Abstract
Gap junctions are widely present in spinal cord white matter; however, their role in modulating the dynamics of axonal dysfunction remains largely unexplored. We hypothesized that inhibition of gap junctions reduces the loss of axonal function during oxygen and glucose deprivation (OGD). The functional role of gap junctions was assessed by electrophysiological recordings of compound action potentials (CAPs) in Wistar rat spinal cord slices with the sucrose gap technique. The in vitro slices were subjected to 30-min OGD. Gap junction connexin (Cx) mRNA expression was determined by qPCR and normalized to β-actin. A 30-min OGD resulted in reduction of CAPs to 14.8 ± 4.6% of their pre-OGD amplitude (n = 5). In the presence of gap junction blockers carbenoxolone (Cbx; 100 μM) and 1-octanol (Oct; 300 μM), the CAP reduction in OGD was to only 35.7 ± 5.7% of pre-OGD amplitude in Cbx (n = 9) and to 37.4 ± 8.9% of pre-OGD amplitude in Oct (n = 10). Both drugs also noticeably prolonged the half-decline time of CAP amplitudes in OGD from 6.0 min in no-drug conditions to 9.6 min in the presence of Cbx and to 7.7 min in the presence of Oct, suggesting that blocking gap junctions reduces conduction loss during OGD. With application of Cbx and Oct in the setting of OGD, expression of Cx30 and Cx43 mRNA was downregulated. Our data provide new insights into the role of gap junctions in white matter ischemia and reveal the necessity of a cautious approach in determining detrimental or beneficial effects of gap junction blockade in white matter ischemia.
Keywords: spinal cord injury, gap junctions, connexins, oxygen-glucose deprivation, electrophysiology
in healthy cns, gap junctions serve an important role in electrochemical and metabolic coupling by forming specialized intercellular connections that mediate transfer of various ions and molecules <1,000 kDa, such as IP3 and possibly Ca2+. Studies using knockouts for glial connexins (protein subunits of gap junctions) have shown gross anatomical deficits, including myelin swelling from the lack of K+/H2O removal from the innermost myelin to astrocytic endfeet mediated by connexins (Menichella et al. 2006; Rash 2010), further illustrating an unequivocal need for connexins in healthy tissue.
While there is an emerging consensus regarding the beneficial role of gap junctions in the normal CNS, there is uncertainty as to whether gap junctions function to aid in recovery after spinal cord injury (SCI) by equalizing ions between coupled cells or hinder recovery by propagating cell injury through degradation of ion gradients and subsequent release of glutamate to the extracellular space (Ye et al. 2003) during anoxia and ischemia. In the brain during stroke, connexin knockout models suggest that open gap junctions are neuroprotective (Kozoriz et al. 2010). However, this finding is yet to be replicated in the white matter tissue in the spinal cord. Evidence suggests that ischemia modeled by oxygen-glucose deprivation (OGD) opens neuronal hemichannels (Thompson et al. 2006) and astrocytic hemichannels via connexin (Cx)43 dephosphorylation (Ye et al. 2003). Opening of astrocytic hemichannels has been shown to release glutamate and reduce glutamate uptake (Ye et al. 2003). Generally, astrocytes in normal tissue maintain low extracellular levels of glutamate, thus providing a good environment for synaptic signal transmission and preventing neuronal overexcitation (Ye et al. 2003). As a result of OGD, opening of hemichannels and glutamate release are implicated in excitotoxicity during ischemia.
After SCI a series of secondary events exacerbate the initial physical and mechanical trauma, resulting in white matter injury that is characterized by damage to the myelin sheath (Park et al. 2003, 2004). During ischemia, the lack of blood flow to the CNS results in a reduction in ATP levels and loss of ionic homeostasis through failure of the Na+-K+-ATPase (Alix 2006). As a result, extracellular levels of K+ rise and noninactivating Na+ channels open, depolarizing the axonal membrane. This depolarization and subsequent Ca2+ influx result in further damage such as mitochondrial swelling, retraction of paranodal myelin, reduction in microtubules, and formation of submyelin vacuoles (Waxman et al. 1995). Identification of the mechanisms involved in the secondary injury is further complicated by glial and neuronal interactions. One proposed mechanism involves glutamate excitotoxicity, which involves AMPA and kainate glutamate receptors (Agrawal and Fehlings 1997; Kanellopoulos et al. 2000; Li et al. 1999; Park et al. 2003), another is the reversal of Na+-dependent glutamate transport (Agrawal and Fehlings 1996; Li et al. 1999; Li and Stys 2001). The myelin sheath, abundant in AMPA receptors, is a target for glutamate excitotoxicity (Li et al. 1999), and AMPA/kainate blockers protect spinal cord white matter (Kanellopoulos et al. 2000). However, glutamate antagonists in general are plagued by clinically intolerable side effects (Alix 2006), and hence alternative approaches to influence secondary injury cascades are desirable. Since little is known regarding the signaling between astrocytes and oligodendrocytes and in particular the functional role of gap junctions in secondary SCI, these areas represent important subjects for investigation.
Given this background, we sought to examine the role of gap junctions in spinal cord ischemic injury that we modeled using OGD on isolated spinal cord slices ex vivo. This is the first study to examine gap junctions in this context, and we present electrophysiological and molecular evidence that strongly implicates gap junctional signaling in the pathophysiology of spinal cord ischemic injury.
MATERIALS AND METHODS
Animals.
All experimental protocols were approved by the Animal Care Committee at the University Health Network and executed in accordance with the guidelines set out by the Canadian Council of Animal Care.
Spinal cord dissection and electrophysiology.
