Abstract
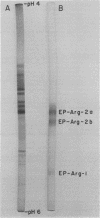
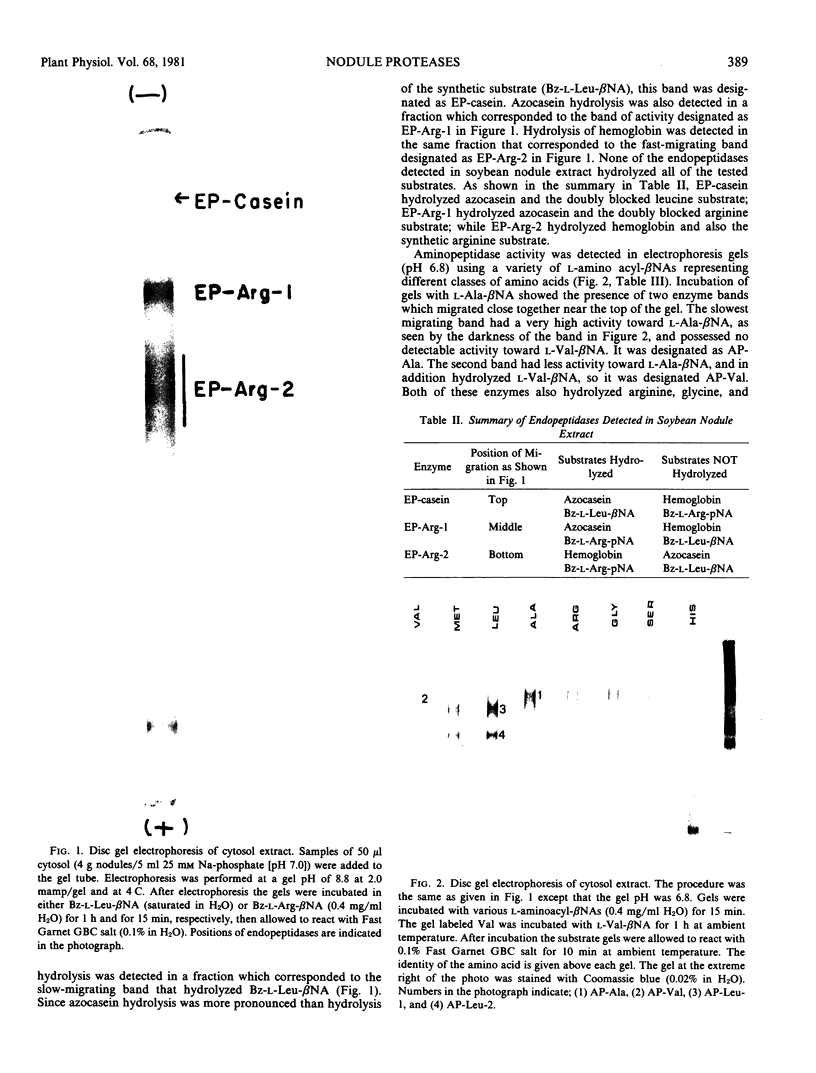
Nodule extracts prepared from Glycine max var Woodworth possessed endopeptidase, aminopeptidase, and carboxypeptidase activities. Three distinct endopeptidase activities could be resolved by disc-gel electrophoresis at pH 8.8. According to their order of increasing electrophoretic mobility, the first of these enzymes hydrolyzed azocasein and n-benzoyl-l-Leu-β-naphthylamide, while the second hydrolyzed n-benzoyl-l-Arg-β-naphthylamine (Bz-l-Arg-βNA), n-benzoyl-l-Arg-p-nitroanilide (Bz-l-Arg-pNA), and azocasein. The third endopeptidase hydrolyzed Bz-l-Arg-βNA, Bz-l-Arg-pNA, and hemoglobin. Fractions of these enzymes extracted from electrophoresis gels were shown to have pH optima from 7.5 to 9.8. All of the endopeptidases were completely inhibited by diisopropylphosphorofluoridate, demonstrating that they were serine proteases.
