Abstract
OBJECTIVE
Glycated hemoglobin (HbA1c), a standard measure of chronic glycemia for managing diabetes, has been proposed to diagnose diabetes and identify people at risk. The Diabetes Prevention Program (DPP) was a 3.2-year randomized clinical trial of preventing type 2 diabetes with a 10-year follow-up study, the DPP Outcomes Study (DPPOS). We evaluated baseline HbA1c as a predictor of diabetes and determined the effects of treatments on diabetes defined by an HbA1c ≥6.5% (48 mmol/mol).
RESEARCH DESIGN AND METHODS
We randomized 3,234 nondiabetic adults at high risk of diabetes to placebo, metformin, or intensive lifestyle intervention and followed them for the development of diabetes as diagnosed by fasting plasma glucose (FPG) and 2-h postload glucose (2hPG) concentrations (1997 American Diabetes Association [ADA] criteria). HbA1c was measured but not used for study eligibility or outcomes. We now evaluate treatment effects in the 2,765 participants who did not have diabetes at baseline according to FPG, 2hPG, or HbA1c (2010 ADA criteria).
RESULTS
Baseline HbA1c predicted incident diabetes in all treatment groups. Diabetes incidence defined by HbA1c ≥6.5% was reduced by 44% by metformin and 49% by lifestyle during the DPP and by 38% by metformin and 29% by lifestyle throughout follow-up. Unlike the primary DPP and DPPOS findings based on glucose criteria, metformin and lifestyle were similarly effective in preventing diabetes defined by HbA1c.
CONCLUSIONS
HbA1c predicted incident diabetes. In contrast to the superiority of the lifestyle intervention on glucose-defined diabetes, metformin and lifestyle interventions had similar effects in preventing HbA1c-defined diabetes. The long-term implications for other health outcomes remain to be determined.
Introduction
The Diabetes Prevention Program (DPP), including its long-term follow-up Diabetes Prevention Program Outcomes Study (DPPOS), was a randomized clinical trial evaluating metformin and an intensive lifestyle (ILS) weight-loss intervention to prevent or delay type 2 diabetes (1–3) defined by 1997 American Diabetes Association (ADA) criteria for fasting plasma glucose (FPG) and 2-h postload glucose (2hPG) (4). Current diagnostic criteria define diabetes using HbA1c ≥6.5% (48 mmol/mol) (5,6). HbA1c <6.5% but ≥6.0% (42 mmol/mol) was recommended to identify persons at high risk of developing diabetes who should be offered preventive interventions (5). The lower limit defining high risk has also been set at ≥5.7% (39 mmol/mol) (6). To compare these different diagnostic criteria and evaluate HbA1c as a risk indicator, we evaluated HbA1c as a predictor of diabetes and as an alternate outcome in the DPP and DPPOS.
Research Design and Methods
Participants, Treatment, and Follow-up
The methods and primary findings have been described (1–3), and protocols are available at https://dppos.bsc.gwu.edu/web/dppos/home. The trial is registered as NCT00004992 (DPP) and NCT00038727 (DPPOS). The 3,234 nondiabetic participants had the following risk factors: BMI ≥24 kg/m2, FPG ≥5.3 mmol/L (95 mg/dL) and <7.0 mmol/L (126 mg/dL), and 2hPG ≥7.8 mmol/L (140 mg/dL) and <11.1 mmol/L (200 mg/dL). There were minor exceptions to these criteria: FPG <7.8 mmol/L before this diagnostic level was lowered with the 1997 ADA criteria, no lower limit of FPG in the American Indian centers, and BMI ≥22 kg/m2 in Asian Americans (1).
Participants were randomly assigned to one of three treatment groups: placebo, metformin 850 mg twice per day, or ILS (1,2). A tentative diabetes diagnosis was made if FPG was ≥7.0 mmol/L (126 mg/dL) at a semiannual examination or 2hPG during the annual 75-g oral glucose tolerance test was ≥11.1 mmol/L (200 mg/dL), according to ADA criteria (4). A diagnosis required confirmation on a repeat of the same test (FPG or oral glucose tolerance test) as that triggering the tentative diagnosis (1). Confirmed diagnoses were reported to the participants and their health-care providers, but study metformin or placebo was still provided unless hyperglycemia worsened to FPG ≥140 mg/dL (7.8 mmol/L) during DPP or HbA1c ≥7.0% (53 mmol/mol) during DPPOS. At this point, the study drug was discontinued, and diabetes management, including metformin or other drug treatment, was transferred to the participant’s own health-care provider. Otherwise, HbA1c results were not used for determining eligibility or outcomes.
