Abstract
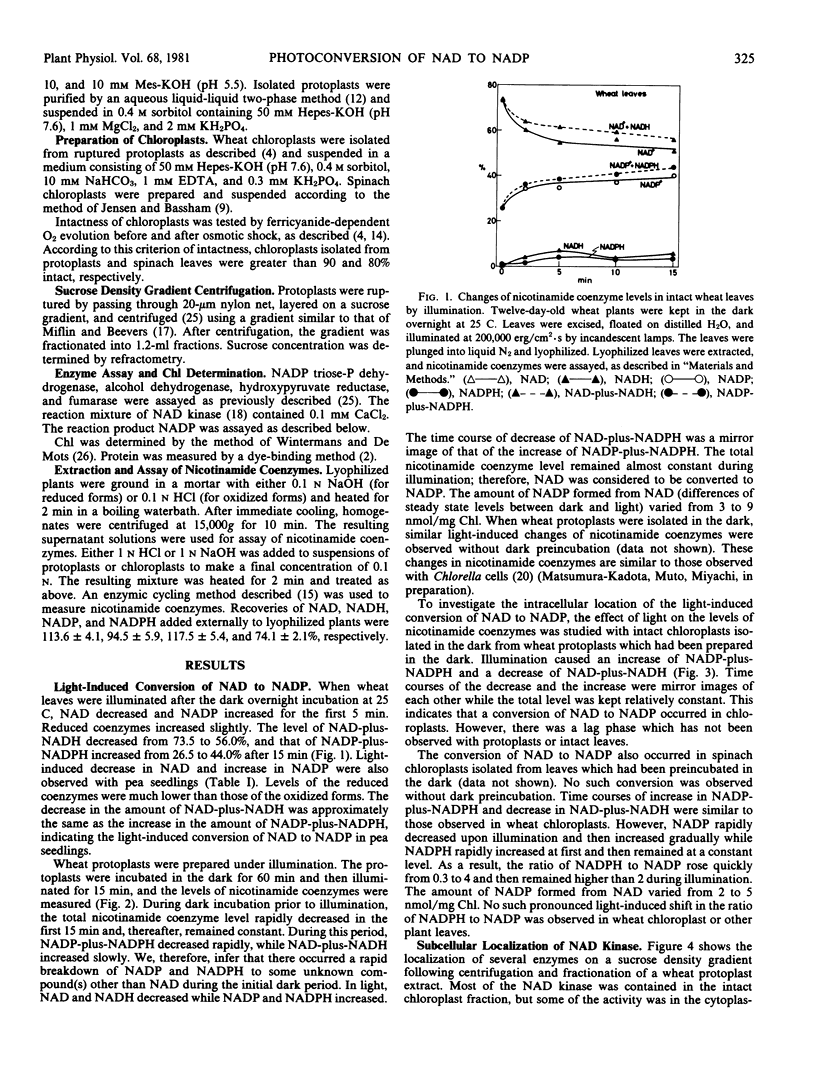
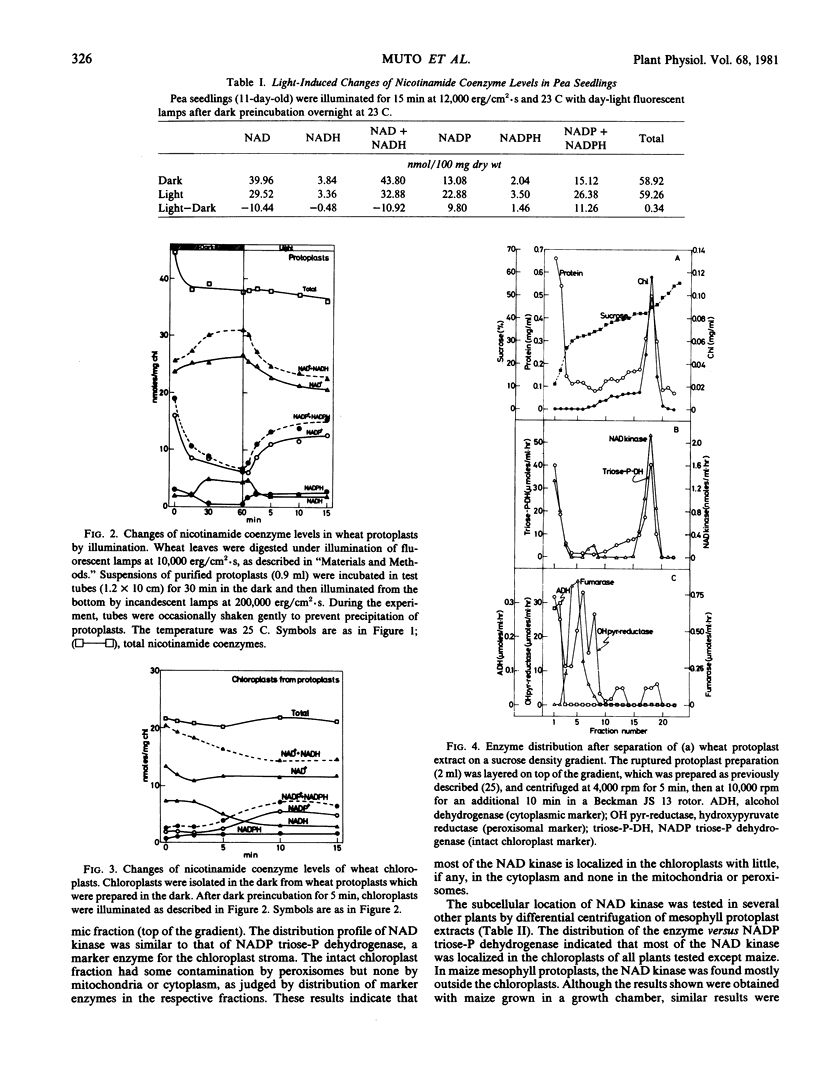
Light-induced conversion of NAD to NADP was investigated in higher plants. Upon illumination, conversion of NAD to NADP was observed in intact leaves of wheat and pea following incubation in the dark. This conversion was also observed in mesophyll protoplasts of wheat leaves when they were isolated in the dark or isolated in light and then preincubated in the dark. Chloroplasts isolated from wheat protoplasts prepared in the dark carried out the conversion. The conversion in the mechanically isolated spinach chloroplasts was observed only when they were isolated in the dark from leaves preincubated in darkness.
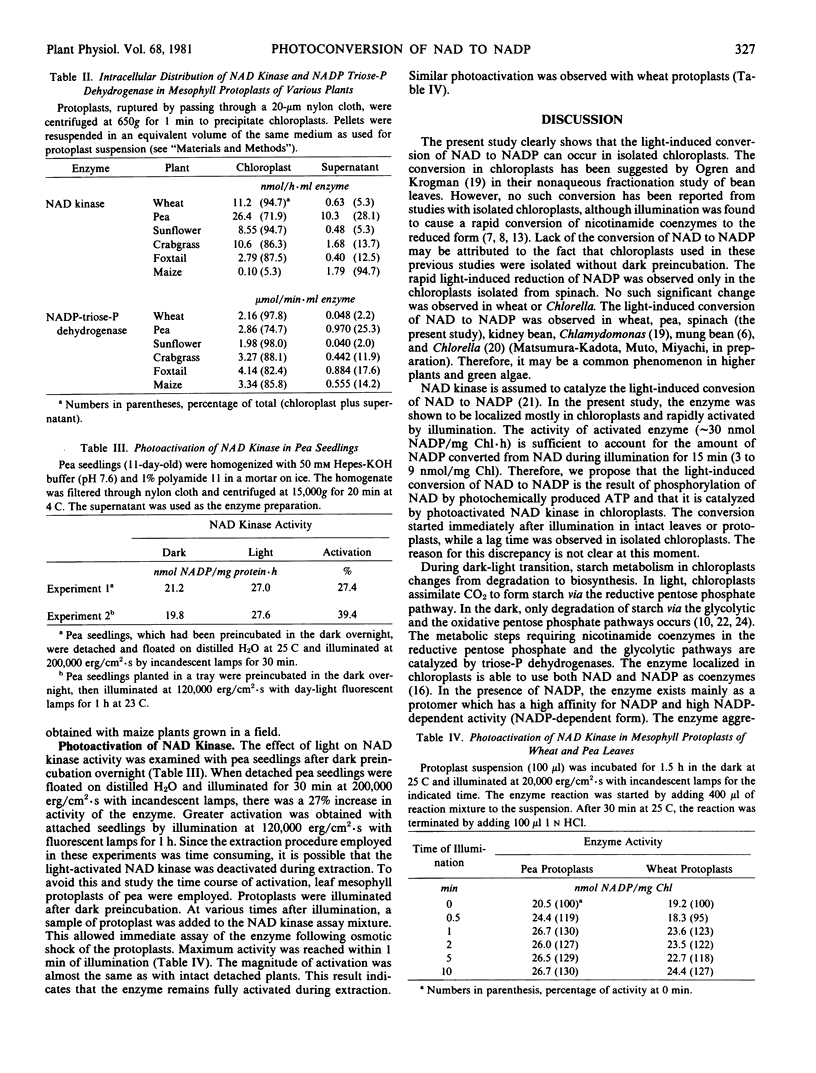
Sucrose density gradient centrifugation of wheat protoplast extracts and differential centrifugation of protoplast extracts from various plants showed that most of the NAD kinase was localized in the chloroplasts. Therefore, the conversion of NAD to NADP is considered to occur in the chloroplasts. However, with extracts of maize mesophyll protoplasts, the enzyme was localized in the extrachloroplast fraction. The NAD kinase was activated some 30% by illumination of leaves or protoplasts of pea and wheat after preincubation in the dark.
