Abstract
Membrane preparation (sedimenting between 13,000g and 80,000g) of germinating radish seeds (Raphanus sativus L.) was active in hydrolyzing ATP and, to a lesser extent, a variety of other phosphorylated compounds. Dicyclohexylcarbodiimide (DCCD) and diethylstilbestrol significantly inhibited the ATPase activity (40%) while their effect on hydrolysis of other phosphorylated compounds was much less.
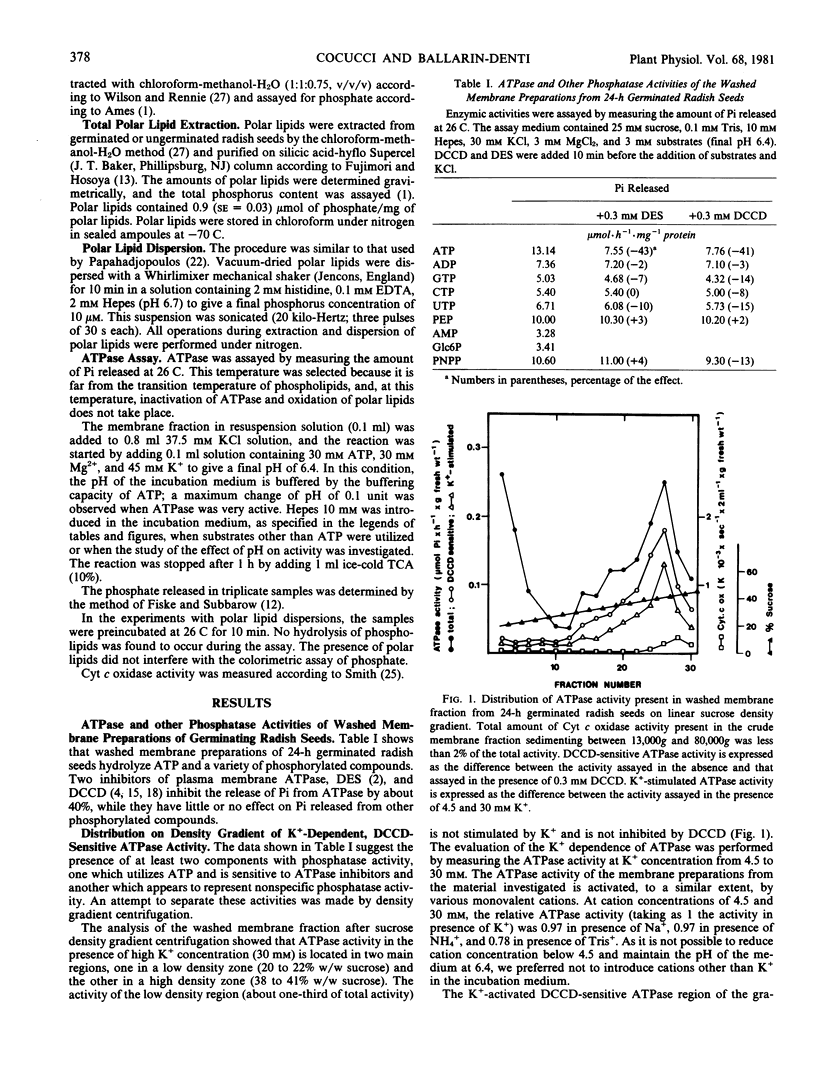
The sucrose density gradient analysis of the membrane preparation showed that the position of the DCCD-sensitive K+-dependent ATPase was similar to that found for plasma membrane of other plant material.