Diabetes incidence rates during the DPP were 11.0 cases/100 person-years in the placebo group, 7.8 in the metformin group, and 4.8 in the ILS group, representing reductions in diabetes incidence of 31% and 58% with metformin and ILS compared with placebo (1). Following drug unmasking and release of these results in 2001, all participants were offered lifestyle intervention (3). Metformin was continued in the original metformin group, placebo was discontinued, and the original ILS group was offered additional lifestyle support. Of the original cohort, 2,766 (88% of those alive and enrolled at the end of the DPP whether or not they had developed diabetes) were enrolled in the DPPOS long-term follow-up (3). During the DPPOS, after all study participants had been offered lifestyle intervention, diabetes rates in the metformin and former placebo groups fell to rates similar to those of the original ILS group, which remained relatively stable (3).
HbA1c was measured at baseline, 6 months, and 12 months and then annually in the level 1 central laboratory at the University of Washington. The high-performance liquid chromatography method was aligned to the National Glycohemoglobin Standardization Program. At the start of the study in 1996, analyses were performed using the Variant Classic instrument (Bio-Rad Laboratories, Inc., Hercules, CA) with an overall interassay coefficient of variance (CV) of 2.3%. In 1999, the Variant Classic was replaced by the Variant II instrument from the same manufacturer with an overall interassay CV of 1.7%. In 2004, the Variant II was replaced by the Tosoh G7 analyzer (Tosoh Bioscience, Inc., San Francisco, CA), with an overall interassay CV of 0.9%. Each transition was monitored by parallel measurements of patient and quality control samples to ensure no difference in the measurements among instruments. Additionally, to monitor for possible assay drift during the course of the study, five blood pools having HbA1c levels of 5%, 6%, 7%, 8%, and 9% (31, 42, 53, 64, and 75 mmol/mol, respectively) were prepared in the laboratory. The pools were aliquotted and stored under liquid nitrogen and analyzed for several days every month, and the mean values were plotted against their assigned values. There was consistently low variation around the target values with no evidence of assay drift over time. Some HbA1c assays are sensitive to hemoglobinopathies, of which HbS and HbF are especially common in African Americans, but the results are not affected by HbS. When a suspected S variant was detected, it was confirmed by an independent method. If HbF was above the instrument threshold, HbA1c results were not reported.
Analysis of HbA1c as a Predictor and as a Study Outcome
The current report includes the participants who did not have diabetes at baseline according to FPG, 2hPG, and HbA1c (2010 ADA criteria), that is, FPG <7.0 mmol/L, 2hPG <11.1 mmol/L, and HbA1c <6.5% (48 mmol/mol). Of the 3,234 individuals randomized, we excluded 54 with FPG ≥7.0 mmol/L (enrolled before the change in ADA diagnostic criteria), 7 with missing HbA1c, and 408 with HbA1c ≥6.5%, leaving an analysis set of 2,765 participants. The participants were grouped by baseline HbA1c <5.5%, 5.5% to <6.0%, or 6.0% to <6.5% (<37, 37 to <42, or 42 to <48 mmol/mol, respectively) to determine the predictive value for diabetes development defined by glucose or HbA1c. Supplementary Fig. 1 shows the follow-up of these participants. HbA1c results were not confirmed with repeat tests; therefore, for these analyses, a single HbA1c ≥6.5% was considered diagnostic.
Statistical Methods
The intention-to-treat analysis compared each intervention group with the placebo group on the modified product-limit life-table distribution using the log-rank test statistic. Treatment groups and study time periods were also compared with incidence rates in cases/100 person-years. Person-years were summed over all participants in a group of time to follow-up before a diagnosis or to end of follow-up if diabetes did not develop during the period of interest. Diabetes hazard rates were stratified by age, sex, and self-reported race/ethnicity, and the covariate effects were assessed by simultaneously evaluating indicator terms for each major group compared with a predefined comparison group with the likelihood ratio test. Risk reduction and interactions between treatment assignments and covariates were assessed by proportional hazards regression.
We present results for the 2,765 participants separately for the 3.0-year median (interquartile range 2.5–3.7) follow-up in the DPP before the study results were announced and the protocol was modified and for the total follow-up period (DPP and DPPOS) from each participant’s randomization until a common closing date of 27 August 2008 (median 9.9 years, interquartile range 9.0–10.5). Statistical tests evaluating both periods must be interpreted while recognizing that they are not independent of each other (the first is contained in the second). Both periods are of interest: the DPP period because intervention effects on diabetes incidence were maximal in this period and it was the only double-blind period (for placebo and metformin) and the total follow-up period to assess effects for as long as possible.
Results
Baseline characteristics of the 2,765 participants are shown in Table 1. None of the diabetes risk factors, including HbA1c and glucose measures, differed among the treatment groups.