Longitudinal slices of spinal cord dorsolateral white matter were prepared from adult female Wistar rats (250–300 g) as described in earlier publications from our laboratory (Agrawal and Fehlings 1997; Fehlings and Nashmi 1996, 1997; Velumian et al. 2010; Fig. 1). The animals were anesthetized with a brief exposure to halothane (1 min) followed by an injection of pentobarbital sodium (60 mg/kg body wt). Rats were intracardially perfused with ice-cold (4°C) 300 mosM artificial cerebrospinal fluid (aCSF) containing 400 μM l-ascorbic acid to protect the fragile spinal cord from free radicals during extrusion (Barabás et al. 1995; Straub et al. 2009). Rats were quickly decapitated, and the spinal cord from upper cervical to lower lumbar level was rapidly removed through hydraulic extrusion (Velumian et al. 2010) and placed into ice-cold oxygenated aCSF, where it was cut into longitudinal slices, roughly 1 mm thick (Fig. 1B). The aCSF contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1.3 MgSO4, 26 NaHCO3, and 10 glucose; pH 7.4 after bubbling with 95% O2+5% CO2 gas mixture. After dissection, the slices were stored at room temperature (∼25°C) in an oxygenated chamber for at least 1 h before use.
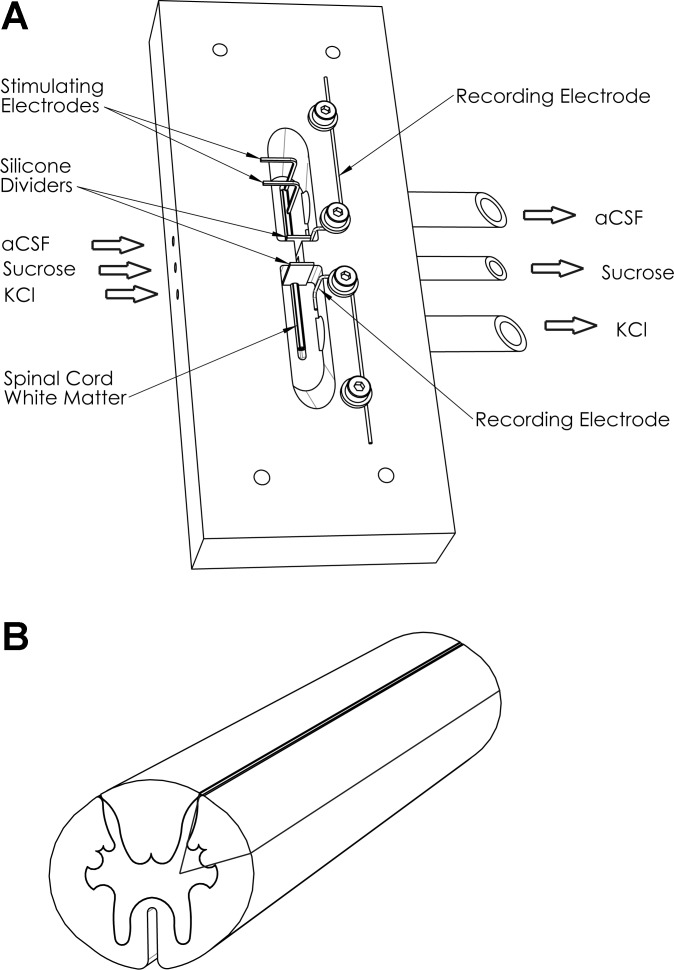
Fig. 1.
Sucrose gap setup and spinal cord slicing. A: schematic diagram of the single sucrose gap apparatus showing 3 adjacent chambers each perfused with isotonic KCl, isotonic sucrose, and artificial cerebrospinal fluid (aCSF), respectively, across which lies a piece of longitudinally sectioned spinal cord. The chambers are separated by silicone dividers that sit tightly around the white matter slice. B: schematic diagram of white matter longitudinal sectioning. Longitudinally cut slices from cervical to lumbar regions, mostly composed of white matter, would either be subjected to electrophysiological manipulation or used for RT-PCR, whereby the slice would be further sectioned as baseline, oxygen-glucose deprivation (OGD), and recovery pieces after each respective condition.
To record compound action potentials (CAPs), we used a sucrose gap technique, which provides higher-amplitude signals with better signal-to-noise signal resolution compared with other extracellular recording techniques (Mert 2007; Peasley and Shi 2002; Utzschneider at al 1991; Velumian et al. 2010, 2011). The sucrose gap apparatus modeled after previous designs published by our group (Fehlings and Nashmi 1997; Nashmi and Fehlings 2001; Sinha et al. 2006) was modified and custom designed to contain three Lexan polycarbonate chambers. The isotonic KCl and oxygenated aCSF chambers were separated by nonionic, isotonic sucrose solution of high specific resistance (for optimal electrical isolation between the isotonic KCl and aCSF compartments) (Mert 2007). Two silver wire chlorided electrodes (Ag/AgCl), placed in aCSF and KCl chambers, respectively, were used as recording electrodes; a double-insulated gold-plated copper wire electrode was used for stimulation. A single slice was placed across the three chambers of the sucrose gap apparatus and held in place with pins on a silicone layer covering the bottom of the chambers. The edges between adjacent chambers were separated with custom-made silicone dividers embalmed in Dow Corning Compound valve lubricant and sealant, and they sat tightly around the longitudinal spinal cord slice (Fig. 1A) to avoid shunting effects (Mert 2007). The slices were perfused at the rate of 3–4 ml/min with warm (35–37°C) oxygenated aCSF, isotonic KCl, and sucrose. The solutions exited the chambers via individual polyurethane tubings, thus avoiding any contact between them.
A stimulating constant current pulse (0.1 ms, 1–15 mA range) was applied every 15 s via the SIU-PSIV6 stimulus isolation unit of a Grass S88 stimulator. The recording electrodes were connected to the Axoprobe 1A amplifier headstage (Axon Instruments), and the signals were amplified 100× in DC mode, digitized via Digidata 1200, processed with pCLAMP6 software, and analyzed with pCLAMP8 software (Axon Instruments).
Oxygen-glucose deprivation.
To model ischemia, the composition of aCSF was altered to deprive white matter slices of glucose and oxygen. To maintain the 300 mosM osmolarity, 10 mM glucose was replaced with 10 mM sucrose in aCSF and the 95% O2+5% CO2 gas mixture was replaced with 95% N2+5% CO2.
Gap junction blockers.
Gap junction blockers (100 μM carbenoxolone and 300 μM 1-octanol; Sigma) were administered 30 min before, during OGD (30 min), and after OGD during the recovery period (1 h after OGD). They were added to the perfusing aCSF solution prior to the onset of the experiment involving the blockers.
Quantitative RT-PCR.
