 resulted in increased apoptosis [83]. Study of Cheng et al. suggested that the upregulated miR-150, miR-152, miR-191, and miR-192 may also be involved in apoptosis [84].
miR-26b was found to be upregulated in GH-secreting pituitary tumors and directly regulate PTEN. Therefore, miR-26b is able to regulate apoptosis through PTEN-Akt pathway. miR-200c, which has been characterized as a tumor suppressor or oncogene in different cancers, also inhibited apoptosis in pituitary adenoma cells by targeting the PTEN/Akt signaling pathway [23]. Intriguingly, a novel marine drug, SZ-685C that was isolated from the secondary metabolites of a mangrove endophytic fungus was reported to induce apoptosis of MMQ pituitary tumor cells by downregulating miR-200c [85].
TGF-β has been shown to inhibit proliferation and induce apoptosis in HP75 cells, a cell line derived from a clinically NFA [86]. Thereby, the miRNAs targeting TGF-β signaling (miR-135a, miR-140-5p, miR-582-3p, miR-582-5p, and miR-938) may have effects in apoptosis [22]. However, as TGF-β can also promote cancer cell invasion by inducing Epithelial-Mesenchymal Transition (EMT) [87], it is rational to conclude that miRNAs targeting TGF-β pathway may suppress invasion and metastasis by blocking EMT, as miR-300 does in human epithelial cancer [88]. Therefore, miRNAs that regulate TGF-β pathway play controversial roles in tumor initiation and progression. Deregulation of BMI1 has been revealed to affect apoptosis; thus, miR-128, which was downregulated in GH-secreting pituitary tumors, could also affect apoptosis by directly regulating BMI1 [18]. These data together lead to the hypothesis that many miRNAs may function in a network to regulate apoptosis in pituitary adenomas.
3.2.3. miRNAs and Invasion or Metastasis of Pituitary Adenomas
Invasion and metastasis are critical lethal factors for malignant cancer. Although invasion and metastasis are rare in pituitary tumors, studies provide some clues of miRNAs' function in pituitary tumor invasion and metastasis (Figure 1).
Significant correlation between HMGA2 overexpression and tumor cell invasion has been detected in breast cancer and gastric cancer [89, 90]. In oral squamous cell carcinomas, strong staining of HMGA2 and loss of E-cadherin expression were observed at the invasive front of tumor [91]. Previous studies also demonstrated that tumor-specific downregulation of E-cadherin and H-cadherin was related to invasiveness of pituitary adenoma [92]. HMGA2 may be involved in tumor cell invasion due to its association with epithelial-mesenchymal transition that facilitates tumor cell invasion. Since let-7 regulates HMGA2 expression in pituitary adenomas, let-7 may also take a role in pituitary adenoma invasion. In Amaral et al.'s study, although no association between miRNAs expression and tumor size was observed, the patients with ACTH-secreting pituitary tumors expressing reduced miR-141 had more chance of remission after transsphenoidal surgery, suggesting that miR-141 may regulate pituitary genes involved in tumor growth and local invasion [27]. PTTG protein 1 is a target of both miR-126 and miR-381, which were downregulated in GH-secreting pituitary adenomas [28]. PTTG is overexpressed in most pituitary adenomas and is involved in tumor invasion [93]. Therefore, miR-126 and miR-381 might regulate pituitary adenoma invasion by targeting PTTG.
Aggressive pituitary adenomas and carcinomas frequently have a deletion in regions near the RB gene [94, 95]. In 2010, Stilling et al. investigated the expression of miRNAs in pituitary carcinomas [96]. In one case, ACTH carcinoma had metastatic carcinomas in three sites. More miRNAs were deregulated between pituitary adenomas and normal pituitaries compared to carcinomas and normal pituitaries. In pituitary carcinomas compared to ACTH adenomas, miR-122 and miR-493 were upregulated, and, in all three metastatic sites of ACTH carcinomas, miR-122 expression was markedly increased.
Recently, Palumbo et al. identified miR-26b to be upregulated and miR-128 to be downregulated in GH-secreting pituitary tumors [18]. Inhibition of miR-26b and overexpression of miR-128 suppressed colony formation and invasiveness of pituitary tumor cells. Interestingly, the inhibition of miR-26b and overexpression of miR-128 had a synergistic effect on suppressing the tumorigenicity and invasiveness of pituitary tumors. Since deregulation of PTEN and BMI1 correlates with the invasive and metastatic phenotype of several human cancer types [97, 98], it is possible that miR-26b and miR-128 regulate invasiveness of pituitary tumor cells by directly targeting PTEN and BMI1, respectively. Although metastatic pituitary carcinomas are rare, these data suggest that altered expression of miRNAs may provide diagnostic information to distinguish pituitary adenomas and carcinomas before they metastasize.