Table 1.
Baseline characteristics at DPP randomization
| Overall | Placebo | Metformin | Lifestyle | |
|---|---|---|---|---|
| Men | 873 (31.6) | 289 (31.0) | 305 (33.5) | 279 (30.3) |
| Women | 1,892 (68.4) | 643 (69.0) | 606 (66.5) | 643 (69.7) |
| Race/ethnicity | ||||
| Caucasian | 1,621 (58.6) | 534 (57.3) | 548 (60.2) | 539 (58.5) |
| African American | ||||
| Hispanic | 422 (15.3) | 135 (14.5) | 147 (16.1) | 140 (15.2) |
| American Indian | 150 (5.4) | 53 (5.7) | 47 (5.2) | 50 (5.4) |
| Asian American | 125 (4.5) | 53 (5.7) | 30 (3.3) | 42 (4.6) |
| HbA1c | ||||
| <5.5% | 532 (19.2) | 186 (20.0) | 164 (18.0) | 182 (19.7) |
| 5.5–5.9% | 1,200 (43.4) | 385 (41.3) | 421 (46.2) | 394 (42.7) |
| 6.0–6.4% | 1,033 (37.4) | 361 (38.7) | 326 (35.8) | 346 (37.5) |
| Age (years) | 50.3 (10.6) | 50.2 (11.3) | 50.4 (10.2) | 50.1 (10.4) |
| Weight (kg) | 93.3 (19.8) | 93.1 (20.3) | 93.4 (19.4) | 93.3 (19.6) |
| BMI (kg/m2) | 33.7 (6.5) | 33.6 (6.6) | 33.6 (6.4) | 33.8 (6.5) |
| Waist circumference (cm) | 104.3 (14.1) | 104.5 (14.5) | 104.1 (13.9) | 104.4 (13.8) |
| FPG (mg/dL) | 105.4 (7.4) | 105.3 (7.3) | 105.2 (7.4) | 105.6 (7.4) |
| 2hPG (mg/dL) | 163.9 (16.9) | 163.5 (16.7) | 164.3 (17.0) | 163.8 (16.9) |
| HbA1c (%) | 5.8 (0.4) | 5.8 (0.4) | 5.8 (0.4) | 5.8 (0.4) |
Data are n (%) or mean (SD). None of the variables differed significantly among treatment groups.
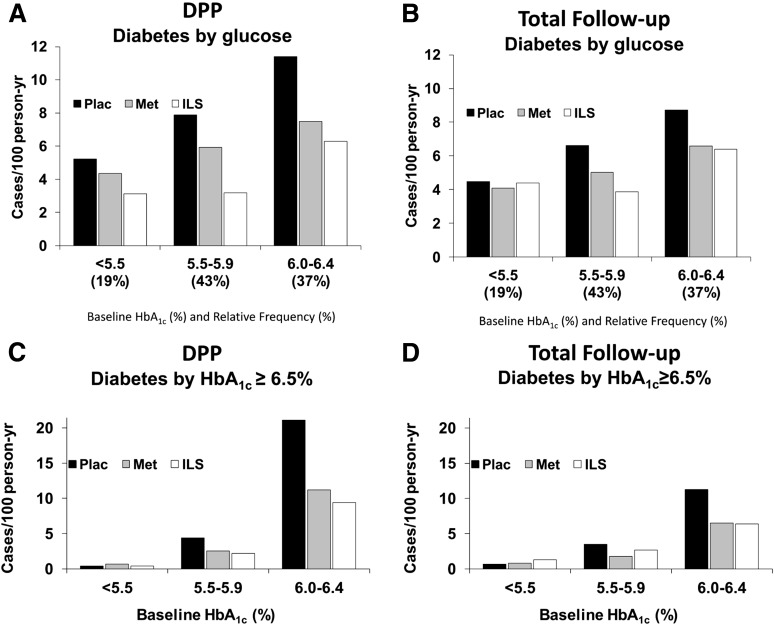
HbA1c at baseline was a strong predictor of the development of glucose-defined diabetes during the DPP and total follow-up periods (Fig. 1A and B). During the DPP and total follow-up periods, the incidence of glucose-defined diabetes was positively related to baseline HbA1c, and stratified by baseline HbA1c, the incidence was reduced by metformin versus placebo (P < 0.001) and by lifestyle versus placebo (P < 0.001), and the reduction by lifestyle was greater than that by metformin (P < 0.001). These relationships were continuous, with no evidence of an HbA1c threshold. In neither period was there a significant interaction of baseline HbA1c with treatment group on incidence of diabetes; that is, treatment effect (as a percent rate reduction) was independent of baseline HbA1c. The absolute effect in reducing diabetes incidence was greater in those with higher baseline HbA1c, however, among whom the incidence rates were higher regardless of treatment assignment.