After spinal cord isolation and 1-h incubation, longitudinal white matter slices were transferred to a custom Styrofoam incubation chamber with petri dishes containing warm aCSF (35–37°C) oxygenated with a 95% O2+5% CO2 gas mixture for 30 min. One-third of the length of each slice randomly selected along the longitudinal axis was then collected for PCR analysis and homogenized in TRIzol (Invitrogen) according to the manufacturer's instructions, and the remaining two-thirds were transferred to a second chamber for 30-min OGD. After exposure to OGD, one half of the length of the tissue fragments from the OGD chamber was homogenized in TRIzol and the other half transferred back to the first incubation chamber for recovery. Upon extraction, RNA was further purified with the RNeasy Micro Kit (Qiagen) and converted to cDNA with 100–500 ng of RNA, 1 μl of oligo(dT), 1 μl of 10 mM dNTP mix, 4 μl of 5× first-strand buffer, 2 μl of 0.1 M DDT, and nuclease-free water, run on the PCR 2400 machine at 42°C for 50 min, and inactivated at 75°C for 15 min as we previously described (Eftekharpour et al. 2005).
Gene-specific gap junction primers, as well as the β-actin control (IDT; Table 1), were designed for real-time PCR with Primer Express Software (PE, Applied Biosystems) and thoroughly tested for efficiency as described previously (Eftekharpour et al. 2005; Mylvaganam et al. 2010).
Table 1.
Primers for real-time PCR (Eftekharpour et al. 2005; Karimi-Abdolrezaee et al. 2004; Mylvaganam et al. 2010)
| Gene | Primer Sequence |
|---|---|
| Cx30 | fw 5′-CTTCATCTTCCGAGTCATGATCCT-3′ |
| rev 3′-CCTGCTCATCTCCCCACACT-3′ | |
| Cx32 | fw 5′-CCTCCGGCATCTGCATTATC-3′ |
| rev 3′-GCCCGGATGATGAGGTACAC-3′ | |
| Cx36 | fw 5′-GGTGGGAGCAAGCGAGAAG-3′ |
| rev 3′-TGGAGCACCCCATTGACAA-3′ | |
| Cx43 | fw 5′-CCCCGACGACAACCAGAAT-3′ |
| rev 5′-GGCTAATGGCTGGAGTTCATG-3′ | |
| β-Actin | fw 5′-CGTGCGTGACATCAAAGAGAA-3′ |
| rev 5′-GGCCATCTCCTGCTCGAA-3′ |
PCR was conducted on the ABI Model 7900 Sequence Detector TM (Perkin-Elmer PE Biosystems). Each plate well contained 4 μl of diluted cDNA template, combined with a mixture of 1× SYBR Green PCR Master Mix—a double-stranded DNA-specific fluorophore (Molecular Probes), each primer (fw + rev) at 0.1 μM, and nuclease-free water. Each sample was tested in triplicate, and after each PCR run the product was checked for primer dimers via a heat dissociation protocol and ensured to have 1 peak on the resulting melting curve. Relative expression levels of the connexin genes were analyzed as fold changes normalized to the β-actin control with the 2−ΔΔCT method (Livak and Schmittgen 2001).
PCR was conducted on the ABI Model 7900 Sequence Detector TM (Perkin-Elmer PE Biosystems). Each plate well contained 4 μl of diluted cDNA template, combined with a mixture of 1× SYBR Green PCR Master Mix—a double-stranded DNA-specific fluorophore (Molecular Probes), each primer (F+R) at 0.1 μM, and nuclease-free water. Each sample was tested in triplicate, and after each PCR run the product was checked for primer dimers via a heat dissociation protocol and ensured to have one peak on the resulting melting curve (Eftekharpour et al. 2005). Relative expression levels of the connexin genes were analyzed as fold changes normalized to the β-actin control with the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis.
All data are reported as means ± SE. Statistical significance between groups was assessed at P < 0.05 by unpaired t-test after Mann-Whitney rank sum test for normality and equal variance with the SigmaStat 3.5 software package (Systat Software).
RESULTS
Experiments were performed at physiological temperatures (35–37°C), with the goal of discerning the effects that two prominent gap junction blockers (carbenoxolone and 1-octanol) have on spinal cord white matter function with and without ischemic injury. The 100 μM carbenoxolone and 300 μM 1-octanol doses were in line with previously established sufficient doses for blocking of gap junctions (Samoilova et al. 2003, 2008; Solomon et al. 2003; Thompson et al. 2006).
General characteristics of CAPs in spinal cord white matter before and after blockade of gap junctions.
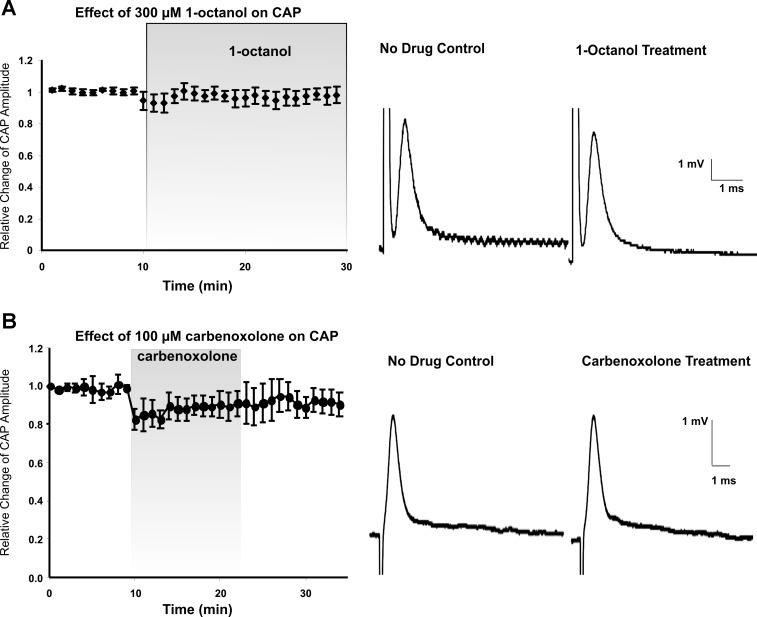
In control aCSF, CAPs recorded from spinal cord white matter had an average amplitude of 8.5 mV ± 1.9 mV (Fig. 2), which is comparable to amplitudes in the submaximal range cited previously by our laboratory with single (Eftekharpour et al. 2005; Fehlings and Nashmi 1997; Nashmi and Fehlings 2001; Sinha et al. 2006) or double (Velumian et al. 2010, 2011) sucrose gap techniques. Adding 300 μM 1-octanol or 100 μM carbenoxolone for 20 min to aCSF had no significant effect on CAP amplitude compared with control by the end of the 20-min period of drug administration (Fig. 2A, P = 0.981, n = 6; Fig. 2B, P = 0.402, n = 5).