3.2.4. miRNAs and Pharmacological Treatment of Pituitary Adenomas
The symptoms of mass effect and hormonal hypersecretion caused by pituitary adenomas could be reversed by surgical resection or debulking of the adenoma, radiotherapy, or medical treatment. Medical treatment is the primary choice for prolactinomas and the secondary option for acromegaly, Cushing's disease, gonadotropin-secreting tumours, and TSH-secreting adenomas [99]. Some studies provide evidence that miRNAs were differentially expressed before and after pharmacological treatment, and the altered miRNA profile could provide useful information of responsiveness of pituitary adenomas patients to pharmacological treatment (Figure 1).
In 2007, a microarray was carried out to analyze the miRNA profiles in pituitary adenomas and normal pituitary samples. To elucidate whether miRNAs profile is altered by pharmacological treatment, differentially expressed miRNAs were identified in NFA from patients with pharmacological treatment or patients without treatment [41]. Six miRNAs were found to be differentially expressed: miR-29b, miR-29c, and miR-200a were upregulated, while miR-134, miR-148, and miR-155 were downregulated after treatment. Cluster analysis showed clear distinction between pharmacological treated and nontreated NFA. Thus, the miRNA expression could differentiate treated patient samples from nontreated patient samples.
In 2010, another study aimed to identify altered expression of miRNAs in GH-secreting pituitary adenomas [28]. Fifteen pituitary adenomas patients were treated with lanreotide for four months before surgery, while six patients did not receive any presurgical medical treatments. Patients with >50% reduction of GH secretion after lanreotide treatment were considered somatostatin analogs (SSA) responders, while patients with <50% GH secretion were considered SSA nonresponders [100]. Thirteen miRNAs were differentially expressed between GH-secreting pituitary adenomas from patients with lanreotide treatment and those without treatment. Eight miRNAs (miR-183, miR-193a-5p, miR-222, miR-516b, miR-524-5p, miR-601, and miR-629, 99b) were upregulated and five miRNAs (miR-124, miR-32, miR-574-5p, miR-744, and miR-96) were downregulated. Moreover, seven miRNAs were differentially expressed between SSA responders and SSA nonresponders. Putative targets of these miRNAs are mainly IGFBP family members, IGFALS, SCP1, and matrix metalloproteinase-9.
4. Conclusion and Future Perspectives
Accumulating evidence demonstrates that a large number of miRNAs have altered expression in pituitary adenomas, and these miRNAs may play important roles in tumor progression by targeting multiple genes. The molecular mechanism of the regulation of miRNAs in pituitary adenomas is still a mystery. Some proofs indicate that genetic or epigenetic alterations may contribute to the deregulated expression of miRNAs. For example, mutations in the miR-16-1 gene have been reported to be partially responsible for its aberrant expression in CLL patients [29], and expressions of miR-124 and miR-203 are decreased because of CpG methylation [101]. Some miRNAs have been demonstrated to target multiple genes, indicating that they may have different roles in pituitary tumors. On the other hand, a gene involved in pituitary adenomas progression can be modulated by more than one miRNA. Therefore, the miRNAs and their targets could regulate pituitary adenomas progression in a complex network.
Advances in the technology to investigate miRNAs make it easier and faster to explore more exactly the roles of miRNAs in pituitary adenomas. As some miRNAs signatures can be used to distinguish pituitary adenomas and normal pituitaries and even subtypes of pituitary tumors, it is also possible to develop miRNA based diagnosis and therapies of pituitary adenomas. The knowledge of pituitary pathogenesis is still limited. Continuing study on miRNAs and their targets will shed more light on mechanisms of pituitary adenomas.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (no. LY14C060002); the fund from the Science and Technology Bureau of Jiaxing (no. 2014AY21022); the Open Fund of Zhejiang Provincial Top Key Discipline of Biology; the fund from Science and Technology of Xiaoshan District (no. 2012121); and the China Natural Science Foundation (no. 31170732 and no. 31270854).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors’ Contribution
Xu-Hui Li and Zhi Rong Qian contributed equally to this work.