Figure 1.
Incidence of diabetes (new cases/100 person-years) by baseline HbA1c, where diabetes was determined by 1997 ADA criteria using FPG and 2hPG concentrations or by HbA1c ≥6.5% (48 mmol/mol). Results are shown for the original masked treatment phase (DPP with mean follow-up of 3.0 years) (A and C) and for the DPP plus long-term follow-up (total follow-up with median follow-up of 9.9 years) (B and D). Met, metformin; Plac, placebo.
Incidence rates by treatment are shown for diabetes defined by HbA1c ≥6.5% in Fig. 1C and D. As with glucose-defined diabetes (Fig. 1A and B), baseline HbA1c strongly predicted HbA1c-defined diabetes, and treatment effects did not differ significantly by baseline HbA1c. During the DPP and total follow-up periods, the incidence of diabetes defined by HbA1c ≥6.5% was positively related to baseline HbA1c, and stratified by baseline HbA1c, the incidence was reduced by metformin versus placebo (P < 0.0001) and by lifestyle versus placebo (P < 0.0001), but the reductions by metformin and lifestyle did not differ significantly from each other. There was a significant interaction (P < 0.01) between baseline HbA1c and the lifestyle versus placebo effect, the effect being greater at higher baseline HbA1c. Indeed, this outcome was so infrequent in those with baseline HbA1c <5.5% that treatment effects could not be estimated precisely in this group.
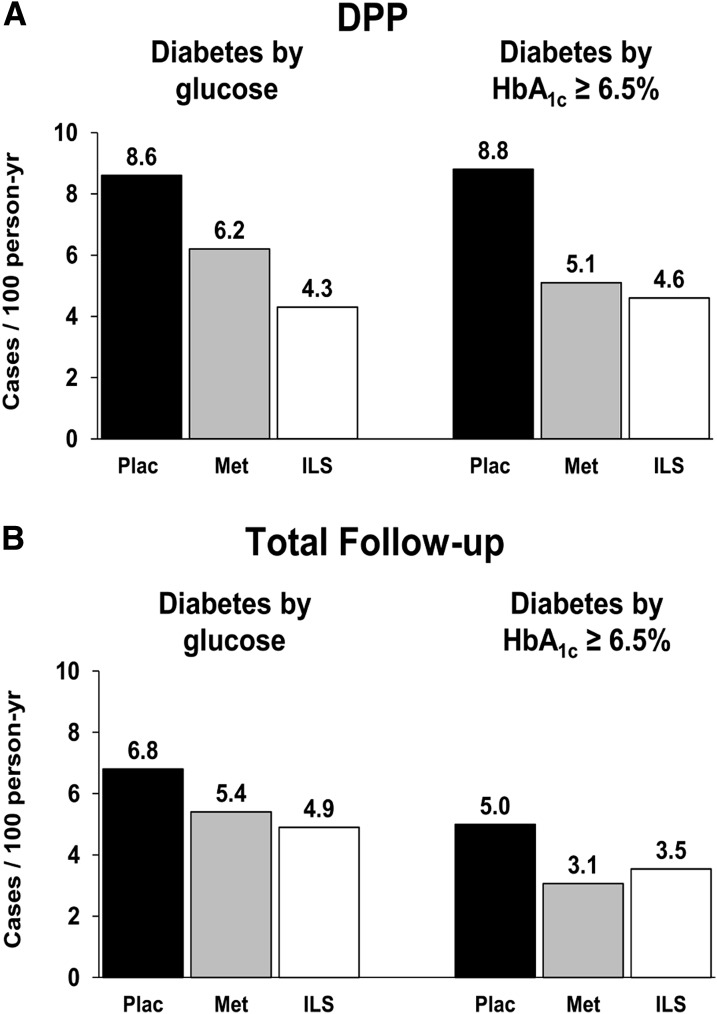
Incidence rates by treatment are compared for the outcomes of glucose- and HbA1c-defined diabetes (Fig. 2). Incidence rates of glucose-defined diabetes were lower in this subset than for all participants as reported previously (2) because of exclusion of the highest risk group with baseline HbA1c ≥6.5%, but the treatment effects persisted, with a reduction by 29% with metformin and by 51% with ILS during the DPP and by 21% with metformin and 28% with ILS in the total follow-up period. During the DPP period, the incidence of diabetes by glucose criteria was reduced by metformin versus placebo (P = 0.0013) and by lifestyle versus placebo (P < 0.0001), and lifestyle intervention reduced it more than metformin (P = 0.0023). During the total follow-up period, the incidence of diabetes by glucose criteria was reduced by metformin versus placebo (P = 0.0014) and by lifestyle versus placebo (P < 0.0001), but the reductions by metformin or lifestyle intervention did not differ significantly from each other. By contrast, for incidence rates of HbA1c-defined diabetes, metformin and ILS resulted in nearly the same rate reductions as each other during the DPP period (44% and 49%, respectively) and during total follow-up (38% and 29%, respectively). During the DPP and total follow-up periods, the incidence of HbA1c ≥6.5% was reduced by metformin versus placebo (P < 0.0001) and by lifestyle versus placebo (P < 0.0001) but did not differ significantly between the metformin and lifestyle interventions.