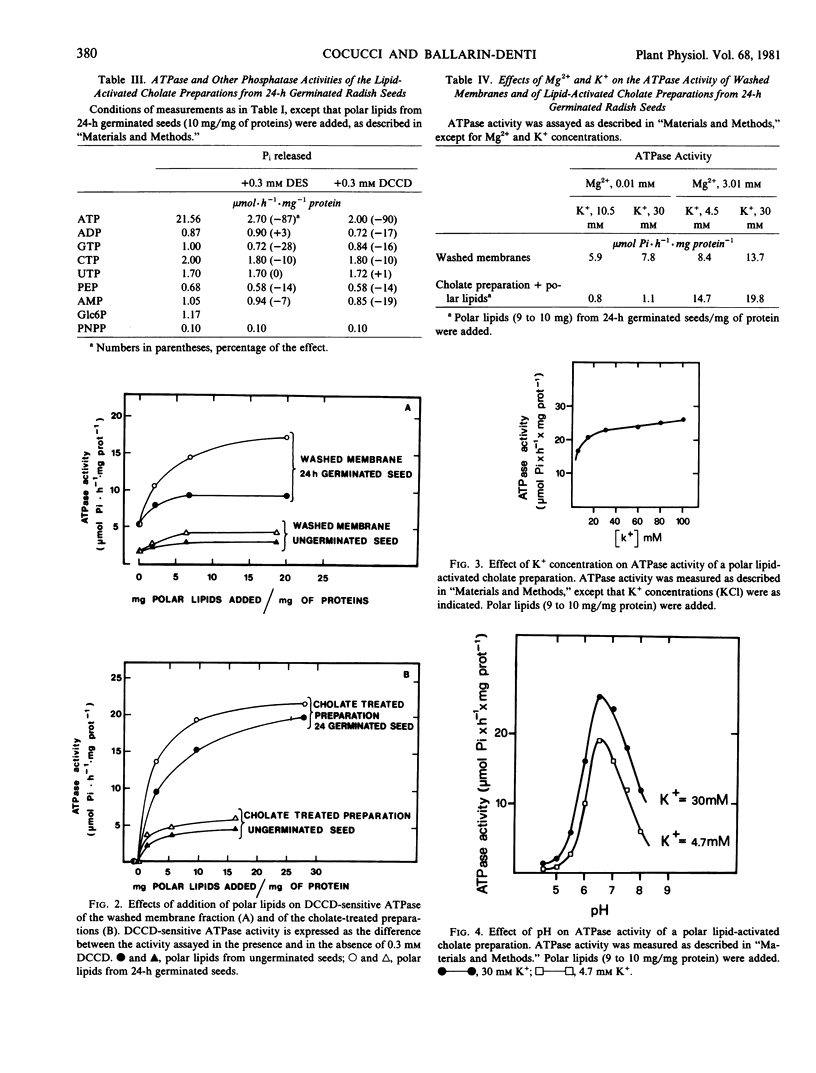
Cholate treatment of membrane preparation removes almost all phospholipids, and ATPase activity is barely detectable. However, the addition of polar lipids completely restores the ATPase activity but does not restore general phosphatase activity.
The ATPase of the polar lipids restored cholate preparation, showed a high sensitivity to DCCD and diethylstilbestrol (up to 90% inhibition), a complete dependence on Mg2+, and a strong dependence on K+ at low concentration; the pH optimum of ATPase was close to 6.5, and the Km for ATP-Mg was 0.51 millimolar. ATPase activity was much greater when polar lipids from 24-hour-germinated seeds were added.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bruni A., van Dijck P. W., de Gier J. The role of phospholipid acyl chains in the activation of mitochondrial ATPase complex. Biochim Biophys Acta. 1975 Oct 6;406(2):315–328. doi: 10.1016/0005-2736(75)90013-9. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N., Quarles R. H. N-acylphosphatidylethanolamine, a phospholipid that is rapidly metabolized during the arly germnation of pea seeds. Biochem J. 1969 Sep;114(2):265–267. doi: 10.1042/bj1140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori A., Hosoya N. Phospholipids in human young placenta. J Biochem. 1966 Apr;59(4):332–339. doi: 10.1093/oxfordjournals.jbchem.a128307. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Nir S., Oki S. Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta. 1972 Jun 20;266(3):561–583. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Purification and properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J Biol Chem. 1975 Oct 10;250(19):7917–7923. [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Phospholipids in the developing soybean seed. Plant Physiol. 1974 Nov;54(5):744–747. doi: 10.1104/pp.54.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pont J. J., van Prooijen-van Eeden A., Bonting S. L. Role of negatively charged phospholipids in highly purified (Na+ + K+)-ATPase from rabbit kidney outer medulla studies on (Na+ + K+)-activated ATPase, XXXIX. Biochim Biophys Acta. 1978 Apr 20;508(3):464–477. doi: 10.1016/0005-2736(78)90092-5. [DOI] [PubMed] [Google Scholar]