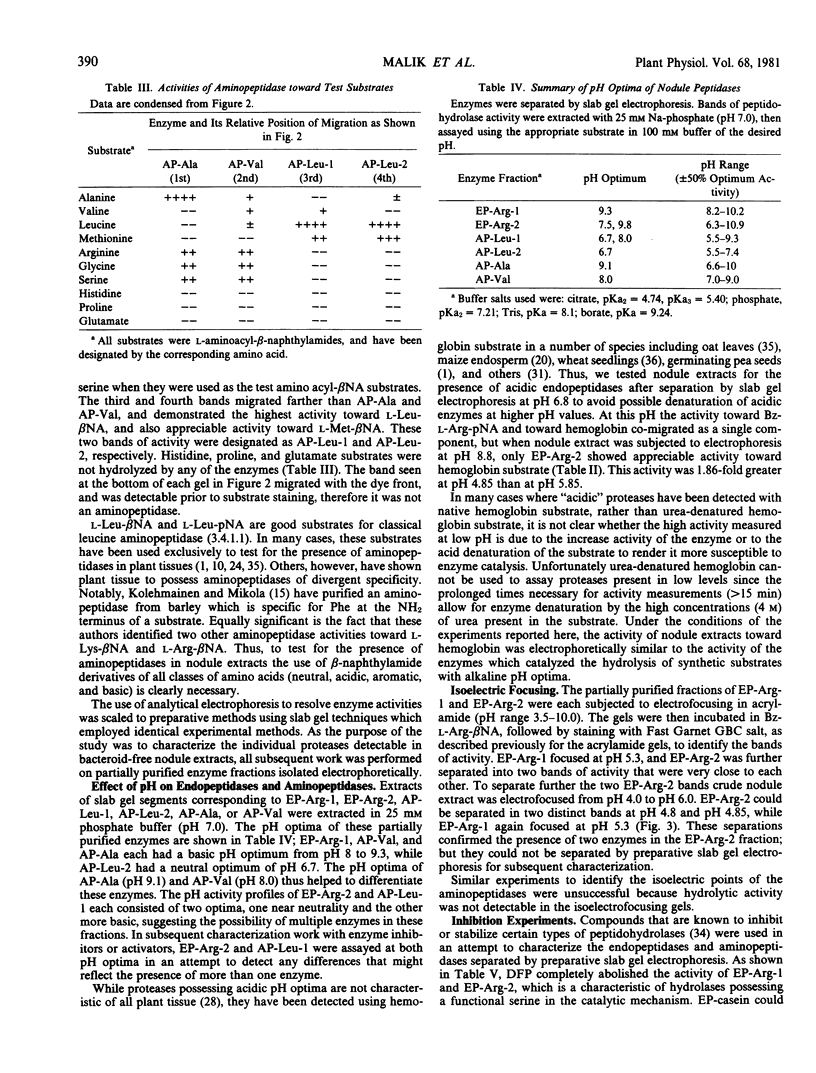
Aminopeptidase activity was measured using amino acyl-β-naphthylamides. Electrophoresis of nodule extracts at pH 6.8 resolved the aminopeptidase activity of nodule extracts into at least four fractions based on mobility and on activities toward amino acyl-β-naphthylamides. The major activity of two of the aminopeptidases was directed toward l-Leu- and l-Met-β-naphthylamide, while the other two aminopeptidases exhibited broader specificity and were capable of hydrolyzing a large number of amino acyl-β-naphthylamides. Two of the aminopeptidases extracted from electrophoresis gels were classified as thiol type enzymes, and all four aminopeptidases had neutral to basic pH optima.