Figure 2.
Comparison of treatment effects on the incidence of diabetes diagnosed by glucose criteria or by HbA1c ≥6.5% (48 mmol/mol). Results are shown for the original masked treatment phase (DPP) (A) and for the total follow-up period (B). Met, metformin; Plac, placebo.
There were significant race/ethnicity effects on the incidence of glucose-defined (during total follow-up) and HbA1c-defined diabetes (during the DPP and total follow-up periods). Across all treatment groups combined, incidence rates were highest among African Americans (Supplementary Table 1) during the total follow-up period when defined by glucose (P = 0.005) and when defined by HbA1c during the DPP and total follow-up periods (all P < 0.001) in models adjusted for sex, age, HbA1c at baseline, FPG and 2hPG at baseline, and treatment assignment.
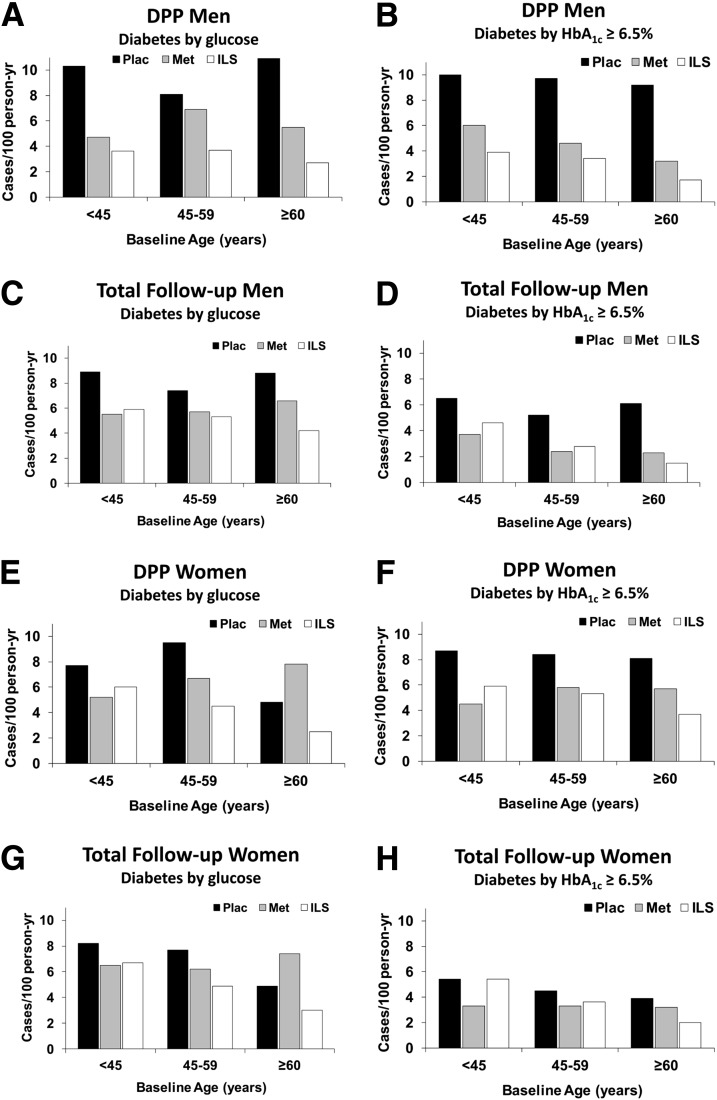
Results are presented stratified by baseline age and sex in Fig. 3. In each age stratum among men and for each outcome (diabetes defined by glucose or HbA1c), rates were lower in the metformin and ILS groups than in the placebo group. Among women ≥60 years old, the incidence of glucose-defined diabetes was higher in the metformin than in the placebo group, but the incidence of HbA1c-defined diabetes was lower in the metformin group. The three-way interaction of sex × age × treatment was not statistically significant, however, indicating that these disparities could be due to chance. There were no significant sex × treatment interactions for incidence of glucose-defined diabetes during DPP or the total follow-up period; however, there were several significant sex × treatment interactions in incidence of HbA1c ≥6.5%. During the DPP, risk reduction by ILS versus placebo was greater in men than in women (70% and 38%, respectively; P = 0.010 for sex × treatment interaction). Reduction in risk of reaching HbA1c ≥6.5% during total follow-up was also greater in men (52% for ILS vs. placebo and 54% for metformin vs. placebo compared with 15% and 29% risk reductions, respectively, in women; both P < 0.05 for sex × treatment interaction).