Fig. 2.
Gap junction blockers do not change the amplitude of compound action potential (CAP) in noninjured tissue. A: relative change of CAP amplitude over time (30 min) in response to the addition of 300 μM 1-octanol (20 min). Right: sample traces of evoked CAPs prior to and during 1-octanol treatment. B: relative change of CAP amplitude over time (35 min) in response to 100 μM carbenoxolone (15 min). Right: sample traces of evoked CAPs prior to and during administration of carbenoxolone. CAP amplitudes at the end of the 20-min period of drug administration did not significantly differ from no-drug values.
CAP amplitude during OGD and effect of gap junction blockers.
During 30-min OGD in control aCSF, the CAP amplitude decreased until the signal disappeared or had a very small amplitude (Fig. 3Aa) and was only partially restored after a recovery period of 50 min.
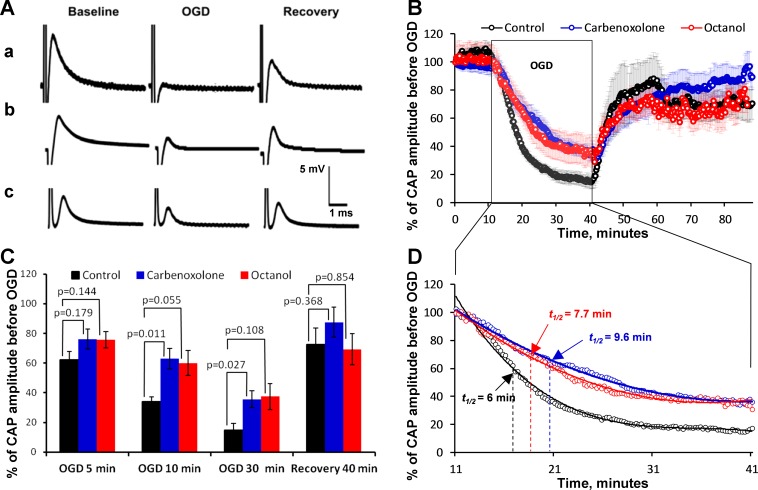
Fig. 3.
OGD-induced reduction in CAP amplitude is attenuated with gap junction blockers. A: traces of CAP recordings for control (a) and carbenoxolone (b)- and octanol (c)-treated spinal cord slices under baseline, OGD, and washout conditions. B: relative change of CAP amplitude during 30-min OGD and 1-h recovery during washout, studied in the presence of 100 μM carbenoxolone (n = 9) and 300 μM octanol (n = 10) compared with the no-drug control (n = 5) condition. Vertical bars, matching the symbols in color, represent SE. C: relative changes of CAP amplitude as % of baseline at selected time points during OGD and recovery upon washout, comparing no-drug control (n = 5), carbenoxolone (n = 9), and octanol (n = 10) conditions. Vertical bars show SE. P values of comparisons between the groups by unpaired t-test were obtained by Mann-Whitney rank sum test after passing normality and equal variance test. D: extended fragment of a portion of the plot shown in B, comparing the rates of CAP amplitude decline during OGD in no-drug control, carbenoxolone, and octanol conditions. The data were fitted with polynomial regression functions with r2 values of 0.991 for octanol, 0.994 for carbenoxolone, and 0.990 for control. The rate of CAP decline in the presence of carbenoxolone and octanol was noticeably slower compared with no-drug control, as manifested by the longer time (9.6 min and 7.7 min) of decline to 50% of total decline during the test (t1/2) compared with no-drug control (6 min).
The addition of carbenoxolone (n = 9; Fig. 3Ab) and 1-octanol (n = 10; Fig. 3Ac) significantly reduced the CAP decline during OGD. The CAPs declined rapidly within minutes after the onset of OGD and decreased to 14.8 ± 4.6% (n = 5) of the original amplitude in the control aCSF (Fig. 3B) by the end of the 30-min OGD period. Similarly, within the 30-min OGD period, the CAP decreased in the spinal cord slices bathed in aCSF with carbenoxolone or octanol. The CAP amplitude after 30 min of OGD in the presence of carbenoxolone was 35.7 ± 5.7% (n = 9) of pre-OGD amplitude, significantly different (P = 0.027) from that in the no-drug control condition. A similar tendency was observed in the presence of octanol, where the CAP amplitude after 30 min of OGD was 37.4 ± 8.9% (n = 10) of pre-OGD amplitude; however, the difference from the no-drug control condition was not statistically significant (P = 0.108).
Upon washout from 30-min OGD, the CAP amplitude began recovering once glucose and oxygen were reintroduced into the solution. This phenomenon was observed both in control aCSF and with either of the two gap junction blockers used. The recovery upon washout was not significantly different between the conditions (Fig. 3C).
Statistically significant differences between CAP amplitudes in the no-drug control and in the presence of carbenoxolone during OGD (Fig. 3C) were obvious at ∼10 min of OGD and lasted up to the end of the 30-min OGD period. Octanol had similar effects, with the time course and extent of CAP amplitude decline during OGD closely following the effects of carbenoxolone (Fig. 3D); however, the difference between corresponding points of octanol and no-drug control curves during OGD was not statistically significant. No statistically significant differences between the three curves was observed during the recovery period upon washout after OGD (Fig. 3C).
The time course of decay of CAP amplitudes in OGD in the presence of carbenoxolone and octanol differed from the no-drug control, as seen in Fig. 3D, which shows the extended part of the charts from Fig. 3B, with error bars removed for clarity. Both drugs noticeably prolonged the half-decline time of CAP amplitudes in OGD from 6.0 min in no-drug conditions to 9.6 min in the presence of carbenoxolone and to 7.7 min in the presence of octanol, suggesting that blocking gap junctions delays conduction loss during OGD.