Full text
PDF


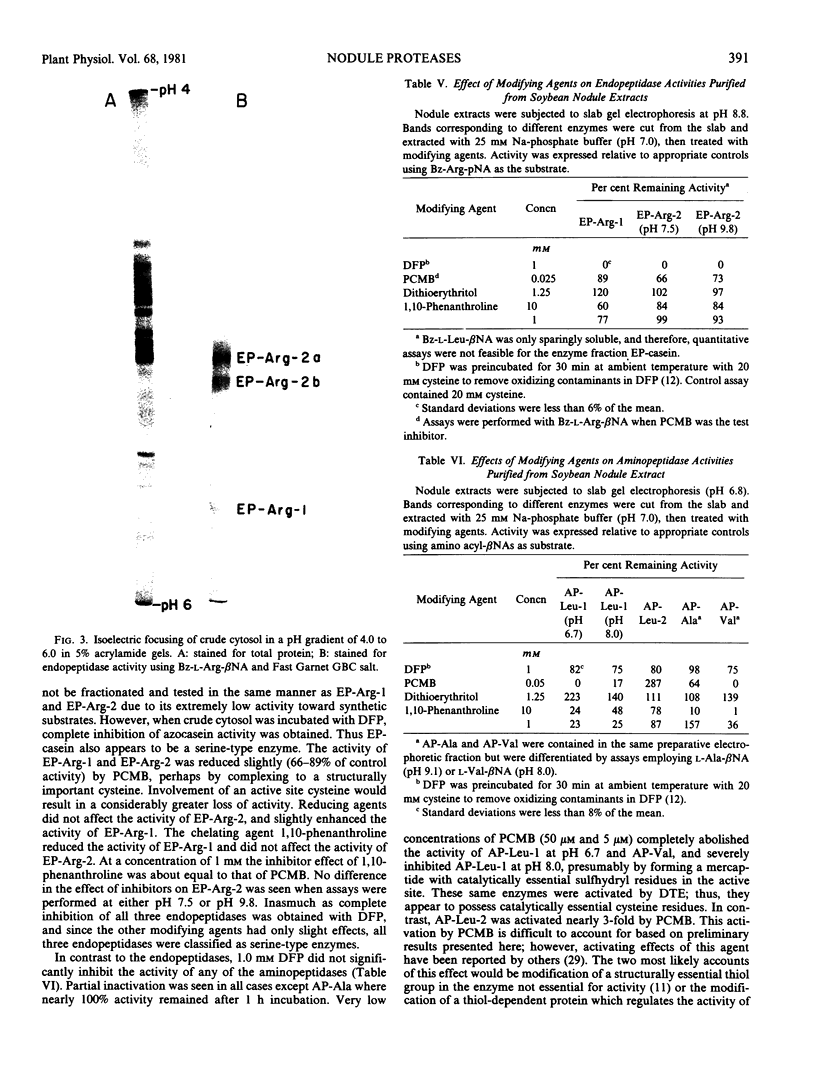

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNDY H. F. CHYMOTRYPSIN-CATALYZED HYDROLYSIS OF N-ACETYL- AND N-BENZOYL-L-TYROSINE P-NITROANILIDES. Arch Biochem Biophys. 1963 Sep;102:416–422. doi: 10.1016/0003-9861(63)90249-2. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kurachi K., Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of Senescing Oat Leaves: II. Reaction to Substrates and Inhibitors. Plant Physiol. 1978 Apr;61(4):501–505. doi: 10.1104/pp.61.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of senescing oat leaves: I. Purification and general properties. Plant Physiol. 1977 Jun;59(6):1059–1063. doi: 10.1104/pp.59.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Feller U. K., Soong T. S., Hageman R. H. Leaf Proteolytic Activities and Senescence during Grain Development of Field-grown Corn (Zea mays L.). Plant Physiol. 1977 Feb;59(2):290–294. doi: 10.1104/pp.59.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- GOULD N. R., LIENER I. E. REACTION OF FICIN WITH DIISOPROPYLPHOSPHOROFLUORIDATE. EVIDENCE FOR A CONTAMINATING INHIBITOR. Biochemistry. 1965 Jan;4:90–98. doi: 10.1021/bi00877a016. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolehmainen L., Mikola J. Partial purification and enzymatic properties of an aminopeptidase from barley. Arch Biochem Biophys. 1971 Aug;145(2):633–642. doi: 10.1016/s0003-9861(71)80023-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M. D., Blakley R. L., Panagou D. Discontinuous buffer systems for analytical and preparative electrophoresis of enzymes on polyacrylamide gel. Anal Biochem. 1972 Jan;45(1):68–85. doi: 10.1016/0003-2697(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Modulation of heart muscle mitochondrial malate dehydrogenase activity. I. Activation and inhibition by p-mercuribenzoate. Biochemistry. 1970 Jan 20;9(2):274–282. doi: 10.1021/bi00804a013. [DOI] [PubMed] [Google Scholar]
- Toncsev H. Detection of some proteolytic enzymes in polyacrylamide gels. Acta Biochim Biophys Acad Sci Hung. 1978;13(1-2):57–61. [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley C. W. Analytical fractionation of plant and animal proteins by gel electrofocusing. J Chromatogr. 1968 Aug 27;36(3):362–365. doi: 10.1016/s0021-9673(01)92959-0. [DOI] [PubMed] [Google Scholar]