Figure 3.
Incidence of diabetes diagnosed by glucose criteria or by HbA1c ≥6.5% (48 mmol/mol) by baseline age and treatment assignment in men (A–D) and women (E–H). Results are shown for the original masked treatment phase (DPP) and for the total follow-up period. Met, metformin; Plac, placebo.
There were significant (P < 0.05) interactions between treatment and baseline age in the incidence of glucose-defined diabetes during the DPP and total follow-up periods, with ILS having a greater advantage over metformin at older ages. Although there was a tendency for such an age interaction on incidence of HbA1c ≥6.5%, the interaction was not as pronounced and not statistically significant in the DPP or total follow-up periods.
The coincidence of diabetes defined by glucose and HbA1c criteria was examined. At the 1,059 examinations that led to a confirmed diabetes diagnosis based on glucose criteria during the total follow-up period, HbA1c was <6.5% in 779 (74%) participants, was ≥6.5% for the first time in 105 (10%), and had been ≥6.5% at a previous examination in 175 (17%). Conversely, at the first examination after baseline at which HbA1c was ≥6.5% (750 occurrences), there was no confirmed glucose-based diagnosis of diabetes in 341 (45%), a confirmed glucose-based diagnosis triggered at the same visit in 105 (14%), and a previously confirmed glucose-based diabetes diagnosis in 304 (41%).
Conclusions
HbA1c is recommended for identifying persons at high risk of developing diabetes and as a diabetes diagnostic criterion (5,6). In this report, we evaluated baseline HbA1c as a predictor of diabetes and analyzed the DPP and DPPOS as if HbA1c ≥6.5% had been the sole outcome. Baseline HbA1c predicted development of glucose- and HbA1c-defined diabetes during the DPP and total follow-up periods, confirming findings in other prevention trials (7–9) that HbA1c below the diagnostic level of 6.5% is directly associated with risk of developing diabetes. The risk relationship is continuous with baseline HbA1c as previously suggested (5), confirming that selection of high-risk cut points of 6.0% (5) or 5.7% (6) is arbitrary. The optimal selection of high-risk characteristics for individuals offered diabetes prevention interventions will depend on available resources, health benefits of preventive measures, and their comparative effectiveness and costs.
In the DPP and DPPOS, diabetes was prevented or delayed with metformin or ILS aimed at weight loss and increased physical activity. The ILS was substantially more effective than metformin in preventing glucose-defined diabetes (2,3). By contrast, if HbA1c ≥6.5% had been the outcome, we would have concluded that both interventions were similarly effective. We would have also accrued fewer events, confirming that HbA1c ≥6.5% alone defines fewer persons as having diabetes than does the combination of FPG or 2hPG, as found in the National Health and Nutrition Examination Survey (10) and the Finnish Diabetes Prevention Study (7). The HbA1c diagnostic cut point was purposefully chosen to favor specificity over sensitivity, recognizing that it would usually lead to fewer diagnoses compared with the 1997 glucose-based criteria (5).
These results add to previous DPP reports that metformin and ILS were similar in affecting FPG, whereas ILS was more effective for 2hPG-defined diabetes (2,3). Metformin lowered FPG (11) consistent with its suppression of endogenous glucose production by the liver (12).
Do these results indicate that the two interventions will have equivalent health benefits in DPP participants; that is, is preventing diabetes defined by HbA1c as clinically important as preventing diabetes defined by FPG or 2hPG? HbA1c and FPG represent usual glycemia better than does 2hPG, which measures response to a nonphysiologic challenge. All three measures, however, have similar associations with microvascular disease (13–16). Therefore, the relative importance for other health outcomes of these glycemic measures is not clear. The current analyses do not address the relative importance of reducing diabetes based on glucose or HbA1c levels. Better understanding of the relative long-term health effects of the two interventions should come from further follow-up, during which intervention effects on microvascular-neuropathic outcomes and cardiovascular disease risk factors will be assessed (3). The analyses performed to date of the treatment effects on other outcomes during DPP, including lipids, blood pressure, and hemostatic factors, suggest that ILS achieves better or similar results with less medication use (3,17,18). Weight loss per se, which was greater with ILS, might have benefits beyond diabetes prevention. Neither the metformin nor the ILS had a significant interaction with a multigene diabetes risk score in predicting glucose-defined diabetes in the DPP (19), providing little support for considering genetic risk in choosing one of these treatment approaches. Evidence suggests that genotype at candidate loci influences success in weight loss intervention (20,21). Such developments and more comprehensive research on the genetics of response to metformin and lifestyle interventions may ultimately lead to tools for selecting optimal interventions for diabetes prevention.