References
- 1.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y., Jeon K., Lee J.-T., Kim S., Kim V. N. MicroRNA maturation: stepwise processing and subcellular localization. The EMBO Journal. 2002;21(17):4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi R., Qin Y., Macara I. G., Cullen B. R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes and Development. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis B. P., Burge C. B., Bartel D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Bartel B. MicroRNAs directing siRNA biogenesis. Nature Structural and Molecular Biology. 2005;12(7):569–571. doi: 10.1038/nsmb0705-569. [DOI] [PubMed] [Google Scholar]
- 6.Filipowicz W., Bhattacharyya S. N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Reviews Genetics. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 7.Vasudevan S., Tong Y., Steitz J. A. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 9.Gartel A. L., Kandel E. S. miRNAs: little known mediators of oncogenesis. Seminars in Cancer Biology. 2008;18(2):103–110. doi: 10.1016/j.semcancer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Calin G. A., Sevignani C., Dumitru C. D., et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson K. M., Weiss G. J. MicroRNAs and cancer: past, present, and potential future. Molecular Cancer Therapeutics. 2008;7(12):3655–3660. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J.-X., Song W., Chen Z.-H., et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. The Lancet Oncology. 2013;14(13):1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 13.Asa S. L., Ezzat S. The pathogenesis of pituitary tumours. Nature Reviews Cancer. 2002;2(11):836–849. doi: 10.1038/nrc926. [DOI] [PubMed] [Google Scholar]
- 14.Ezzat S., Asa S. L. Mechanisms of disease: the pathogenesis of pituitary tumors. Nature Clinical Practice Endocrinology & Metabolism. 2006;2(4):220–230. doi: 10.1038/ncpendmet0159. [DOI] [PubMed] [Google Scholar]
- 15.Qian Z. R., Asa S. L., Siomi H., Siomi M. C., Yoshimoto K., Yamada S., Wang E. L., Rahman M. M., Inoue H., Itakura M., Kudo E., Sano T. Overexpression of HMGA2 relates to reduction of the let-7 and its relationship to clinicopathological features in pituitary adenomas. Modern Pathology. 2009;22(3):431–441. doi: 10.1038/modpathol.2008.202. [DOI] [PubMed] [Google Scholar]
- 16.Leone V., Langella C., D'Angelo D., Mussnich P., Wierinckx A., Terracciano L., Raverot G., Lachuer J., Rotondi S., Jaffrain-Rea M.-L., Trouillas J., Fusco A. MiR-23b and miR-130b expression is downregulated in pituitary adenomas. Molecular and Cellular Endocrinology. 2014;390(1-2):1–7. doi: 10.1016/j.mce.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Gentilin E., Tagliati F., Filieri C., Molé D., Minoia M., Ambrosio M. R., Uberti E. C. D., Zatelli M. C. MiR-26a plays an important role in cell cycle regulation in ACTH-secreting pituitary adenomas by modulating protein kinase Cdelta. Endocrinology. 2013;154(5):1690–1700. doi: 10.1210/en.2012-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palumbo T., Faucz F. R., Azevedo M., Xekouki P., Iliopoulos D., Stratakis C. A. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway. Oncogene. 2013;32(13):1651–1659. doi: 10.1038/onc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Angelo D., Palmieri D., Mussnich P., et al. Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. Journal of Clinical Endocrinology and Metabolism. 2012;97(7):E1128–E1138. doi: 10.1210/jc.2011-3482. [DOI] [PubMed] [Google Scholar]
- 20.Trivellin G., Butz H., Delhove J., Igreja S., Chahal H. S., Zivkovic V., McKay T., Patócs A., Grossman A. B., Korbonits M. MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. The American Journal of Physiology—Endocrinology and Metabolism. 2012;303(6):E708–E719. doi: 10.1152/ajpendo.00546.2011. [DOI] [PubMed] [Google Scholar]
- 21.Butz H., Likó I., Czirják S., et al. Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. The Journal of Clinical Endocrinology & Metabolism. 2010;95(10):E181–E191. doi: 10.1210/jc.2010-0581. [DOI] [PubMed] [Google Scholar]
- 22.Butz H., Likó I., Czirják S., Igaz P., Korbonits M., Rácz K., Patócs A. MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary. 2011;14(2):112–124. doi: 10.1007/s11102-010-0268-x. [DOI] [PubMed] [Google Scholar]
- 23.Liao C., Chen W., Fan X., Jiang X., Qiu L., Chen C., Zhu Y., Wang H. MicroRNA-200c inhibits apoptosis in pituitary adenoma cells by targeting the PTEN/Akt signaling pathway. Oncology Research. 2014;21:129–136. doi: 10.3727/096504013X13832473329999. [DOI] [PubMed] [Google Scholar]
