Abstract
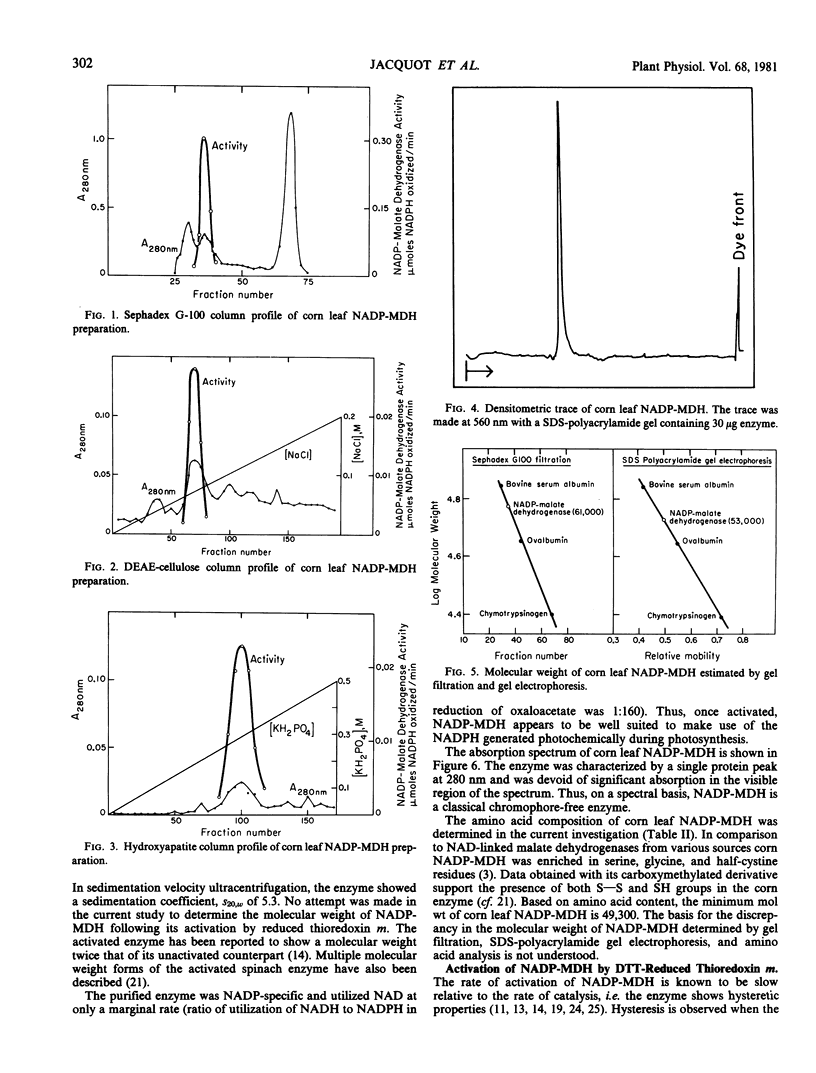
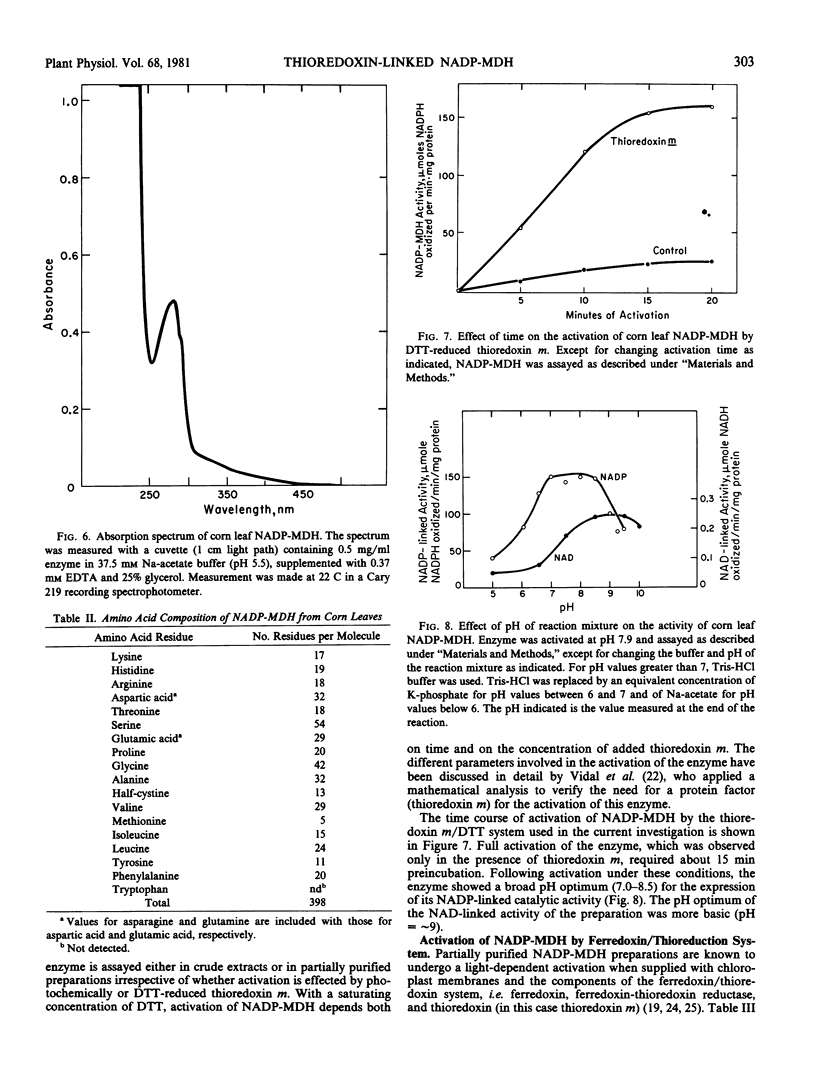
NADP-malate dehydrogenase, a light-modulated enzyme of C4 photosynthesis, was purified to homogeneity from leaves of corn. The pure enzyme was activated by thioredoxin m that was reduced either photochemically (with ferredoxin and ferredoxin-thioredoxin reductase) or chemically (with dithiothreitol). Unactivated corn leaf NADP-malate dehydrogenase had a molecular weight of 50,000 to 60,000 and was chromophorefree. The enzyme appeared to have a high content of serine and glycine and to contain both S—S and SH groups. Consequently, NADP-malate dehydrogenase seems to be capable of undergoing reversible oxidation/reduction during its photoregulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Kalberer P. P., Arnon D. I. Ferredoxin-activated fructose diphosphatase in isolated chloroplasts. Biochem Biophys Res Commun. 1967 Oct 11;29(1):74–79. doi: 10.1016/0006-291x(67)90543-8. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Jacquot J. P., Vidal J., Gadal P. Identification of a protein factor involved in dithiothreitol activation of NADP malate dehydrogenase from French bean leaves. FEBS Lett. 1976 Dec 1;71(2):223–227. doi: 10.1016/0014-5793(76)80937-4. [DOI] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Regulation of C4 photosynthesis: characterization of a protein factor mediating the activation and inactivation of NADP-malate dehydrogenase. Arch Biochem Biophys. 