Not only did treatment effects on glucose-defined and HbA1c-defined diabetes differ, but the participants diagnosed by each criterion did not fully overlap. Only 26% of those diagnosed by FPG or 2hPG had a previous or simultaneous HbA1c ≥6.5%. Conversely, 55% of those first attaining an HbA1c ≥6.5% had a current or previous diagnosis of diabetes by glucose criteria. We cannot determine whether those diagnosed by FPG or 2hPG with HbA1c <6.5% would have subsequently met this level had they not been diagnosed by glucose levels and diabetes management subsequently initiated.
There were significant treatment interactions with age and sex. We previously reported that the effects of the active DPP interventions differed by age, with ILS being exceptionally effective and metformin ineffective among participants ≥60 years old at baseline (22). Furthermore, among those developing diabetes during DPP, the older group was more likely than the young or middle-aged groups to be diagnosed by 2hPG, on which metformin may have less effect. In the current analysis of HbA1c-defined diabetes, the greater effect of ILS with older age was maintained, and metformin was effective in all age-groups, particularly in men. The apparent adverse effect of metformin on incidence of glucose-defined diabetes in older women was not observed for HbA1c-defined diabetes.
Racial differences in the relationships between HbA1c and FPG and 2hPG have been reported (23,24). Other studies, however, suggested that interracial differences in HbA1c parallel differences in other measures of chronic glycemia (25) and that the relationships of HbA1c with retinopathy (26) and macrovascular disease and death (27) are the same in American blacks and whites. Moreover, differences in HbA1c between races are not explained by ancestry-informative genetic markers (28) or by allele frequency differences in genes associated with HbA1c (29). In the current analyses over the total follow-up period, the incidence of diabetes in the African Americans was higher than in the other race/ethnicity groups whether diabetes was defined by glucose or HbA1c. In addition, treatment effects on HbA1c-defined diabetes were similar among the race/ethnicity groups. Despite potential race/ethnicity differences in HbA1c, these data suggest treatment efficacy in all the race/ethnicity groups.
There are three important limitations to these analyses. First, we cannot strictly compare the performance of the different tests for diabetes because by protocol, diagnoses made by FPG or 2hPG required confirmation and the HbA1c tests did not. Second, we cannot determine to what extent elevation of HbA1c to ≥6.5% was prevented or delayed by the diagnosis of diabetes by glucose criteria and subsequent management or behavioral changes. Third, the eligibility criteria, including adults with BMI ≥24 kg/m2, with elevated FPG and 2hPG but without limitations on HbA1c, limit generalizability. Among persons not selected by BMI and glucose criteria as in the DPP, it is not known to what extent HbA1c would predict diabetes or response to interventions. For example, persons with HbA1c in the range of 6.0–6.4% who do not meet the other criteria may be at lower risk of diabetes than indicated herein. Given the uniformity of treatment effects according to baseline FPG and 2hPG reported previously (2) and HbA1c in the current study, we suspect that the study interventions would reduce diabetes risk similarly regardless of these baseline factors but that the absolute risk reduction (or numbers of cases prevented per number treated) would be lower in persons with lower levels of risk factors. Because eligibility criteria in prevention trials have been so restricted, we do not know how best to select persons who should be offered preventive interventions (30).
In summary, HbA1c measured at DPP entry predicted incidence of diabetes, and study treatment effects were uniform with respect to baseline HbA1c (i.e., there were no significant baseline HbA1c by treatment interactions). By contrast, although ILS was superior to metformin for preventing the development of glucose-defined diabetes, the effects of the two study treatments were similar in preventing diabetes defined by HbA1c. The health implications of these treatment and diagnostic differences await further assessment of long-term health outcomes.
Supplementary Material
Article Information
Acknowledgments. The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding. During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the coordinating center for the design and conduct of the study and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding was also provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Institute on Aging; the National Eye Institute; the National Heart, Lung, and Blood Institute; the Office of Research on Women’s Health; the National Institute on Minority Health and Health Disparities; the Centers for Disease Control and Prevention; and the ADA.