- 24.Calin G. A., Dumitru C. D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., Rassenti L., Kipps T., Negrini M., Bullrich F., Croce C. M. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei L., Melmed S., Scheithauer B., Kovacs K., Benedict W. F., Prager D. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary tumors: evidence for a chromosome 13 tumor suppressor gene other than RB. Cancer Research. 1995;55(8):1613–1616. [PubMed] [Google Scholar]
- 26.Bottoni A., Piccin D., Tagliati F., Luchin A., Zatelli M. C., Uberti E. C. D. miR-15a and miR-16-1 down-regulation in pituitary adenomas. Journal of Cellular Physiology. 2005;204(1):280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 27.Amaral F. C., Torres N., Saggioro F., Neder L., Machado H. R., Silva W. A., Jr., Moreira A. C., Castro M. MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. Journal of Clinical Endocrinology and Metabolism. 2009;94(1):320–323. doi: 10.1210/jc.2008-1451. [DOI] [PubMed] [Google Scholar]
- 28.Mao Z.-G., He D.-S., Zhou J., Yao B., Xiao W.-W., Chen C.-H., Zhu Y.-H., Wang H.-J. Differential expression of microRNAs in GH-secreting pituitary adenomas. Diagnostic Pathology. 2010;5(1, article 79) doi: 10.1186/1746-1596-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calin G. A., Ferracin M., Cimmino A., et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. The New England Journal of Medicine. 2005;353(17):1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 30.Iorio M. V., Ferracin M., Liu C.-G., Veronese A., Spizzo R., Sabbioni S., Magri E., Pedriali M., Fabbri M., Campiglio M., Ménard S., Palazzo J. P., Rosenberg A., Musiani P., Volinia S., Nenci I., Calin G. A., Querzoli P., Negrini M., Croce C. M. MicroRNA gene expression deregulation in human breast cancer. Cancer Research. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 31.Takamizawa J., Konishi H., Yanagisawa K., Tomida S., Osada H., Endoh H., Harano T., Yatabe Y., Nagino M., Nimura Y., Mitsudomi T., Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Research. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 32.Akao Y., Nakagawa Y., Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biological & Pharmaceutical Bulletin. 2006;29(5):903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 33.Shell S., Park S.-M., Radjabi A. R., Schickel R., Kistner E. O., Jewell D. A., Feig C., Lengyel E., Peter M. E. Let-7 expression defines two differentiation stages of cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Mayr C., Hemann M. T., Bartel D. P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315(5818):1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y. S., Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes & Development. 2007;21(9):1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sgarra R., Rustighi A., Tessari M. A., et al. Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Letters. 2004;574(1–3):1–8. doi: 10.1016/j.febslet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Fusco A., Fedele M. Roles of HMGA proteins in cancer. Nature Reviews Cancer. 2007;7(12):899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- 39.Finelli P., Pierantoni G. M., Giardino D., Losa M., Rodeschini O., Fedele M., Valtorta E., Mortini P., Croce C. M., Larizza L., Fusco A. The High Mobility Group A2 gene is amplified and overexpressed in human prolactinomas. Cancer Research. 2002;62(8):2398–2405. [PubMed] [Google Scholar]
- 40.Fedele M., Battista S., Kenyon L., Baldassarre G., Fidanza V., Klein-Szanto A. J. P., Parlow A. F., Visone R., Pierantoni G. M., Outwater E., Santoro M., Croce C. M., Fusco A. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21(20):3190–3198. doi: 10.1038/sj/onc/1205428. [DOI] [PubMed] [Google Scholar]
- 41.Bottoni A., Zatelli M. C., Ferrcin M., Tagliati F., Piccin D., Vignali C., Calin G. A., Negrini M., Croce C. M., Degli Uberti E. C. Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas. Journal of Cellular Physiology. 2007;210(2):370–377. doi: 10.1002/jcp.20832. [DOI] [PubMed] [Google Scholar]