1977 Nov;184(1):290–297. doi: 10.1016/0003-9861(77)90353-8. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E. Biosynthesis of delta-Aminolevulinic Acid from Glutamate in Agmenellum quadruplicatum. Plant Physiol. 1980 Jan;65(1):126–128. doi: 10.1104/pp.65.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Malate dehydrogenases of leaf tissue from Spinacia oleracea: properties of three isoenzymes. Arch Biochem Biophys. 1971 Nov;147(1):114–122. doi: 10.1016/0003-9861(71)90316-x. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Jacquot J. P. Improved in vitro light activation and assay systems for two spinach chloroplast enzymes. Biochim Biophys Acta. 1979 Aug 15;569(2):309–312. doi: 10.1016/0005-2744(79)90067-6. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Rocha V. NADP-specific malate dehydrogenase of green spinach leaf tissue. Arch Biochem Biophys. 1971 Nov;147(1):156–164. doi: 10.1016/0003-9861(71)90322-5. [DOI] [PubMed] [Google Scholar]
- Wolosiuk R. A., Crawford N. A., Yee B. C., Buchanan B. B. Isolation of three thioredoxins from spinach leaves. J Biol Chem. 1979 Mar 10;254(5):1627–1632. [PubMed] [Google Scholar]
- de la Torre A., Lara C., Wolosiuk R. A., Buchanan B. B. Ferredoxin-thioredoxin reductase: a chromophore-free protein of chloroplasts. FEBS Lett. 1979 Nov 1;107(1):141–145. doi: 10.1016/0014-5793(79)80482-2. [DOI] [PubMed] [Google Scholar]