Gap junction blockers alter Cx43 and Cx30 mRNA expression during OGD.
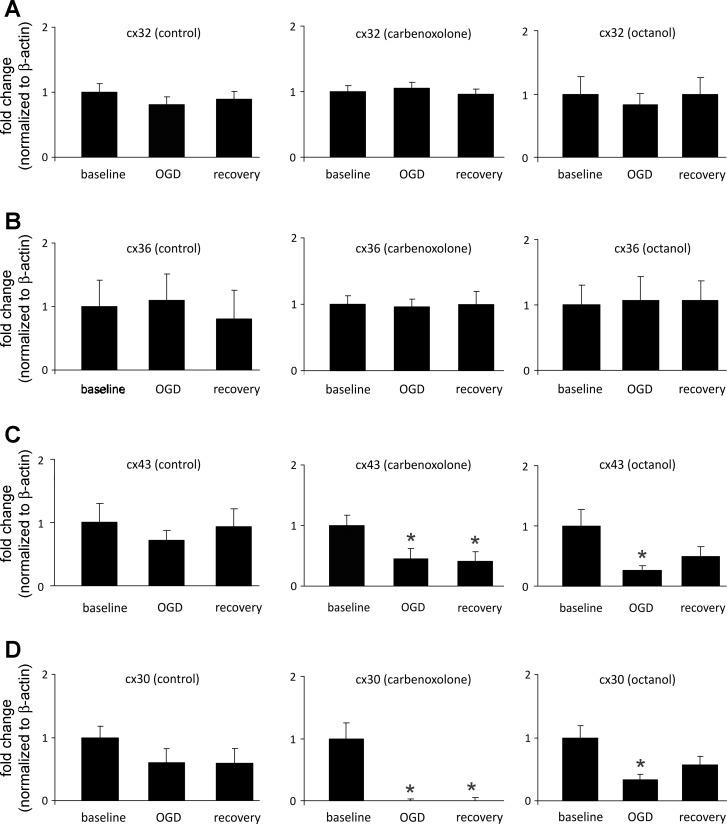
The effect of anoxic/ischemic injury and gap junction blockers on connexin mRNA expression was examined for Cx32, 36, 30, and 43; overall, it appears that OGD alone does not alter their expression (Fig. 4). Cx32 and Cx36 mRNA expression do not change with the addition of the blockers carbenoxolone and octanol. In contrast, the presence of carbenoxolone alters mRNA expression levels of Cx30 and Cx43 by causing a significant downregulation during OGD and recovery compared with baseline levels with the drug (Fig. 4, C and D). Similarly, octanol causes a downregulation of Cx30 and Cx43 during OGD (Fig. 4, C and D), while the recovery under this drug returned their mRNA to levels that were not statistically different from baseline levels.
Fig. 4.
RT-PCR comparison of fold changes during OGD and recovery periods relative to baseline conditions for control, carbenoxolone, and 1-octanol treatments. Average fold change ± SE is normalized to β-actin. A: comparison of fold changes for connexin 32 under control (n = 15, 10, 14), carbenoxolone (n = 11, 9, 10), and 1-octanol (n = 14, 9, 12) conditions. There were no significant fold change differences for all conditions. B: comparison of fold changes for connexin 36 under control (n = 11, 10, 10), carbenoxolone (n = 8, 6, 8), and 1-octanol (n = 12, 8, 12) conditions. There were no significant fold change differences for all conditions. C: comparison of fold changes for connexin 43 under control (n = 9, 11, 14), carbenoxolone (n = 13, 8, 9), and 1-octanol (n = 15, 9, 13) conditions. There was a significant downregulation of expression during OGD and recovery with carbenoxolone treatment (P < 0.05) and during OGD only with 1-octanol treatment (P < 0.05). D: comparison of fold changes for connexin 30 under control (n = 7, 5, 10), carbenoxolone (n = 6, 7, 8), and 1-octanol (n = 16, 12, 15) conditions. Similar to connexin 43, there was a significant downregulation of expression during OGD and recovery with carbenoxolone treatment (P < 0.05) and during OGD only with 1-octanol treatment (P < 0.05).
DISCUSSION
While the role of gap junctional communications in CNS injury is well studied in gray matter (Chew et al. 2010; Giaume et al. 2010), only a few studies have addressed this in the white matter. Upregulation of Cx43 was detected in traumatic SCI models (Cronin et al. 2008; Lee et al. 2005; O'Carroll et al. 2008, 2013; Theriault et al. 1997) and in cultured optic nerve after ischemia (Danesh-Meyer et al. 2008). Suppression of Cx43 upregulation with an Cx43-antisense oligodeoxynucleotide (Cronin et al. 2008; Danesh-Meyer et al. 2008) or blocking astrocytic gap junctional communication with Cx43 mimetic peptide (O'Carroll et al. 2008, 2013) demonstrated beneficial effects after injury.
This is the first study to examine the role of gap junctions in white matter ischemia, modeled in vitro by OGD in spinal cord slices. We have shown that a 30-min OGD period causes a severe decrease in the amplitude of the CAP that is not fully reversible after a 1-h recovery period. The decrease of CAPs in OGD may be due to several causes, such as initiation failures at the site of stimulation, conduction failure en route to the recording electrode, or the amplitude and synchrony of the spikes arriving at the recording site. While many of these factors remain incompletely understood, the fact that the CAP threshold does not change in OGD (see Fig. 7G in Velumian et al. 2011) apparently rules out the possible initiation failure. A reduced synchrony would be expected to be manifested in a broader shape of recorded CAPs, which is not the case, and in fact the CAP rise times do not change in OGD, while their decay becomes faster (Velumian et al. 2011). Therefore the CAP reduction in OGD can be due to either a reduced number of conducting axons or a reduced amplitude of action potentials in individual axons. Further studies are needed to differentiate between these two possibilities.