- 42.Hebert C., Norris K., Scheper M. A., Nikitakis N., Sauk J. J. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Molecular Cancer. 2007;6, article 5 doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Repetto E., Briata P., Kuziner N., Harfe B. D., McManus M. T., Gherzi R., Rosenfeld M. G., Trabucchi M. Let-7b/c enhance the stability of a tissue-specific mRNA during mammalian organogenesis as part of a feedback loop involving KSRP. PLoS Genetics. 2012;8(7) doi: 10.1371/journal.pgen.1002823.e1002823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Y., Xu Y., Ding L., Yao H., Yu H., Zhou T., Si J. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. Journal of Gastroenterology. 2009;44(6):556–561. doi: 10.1007/s00535-009-0037-7. [DOI] [PubMed] [Google Scholar]
- 45.Nakada C., Matsuura K., Tsukamoto Y., et al. Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c. The Journal of Pathology. 2008;216(4):418–427. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 46.Yanaihara N., Caplen N., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R. M., Okamoto A., Yokota J., Tanaka T., Calin G. A., Liu C.-G., Croce C. M., Harris C. C. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Michael M. Z., O'Connor S. M., van Holst Pellekaan N. G., Young G. P., James R. J. Reduced accumulation of specific microRNAs in colorectal neoplasia. Molecular Cancer Research. 2003;1(12):882–891. [PubMed] [Google Scholar]
- 48.Chen X., Guo X., Zhang H., et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28(10):1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 49.Bandrés E., Cubedo E., Agirre X., Malumbres R., Zárate R., Ramirez N., Abajo A., Navarro A., Moreno I., Monzó M., García-Foncillas J. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Molecular Cancer. 2006;5, article 29 doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kano M., Seki N., Kikkawa N., et al. miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. International Journal of Cancer. 2010;127(12):2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S., Wu Y., Feng D., Zhang Z., Jiang F., Yin R., Xu L. miR-145 inhibits lung adenocarcinoma stem cells proliferation by targeting OCT4 gene. Zhongguo Fei Ai Za Zhi. 2011;14(4):317–322. doi: 10.3779/j.issn.1009-3419.2011.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho W. C. S., Chow A. S. C., Au J. S. K. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biology. 2011;8(1):125–131. doi: 10.4161/rna.8.1.14259. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Guo H., Zhang H., Wang H., Qian G., Fan X., Hoffman A. R., Hu J.-F., Ge S. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene friend leukemia virus integration 1 gene. Cancer. 2011;117(1):86–95. doi: 10.1002/cncr.25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boucher J. M., Peterson S. M., Urs S., Zhang C., Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. The Journal of Biological Chemistry. 2011;286(32):28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou B., Wang S., Mayr C., Bartel D. P., Lodish H. F. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(17):7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghisi M., Corradin A., Basso K., et al. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood. 2011;117(26):7053–7062. doi: 10.1182/blood-2010-12-326629. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z., Florez S., Gutierrez-Hartmann A., Martin J. F., Amendt B. A. MicroRNAs regulate pituitary development, and microRNA 26b specifically targets lymphoid enhancer factor 1 (Lef-1), which modulates pituitary transcription factor 1 (Pit-1) expression. The Journal of Biological Chemistry. 2010;285(45):34718–34728. doi: 10.1074/jbc.M110.126441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pais H., Nicolas F. E., Soond S. M., et al. Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. RNA. 2010;16(3):489–494. doi: 10.1261/rna.1701210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang S., Chen L., Huang H., Zhi D. The experimental study of miRNA in pituitary adenomas. Turkish Neurosurgery. 2013;23(6):721–727. doi: 10.5137/1019-5149.JTN.7425-12.1. [DOI] [PubMed] [Google Scholar]
- 60.Godoy J., Nishimura M., Webster N. J. G. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized L2 T2 pituitary gonadotrope cells. Molecular Endocrinology. 2011;25(5):810–820. doi: 10.1210/me.2010-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermeulen K., Van Bockstaele D. R., Berneman Z. N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Proliferation. 2003;36(3):131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGowan C. H., Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. The EMBO Journal. 1993;12(1):75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evangelisti C., Florian M. C., Massimi I., Dominici C., Giannini G., Galardi S., Buè M. C., Massalini S., McDowell H. P., Messi E., Gulino A., Farace M. G., Ciafrè S. A. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB Journal. 2009;23(12):4276–4287. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 64.Faraoni I., Antonetti F. R., Cardone J., Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochimica et Biophysica Acta: Molecular Basis of Disease. 2009;1792(6):497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Godlewski J., Nowicki M. O., Bronisz A., Williams S., Otsuki A., Nuovo G., RayChaudhury A., Newton H. B., Chiocca E. A., Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by MicroRNA-128 inhibits glioma proliferation and self-renewal. Cancer Research. 2008;68(22):9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 66.Foekens J. A., Sieuwerts A. M., Smid M., Look M. P., De Weerd V., Boersma A. W. M., Klijn J. G. M., Wiemer E. A. C., Martens J. W. M. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tessari M. A., Gostissa M., Altamura S., Sgarra R., Rustighi A., Salvagno C., Caretti G., Imbriano C., Mantovani R., Del Sal G., Giancotti V., Manfioletti G. Transcriptional activation of the cyclin A gene by the architectural transcription factor HMGA2. Molecular and Cellular Biology. 2003;23(24):9104–9116. doi: 10.1128/MCB.23.24.9104-9116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonci D., Coppola V., Musumeci M., Addario A., Giuffrida R., Memeo L., D'Urso L., Pagliuca A., Biffoni M., Labbaye C., Bartucci M., Muto G., Peschle C., De Maria R. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature Medicine. 2008;14(11):1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 69.Guo C., Sah J. F., Beard L., Willson J. K. V., Markowitz S. D., Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes and Cancer. 2008;47(11):939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang Q., Feng M.-G., Mo Y.-Y. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer. 2009;9, article 194 doi: 10.1186/1471-2407-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun H., Lesche R., Li D.-M., Liliental J., Zhang H., Gao J., Gavrilova N., Mueller B., Liu X., Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(11):6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams J. M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cimmino A., Calin G. A., Fabbri M., Iorio M. V., Ferracin M., Shimizu M., Wojcik S. E., Aqeilan R. I., Zupo S., Dono M., Rassenti L., Alder H., Volinia S., Liu C.-G., Kipps T. J., Negrini M., Croce C. M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sánchez-Beato M., Sánchez-Aguilera A., Piris M. A. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101(4):1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 75.Volinia S., Calin G. A., Liu C.-G., et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corsten M. F., Miranda R., Kasmieh R., Krichevsky A. M., Weissleder R., Shah K. MicroRNA-21 knockdown disrupts glioma growth In vivo and displays synergistic cytotoxicity with neural precursor cell-delivered S-TRAIL in human gliomas. Cancer Research. 2007;67(19):8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 77.Asangani I. A., Rasheed S. A. K., Nikolova D. A., et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 78.Bai H., Xu R., Cao Z., Wei D., Wang C. Involvement of miR-21 in resistance to daunorubicin by regulating PTEN expression in the leukaemia K562 cell line. FEBS Letters. 2011;585(2):402–408. doi: 10.1016/j.febslet.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 79.Afonja O., Juste D., Das S., Matsuhashi S., Samuels H. H. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene. 2004;23(49):8135–8145. doi: 10.1038/sj.onc.1207983. [DOI] [PubMed] [Google Scholar]
- 80.Weng L.-P., Brown J. L., Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Human Molecular Genetics. 2001;10(3):237–242. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- 81.Frezzetti D., de Menna M., Zoppoli P., et al. Upregulation of miR-21 by Ras in vivo and its role in tumor growth. Oncogene. 2011;30(3):275–286. doi: 10.1038/onc.2010.416. [DOI] [PubMed] [Google Scholar]