The primary sponsor, the NIDDK, was represented on the steering committee and played a part in study design, management, and publication. The sponsors were not members of the writing group, although all members of the steering committee had input into the article’s contents and reviewed the manuscript.
Duality of Interest. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck Santé) provided medication, and LifeScan Inc. donated materials during the DPP and DPPOS. No other potential conflicts of interest relevant to this article were reported.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of centers, investigators, and staff can be found in the Supplementary Data online.
Author Contributions. The Writing Group, W.C.K., S.L.E., R.B.G., R.T.A., J.P.C., J.C.F., S.E.F., W.H.H., E.S.H., S.E.K., K.J.M., and D.M.N., had access to all data. W.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, FL, 25–29 June 2010.
Appendix
This article was prepared by William C. Knowler (National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ), Sharon L. Edelstein (Biostatistics Center, George Washington University, Rockville, MD), Ronald B. Goldberg (University of Miami, Miami, FL), Ronald T. Ackermann (Northwestern University, Chicago, IL), Jill P. Crandall (Albert Einstein College of Medicine, New York, NY), Jose C. Florez (Massachusetts General Hospital and Harvard Medical School, Boston, MA), Sarah E. Fowler (Biostatistics Center, George Washington University, Rockville, MD), William H. Herman (University of Michigan, Ann Arbor, MI), Edward S. Horton (Joslin Diabetes Center and Harvard Medical School, Boston, MA), Steven E. Kahn (VA Puget Sound Health Care System and University of Washington, Seattle, WA), Kieren J. Mather (Indiana University, Indianapolis, IN), and David M. Nathan (Massachusetts General Hospital and Harvard Medical School, Boston, MA).
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0886/-/DC1.
Clinical trial reg. nos. NCT00004992, clinicaltrials.gov, and NCT00038727, clinicaltrials.gov.
A complete list of the members of the Diabetes Prevention Program Research Group can be found in the Supplementary Data online, and a complete list of the members of the Writing Group is available in the appendix.
References
- 1.The Diabetes Prevention Program Research Group . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 5.International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajunen P, Peltonen M, Eriksson JG, et al. Finnish Diabetes Prevention Study . HbA(1c) in diagnosing and predicting type 2 diabetes in impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabet Med 2011;28:36–42 [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Watanabe M, Nishida J, et al. Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran A, Snehalatha C, Samith Shetty A, Nanditha A. Predictive value of HbA1c for incident diabetes among subjects with impaired glucose tolerance—analysis of the Indian Diabetes Prevention Programmes. Diabet Med 2012;29:94–98 [DOI] [PubMed] [Google Scholar]
- 10.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCance DR, Hanson RL, Charles M-A, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994;308:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE, Australian Diabetes Obesity and Lifestyle Study Group . Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008;31:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colagiuri S, Lee CMY, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011;34:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 2011;60:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner S, Temprosa M, Crandall J, et al. Diabetes Prevention Program Research Group . Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Diabetes Prevention Program Outcomes Study Research Group . Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hivert MF, Jablonski KA, Perreault L, et al. DIAGRAM Consortium. Diabetes Prevention Program Research Group . Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the Diabetes Prevention Program. Diabetes 2011;60:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franks PW, Jablonski KA, Delahanty LM, et al. Diabetes Prevention Program Research Group . Assessing gene-treatment interactions at the FTO and INSIG2 loci on obesity-related traits in the Diabetes Prevention Program. Diabetologia 2008;51:2214–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Q, Delahanty LM, Jablonski KA, et al. Diabetes Prevention Program Research Group . Variation at the melanocortin 4 receptor gene and response to weight-loss interventions in the Diabetes Prevention Program. Obesity (Silver Spring) 2013;21:E520–E526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crandall J, Schade D, Ma Y, et al. Diabetes Prevention Program Research Group . The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006;61:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman WH, Ma Y, Uwaifo G, et al. Diabetes Prevention Program Research Group . Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med 2010;152:770–777 [DOI] [PubMed] [Google Scholar]
- 25.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 2011;154:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med 2012;157:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruthur NM, Kao WHL, Clark JM, et al. Does genetic ancestry explain higher values of glycated hemoglobin in African Americans? Diabetes 2011;60:2434–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimsby JL, Porneala BC, Vassy JL, et al. MAGIC Investigators . Race-ethnic differences in the association of genetic loci with HbA1c levels and mortality in U.S. adults: the third National Health and Nutrition Examination Survey (NHANES III). BMC Med Genet 2012;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowler WC. Prevention of type 2 diabetes: comment on “Lifestyle Modification and Prevention of Type 2 Diabetes in Overweight Japanese With Impaired Fasting Glucose Levels.” Arch Intern Med 2011;171:1361–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.