Our finding is consistent with previous studies that showed a decline in the CAP amplitude caused by 30-min ischemic periods in the CNS (Baltan 2009; Malek et al. 2003; Ouardouz et al. 2006; Peasley and Shi 2002; Tekkok et al. 2007; Utzschneider et al. 1991; Velumian et al. 2010). Typically, the CAP amplitude remains steady 30–40 min after anoxia, with shorter anoxic periods resulting in more rapid and higher recovery of the CAP magnitude (Stys 1998). A 60-min in vitro anoxia yielded a recovery to 20–30% of the baseline CAP and failed to improve further (Stys 1998); as a result, we chose a 30-min OGD model where a higher recovery of CAP was more likely.
The recovery period may give insight into the extent of the injury at a molecular level. That is, a full recovery would indicate a transient effect of OGD, whereby only electrogenesis and initiation of action potentials is affected and fully restored when the necessary ATP is available to restore the ion gradients needed to initiate action potentials (Stys et al. 1990). This is evident in a short, 10- to 15-min period of anoxia (Stys et al. 1990). Conversely, a low recovery is indicative of a large internal accumulation of Ca2+ that, even with reoxygenation and reinstitution of oxidative phosphorylation, only further damages the axons, resulting in free radical formation and activation of pathways leading to necrotic cell death (Stys 1998). The latter is evident with 60 min of anoxia. Since the recovery from 30-min OGD in our experiments was only partial, an internal accumulation of Ca2+ is likely involved in the CAP reduction during OGD.
The cellular, molecular, and ionic mechanisms of ischemic injury of white matter are complex (Bakiri et al. 2008; Matute 2011; Park et al. 2004; Ransom et al. 2011; Stirling and Stys 2010; Stys and Waxman 2004) and involve release of neurotransmitters, such as glutamate and ATP, possibly through hemichannels (half gap junctions) (Domercq et al. 2010; Ye et al. 2003). However, there have been no conclusive studies regarding the role of gap junctions and their constituent components, connexins, on axonal function after ischemic SCI.
Analysis of CAP recordings showed that there was no significant effect of either carbenoxolone or octanol on axonal signaling in normoxic spinal cord slices (Fig. 2), indicating that gap junctions do not have a detrimental function under normal conditions During OGD, carbenoxolone and octanol attenuated the decline in the CAP amplitude (Fig. 3).
The ability of gap junction blockers to attenuate CAP decline during OGD demonstrated in the present study (Fig. 3) suggests that functional gap junctions and/or hemichannels (half gap junctions) contribute to loss of axonal function during initial ischemia. Ischemia can open hemichannels, as demonstrated in pyramidal neurons (Thompson et al. 2006) and oligodendrocytes derived from optic nerves (Domercq et al. 2010), contributing to the profound ionic dysregulation and ischemic cell death directly or by inducing massive ATP release. Indeed, in the optic nerve OGD-induced ATP release was found to be reduced by carbenoxolone (Domercq et al. 2010). This study also demonstrated that ATP application caused a 50% reduction in CAP area through the activation of Ca2+-permeable ionotropic purinergic receptors P2X7 and suggested that during ischemia the ATP release, at least partly through hemichannels, and subsequent activation of these receptors are critical to white matter demise during stroke, thus pointing to a possible harmful role of gap junctions.
None of the pharmacological agents studied so far provided complete protection against white matter axonal dysfunction. Previously, it was shown that blockade of ionotropic glutamate (Tekkok et al. 2007; Tekkok and Goldberg 2001) or purinergic (Domercq et al. 2010) receptors or inhibition of histone deacetylase (Baltan et al. 2011) preserves white matter function during OGD, manifested by the delay of CAP decline and preserved residual CAP during OGD, similar to that observed in the present study. However, in contrast to these studies that also demonstrated a significant or even full recovery of axonal function during reperfusion after OGD, we did not detect any improvements in CAP recovery in the presence of gap junction blockers during reperfusion (Fig. 3). Furthermore, despite the fact that residual CAP amplitudes at the end of the OGD period were higher in the presence of carbenoxolone compared with controls (36% vs. 15% of initial pre-OGD values), recovery after OGD brought their amplitudes to the same level as in controls, suggesting that functional gap junctions and/or hemichannels are needed for improved recovery. Overall, the differences in the time course of CAP amplitude decline during OGD and its recovery during reperfusion with or without gap junction blockers suggest that the role of gap junctions may be different at different stages of ischemic insult/recovery.
It has been proposed that gap junctions play an important role in white matter, providing spatial buffering of K+ released from myelinated axons (Rash 2010) and a trophic support of the axons (Nave 2010). The importance of gap junctions in pathophysiology of myelinated axons is also evident from human disorders, such Pelizaeus-Merzbacher-like diseases or hereditary spastic paraplegia (CNS; mutations affecting Cx47) (Kleopa et al. 2010; Nave 2010; Rash 2010).
While further studies are needed to distinguish between beneficial and deleterious effects of gap junctions in OGD, it is feasible to suggest that the demand for their supporting functions in white matter dramatically increases during postischemic reperfusion. Inhibition of gap junctions at this stage can be deleterious for axon function and can contribute to the absence of improved recovery of CAP amplitude observed in our study (Fig. 3C).
The turnover of connexin proteins has previously been shown to be quite rapid (Hervé et al. 2007; Leithe and Rivedal 2007; Vanslyke and Musil 2005), with the half-life of connexin subunits ranging from 1.5 to 3 h (Hervé et al. 2007). As a result, the potential underlying changes in connexin expression and regulation during injury may affect their function, which directly impacts axonal function. However, the mRNAs may have even faster turnover rates compared with Cx protein expression. For example, some mRNAs may turn over within the range of 3–90 min (e.g., Wang et al. 2002). We have found that there are no changes in mRNA expression of Cx32, commonly found in oligodendrocytes, and Cx36, believed to be principally expressed by neurons (Nagy et al. 2004; Oguro et al. 2001; Rash et al. 2007a, 2007b; Rouach et al. 2002), after OGD either without or with the application of gap junction blockers. Interestingly, Cx30 and Cx43, unequivocally believed to be localized to astrocytes (Koulakoff et al. 2008; Nagy et al. 1997; Parys et al. 2010), appear to have reduced mRNA expression during OGD with the administration of gap junction blockers. It was previously demonstrated that Cx43 gap junctions are able to modulate their own transcription and, in the presence of the gap junction blocker oleamide, alter their gene transcription in favor of transcription repression (Stains et al. 2003).