- 82.Stegh A. H., Schickling O., Ehret A., Scaffidi C., Peterhänsel C., Hofmann T. G., Grummt I., Krammer P. H., Peter M. E. DEDD, a novel death effector domain-containing protein, targeted to the nucleolus. The EMBO Journal. 1998;17(20):5974–5986. doi: 10.1093/emboj/17.20.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J., Ryan D. G., Getsios S., Oliveira-Fernandes M., Fatima A., Lavker R. M. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19300–19305. doi: 10.1073/pnas.0803992105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng A. M., Byrom M. W., Shelton J., Ford L. P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Research. 2005;33(4):1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen C.-H., Xiao W.-W., Jiang X.-B., Wang J.-W., Mao Z.-G., Lei N., Fan X., Song B.-B., Liao C.-X., Wang H.-J., She Z.-G., Zhu Y.-H. A novel marine drug, sz-685c, induces apoptosis of mmq pituitary tumor cells by downregulating mir-200c. Current Medicinal Chemistry. 2013;20(16):2145–2154. doi: 10.2174/0929867311320160007. [DOI] [PubMed] [Google Scholar]
- 86.Kulig E., Jin L., Qian X., Horvath E., Kovacs K., Stefaneanu L., Scheithauer B. W., Lloyd R. V. Apoptosis in nontumorous and neoplastic human pituitaries: expression of the Bcl-2 family of proteins. The American Journal of Pathology. 1999;154(3):767–774. doi: 10.1016/S0002-9440(10)65323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kasai H., Allen J. T., Mason R. M., Kamimura T., Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respiratory Research. 2005;6, article 56 doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu J., Xie F., Bao X., Chen W., Xu Q. miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Molecular Cancer. 2014;13, article 121 doi: 10.1186/1476-4598-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fabjani G., Tong D., Wolf A., Roka S., Leodolter S., Hoecker P., Fischer M. B., Jakesz R., Zeillinger R. HMGA2 is associated with invasiveness but not a suitable marker for the detection of circulating tumor cells in breast cancer. Oncology Reports. 2005;14(3):737–741. [PubMed] [Google Scholar]
- 90.Motoyama K., Inoue H., Nakamura Y., Uetake H., Sugihara K., Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clinical Cancer Research. 2008;14(8):2334–2340. doi: 10.1158/1078-0432.CCR-07-4667. [DOI] [PubMed] [Google Scholar]
- 91.Miyazawa J., Mitoro A., Kawashiri S., Chada K. K., Imai K. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Research. 2004;64(6):2024–2029. doi: 10.1158/0008-5472.CAN-03-1855. [DOI] [PubMed] [Google Scholar]
- 92.Qian Z. R., Sano T., Yoshimoto K., Asa S. L., Yamada S., Mizusawa N., Kudo E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Modern Pathology. 2007;20(12):1269–1277. doi: 10.1038/modpathol.3800965. [DOI] [PubMed] [Google Scholar]
- 93.Salehi F., Kovacs K., Scheithauer B. W., Lloyd R. V., Cusimano M. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocrine-Related Cancer. 2008;15(3):721–743. doi: 10.1677/ERC-08-0012. [DOI] [PubMed] [Google Scholar]
- 94.Bates A. S., Farrell W. E., Bicknell E. J., McNicol A. M., Talbot A. J., Broome J. C., Perrett C. W., Thakker R. V., Clayton R. N. Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. Journal of Clinical Endocrinology and Metabolism. 1997;82(3):818–824. doi: 10.1210/jc.82.3.818. [DOI] [PubMed] [Google Scholar]
- 95.Fan X., Paetau A., Aalto Y., Välimäki M., Sane T., Poranen A., Castresana J. S., Knuutila S. Gain of chromosome 3 and loss of 13q are frequent alterations in pituitary adenomas. Cancer Genetics and Cytogenetics. 2001;128(2):97–103. doi: 10.1016/S0165-4608(01)00398-3. [DOI] [PubMed] [Google Scholar]
- 96.Stilling G., Sun Z., Zhang S., Jin L., Righi A., Kovacs G., Korbonits M., Scheithauer B. W., Kovacs K., Lloyd R. V. MicroRNA expression in ACTH-producing pituitary tumors: up-regulation of microRNA-122 and -493 in pituitary carcinomas. Endocrine. 2010;38(1):67–75. doi: 10.1007/s12020-010-9346-0. [DOI] [PubMed] [Google Scholar]
- 97.Guo B.-H., Feng Y., Zhang R., et al. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Molecular Cancer. 2011;10, article 10 doi: 10.1186/1476-4598-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamura M., Gu J., Takino T., Yamada K. M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130(Cas) Cancer Research. 1999;59(2):442–449. [PubMed] [Google Scholar]
- 99.Orrego J. J., Barkan A. L. Pituitary disorders: drug treatment options. Drugs. 2000;59(1):93–106. doi: 10.2165/00003495-200059010-00006. [DOI] [PubMed] [Google Scholar]
- 100.Plöckinger U., Albrecht S., Mawrin C., et al. Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. The Journal of Clinical Endocrinology & Metabolism. 2008;93(4):1203–1210. doi: 10.1210/jc.2007-1986. [DOI] [PubMed] [Google Scholar]
- 101.Furuta M., Kozaki K.-I., Tanaka S., Arii S., Imoto I., Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2009;31(5):766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]