These results suggest that, in general, the light-induced conversion of NAD to NADP occurs in the chloroplast and is catalyzed by photoactivated NAD kinase using photochemically produced ATP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. A requirement for chelation in obtaining functional chloroplasts of sunflower and wheat. Arch Biochem Biophys. 1978 Oct;190(2):421–433. doi: 10.1016/0003-9861(78)90295-3. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Bassham J. A. Light-Dark Regulation of Starch Metabolism in Chloroplasts: I. Levels of Metabolites in Chloroplasts and Medium during Light-Dark Transition. Plant Physiol. 1979 Jan;63(1):105–108. doi: 10.1104/pp.63.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendzian K., Bassham J. A. Regulation of glucose-6-phosphate dehydrogenase in spinach chloroplasts by ribulose 1,5-diphosphate and NADPH/NADP+ ratios. Biochim Biophys Acta. 1975 Aug 11;396(2):260–275. doi: 10.1016/0005-2728(75)90040-7. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Pupillo P., Baccarini-Melandri A. D-glyceraldehyde-3-phosphate dehydrogenase in photosynthetic cells. I. The reversible light-induced activation in vivo of NADP-dependent enzyme and its relationship to NAD-dependent activities. Biochim Biophys Acta. 1970 Nov 11;220(2):178–189. doi: 10.1016/0005-2744(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Miyachi S. Properties of a Protein Activator of NAD Kinase from Plants. Plant Physiol. 1977 Jan;59(1):55–60. doi: 10.1104/pp.59.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OH-HAMA T., MIYACHI S. Effects of illumination and oxygen supply upon the levels of pyridine nucleotides in Chlorella cells. Biochim Biophys Acta. 1959 Jul;34:202–210. doi: 10.1016/0006-3002(59)90248-3. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- Peavey D. G., Steup M., Gibbs M. Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol. 1977 Aug;60(2):305–308. doi: 10.1104/pp.60.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo P., Giuliani Piccari G. The reversible depolymerization of spinach chloroplast glyceraldehyde-phosphate dehydrogenase. Interaction with nucleotides and dithiothreitol. Eur J Biochem. 1975 Feb 21;51(2):475–482. doi: 10.1111/j.1432-1033.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Rees T. A. Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta. 1980 Jan 17;627(2):131–143. doi: 10.1016/0304-4165(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