Our data on the reduced levels of astrocytic connexin (Cx43 and Cx30) mRNA during/after OGD in the presence of gap junction blockers (Fig. 4) reveal another possible impact of gap junction blockade. If translated to reduced protein expression and impaired gap junctional communication, this could lead to further white matter pathology, as demonstrated in animal models with the deletion of astrocytic connexins Cx43 and Cx30 (Lutz et al. 2009) or astrocytic Cx43 and oligodendrocytic Cx32 (Magnotti et al. 2011).
Conclusions.
Our data provide new insights into the role of gap junctions in white matter ischemia and reveal the necessity of a cautious approach in determining detrimental or beneficial effects of gap junction blockade in white matter ischemia. Further studies should focus on deducing the interaction between astrocytic gap junctions and oligodendrocytes and neurons to better understand when blocking gap junctions during SCI is beneficial.
GRANTS
The work reported in this article was supported by funding from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Krembil Foundation, and the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.G., E.E., A.A.V., P.L.C., and M.G.F. conception and design of research; K.G. performed experiments; K.G., A.A.V., P.L.C., and M.G.F. interpreted results of experiments; K.G. prepared figures; K.G. drafted manuscript; K.G., A.A.V., P.L.C., and M.G.F. edited and revised manuscript; K.G., A.A.V., and M.G.F. approved final version of manuscript; A.A.V. analyzed data.
ACKNOWLEDGMENTS
The authors thank Dr. Marina Samoilova for her valuable advice on connexin expression and functions and the interpretation of the data.
REFERENCES
- Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na+-K+-ATPase, the Na+-H+ exchanger, and the Na+-Ca2+ exchanger. J Neurosci 16: 545–552, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci 17: 1055–1063, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix JJ. The pathophysiology of ischemic injury to developing white matter. McGill J Med 9: 134–140, 2006. [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Karadottir R, Attwell D. Glutamatergic signaling in the brain's white matter. Neuroscience 158: 266–274, 2008. [DOI] [PubMed] [Google Scholar]
- Baltan S. Ischemic injury to white matter: an age-dependent process. Neuroscientist 15: 126–133, 2009. [DOI] [PubMed] [Google Scholar]
- Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci 31: 3990–3999, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas J, Nagy E, Degrell I. Ascorbic acid in cerebrospinal fluid—a possible protection against free radicals in the brain. Arch Gerontol Geriatr 21: 43–48, 1995. [DOI] [PubMed] [Google Scholar]
- Chew SS, Johnson CS, Green CR, Danesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp Neurol 225: 250–261, 2010. [DOI] [PubMed] [Google Scholar]
- Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci 39: 152–160, 2008. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Huang R, Nicholson LF, Green CR. Connexin43 antisense oligodeoxynucleotide treatment down-regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J Clin Neurosci 15: 1253–1263, 2008. [DOI] [PubMed] [Google Scholar]
- Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 58: 730–740, 2010. [DOI] [PubMed] [Google Scholar]
- Eftekharpour E, Karimi-Abdolrezaee S, Sinha K, Velumian AA, Kwiecien JM, Fehlings MG. Structural and functional alterations of spinal cord axons in adult Long Evans Shaker (LES) dysmyelinated rats. Exp Neurol 193: 334–349, 2005. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Nashmi R. Changes in pharmacological sensitivity of the spinal cord to potassium channel blockers following acute spinal cord injury. Brain Res 736: 135–145, 1996. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Nashmi R. A novel model of acute compressive spinal cord injury in vitro. J Neurosci Methods 71: 215–224, 1997. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11: 87–99, 2010. [DOI] [PubMed] [Google Scholar]
- Hervé JC, Derangeon M, Bahbouhi B, Mesnil M, Sarrouilhe D. The connexin turnover, an important modulating factor of the level of cell-to-cell junctional communication: comparison with other integral membrane proteins. J Membr Biol 217: 21–33, 2007. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos GK, Xu XM, Hsu CY, Lu X, Sundt TM, Kouchoukos NT, Chan PH. White matter injury in spinal cord ischemia: protection by AMPA/kainate glutamate receptor antagonism. Stroke 31: 1945–1952, 2000. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Fehlings MG. Temporal and spatial patterns of Kv1.1 and Kv1.2 protein and gene expression in spinal cord white matter after acute and chronic spinal cord injury in rats: implications for axonal pathophysiology after neurotrauma. Neuroscience 19: 577–589, 2004. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Orthmann-Murphy JL, Sargiannidou I. Gap junction disorders of myelinating cells. Rev Neurosci 21: 397–419, 2010. [DOI] [PubMed] [Google Scholar]
- Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia 56: 1299–1311, 2008. [DOI] [PubMed] [Google Scholar]
- Kozoriz MG, Bechberger JF, Bechberger GR, Suen MW, Moreno AP, Maass K, Willecke K, Naus CC. The connexin 43 C-terminal region mediates neuroprotection during stroke. J Neuropathol Exp Neurol 69: 196–206, 2010. [DOI] [PubMed] [Google Scholar]
- Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L. Glia and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol 489: 1–10, 2005. [DOI] [PubMed] [Google Scholar]
- Leithe E, Rivedal E. Ubiquitination of gap junction proteins. J Membr Biol 217: 43–51, 2007. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci 19: RC16, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Stys PK. Na+-K+-ATPase inhibition and depolarization induce glutamate release via reverse Na+-dependent transport in spinal cord white matter. Neuroscience 107: 675–683, 2001. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−DeltaDeltaCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29: 7743–7752, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti LM, Goodenough DA, Paul DL. Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia 59: 1064–1074, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek SA, Coderre E, Stys PK. Aberrant chloride transport contributes to anoxic/ischemic white matter injury. J Neurosci 23: 3826–3836, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat 219: 53–64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci 26: 10984–10991, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert T. Sucrose-gap technique: advantages and limitations. Neurophysiology 39: 237–241, 2007. [Google Scholar]
- Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 119: 5193–5203, 2006. [DOI] [PubMed] [Google Scholar]
- Mylvaganam S, Zhang L, Wu C, Zhang ZJ, Samoilova M, Eubanks J, Carlen PL, Poulter MO. Hippocampal seizures alter the expression of the pannexin and connexin transcriptome. J Neurochem 112: 92–102, 2010. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev 47: 191–215, 2004. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ochalski PAY, Li J, Hertzberg EL. Evidence for the co-localization of another connexin with connexin-43 at astrocytic gap junctions in rat brain. Neuroscience 78: 533–548, 1997. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience 104: 235–51, 2001. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci 11: 275–283, 2010. [DOI] [PubMed] [Google Scholar]
- O'Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes 15: 27–42, 2008. [DOI] [PubMed] [Google Scholar]
- O'Carroll SJ, Gorrie CA, Velamoor S, Green CR, Nicholson LF. Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci Res 75: 256–267, 2013. [DOI] [PubMed] [Google Scholar]
- Oguro K, Jover T, Tanaka H, Lin Y, Kojima T, Oguro N, Grooms SY, Bennett MV, Zukin RS. Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci 21: 7534–7542, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Malek S, Coderre E, Stys PK. Complex interplay between glutamate receptors and intracellular Ca2+ stores during ischemia in rat spinal cord white matter. J Physiol 577: 191–204, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Liu Y, Fehlings MG. Changes in glial cell white matter AMPA receptor expression after spinal cord injury and relationship to apoptotic cell death. Exp Neurol 182: 35–48, 2003. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21: 754–774, 2004. [DOI] [PubMed] [Google Scholar]
- Parys B, Côté A, Gallo V, De Koninck P, Sík A. Intercellular calcium signalling between astrocytes and oligodendrocytes via gap junctions in culture. Neuroscience 167: 1032–1043, 2010. [DOI] [PubMed] [Google Scholar]
- Peasley MA, Shi R. Resistance of isolated mammalian spinal cord white matter to oxygen-glucose deprivation. Am J Physiol Cell Physiol 283: C980–C989, 2002. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Goldberg MP, Baltan S. Molecular pathophysiology of white matter anoxic-ischemic injury. In: Stroke: Pathophysiology, Diagnosis, and Management, edited by Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg MR, von K, ummer R. Philadelphia: Elsevier, 2011, p. 122–137. [Google Scholar]
- Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience 168: 982–1008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Davidson KG, Yasumura T, Kamasawa N, Nagy JI. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience 147: 938–956, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Pouliot WA, Davidson KG, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36 vs. connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience 149: 350–371, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Avignone E, Même W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell 94: 457–475, 2002. [DOI] [PubMed] [Google Scholar]
- Samoilova M, Li J, Pelletier MR, Wentlandt K, Adamchik Y, Naus CC, Carlen PL. Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem 86: 687–699, 2003. [DOI] [PubMed] [Google Scholar]
- Samoilova M, Wentlandt K, Adamchik Y, Velumian AA, Carlen PL. Connexin 43 mimetic peptides inhibit spontaneous epileptiform activity in organotypic hippocampal slice cultures. Exp Neurol 210: 762–775, 2008. [DOI] [PubMed] [Google Scholar]
- Sinha K, Karimi-Abdolrezaee S, Velumian AA, Fehlings MG. Functional changes in genetically dysmyelinated spinal cord axons of shiverer mice: role of juxtaparanodal Kv1 family K+ channels. J Neurophysiol 95: 1683–1695, 2006. [DOI] [PubMed] [Google Scholar]
- Solomon I, Chon KH, Rodriguez MN. Blockade of brain stem gap junctions increases phrenic burst frequency and reduces phrenic burst synchronization in adult rat. J Neurophysiol 89: 135–149, 2003. [DOI] [PubMed] [Google Scholar]
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem 278: 24377–24387, 2003. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med 16: 160–170, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by Kv channels: effects of glucose and PKC. Am J Physiol Cell Physiol 297: C788–C796, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. Anoxic and ischemic injury of myelinated axons in the CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metabol 18: 2–25, 1998. [DOI] [PubMed] [Google Scholar]
- Stys P, Ransom BR, Waxman SG, Davis PK. Role of extracellular calcium in anoxic injury of mammalian central white matter. Proc Natl Acad Sci USA 87: 4212–4216, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, Waxman SG. Ischemic white matter damage. In: Myelin Biology and Disorders, edited by Lazzarini RA, Griffin JW, Lassman H, Nave KA, Miller R, Trapp BD. San Diego, CA: Academic, 2004, p. 985–1007. [Google Scholar]
- Tekkok SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci 21: 4237–4248, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Ye Z, Ransom BR. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metabol 27: 1540–1552, 2007. [DOI] [PubMed] [Google Scholar]
- Theriault E, Frankenstein UN, Hertzberg EL, Nagy JI. Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J Comp Neurol 382: 199–214, 1997. [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar B. Ischemia opens neuronal gap junction hemichannels. Science 312: 924–927, 2006. [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Kocsis JD, Waxman SG. Differential sensitivity to hypoxia of the peripheral versus central trajectory of primary afferent axons. Brain Res 551: 136–141, 1991. [DOI] [PubMed] [Google Scholar]
- Vanslyke JK, Musil LS. Cytosolic stress reduces degradation of connexin43 internalized from the cell surface and enhances gap junction formation and function. Mol Biol Cell 16: 5247–5257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velumian AA, Wan Y, Samoilova M, Fehlings MG. Modular double sucrose gap apparatus for improved recording of compound action potentials from rat and mouse spinal cord white matter preparations. J Neurosci Methods 187: 33–40, 2010. [DOI] [PubMed] [Google Scholar]
- Velumian AA, Wan Y, Samoilova M, Fehlings MG. Contribution of fast and slow conducting myelinated axons to single-peak compound action potentials in rat spinal cord white matter preparations. J Neurophysiol 105: 929–941, 2011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu CL, Storey JD, Tibshirani RJ, Herschlag D, Brown PO. Precision and functional specificity in mRNA decay. Proc Natl Acad Sci USA 99: 5860–5865, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Stys PK. The Axon: Structure, Function and Pathophysiology. New York: Oxford Univ. Press; 1995. [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23: 3588–3596, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]