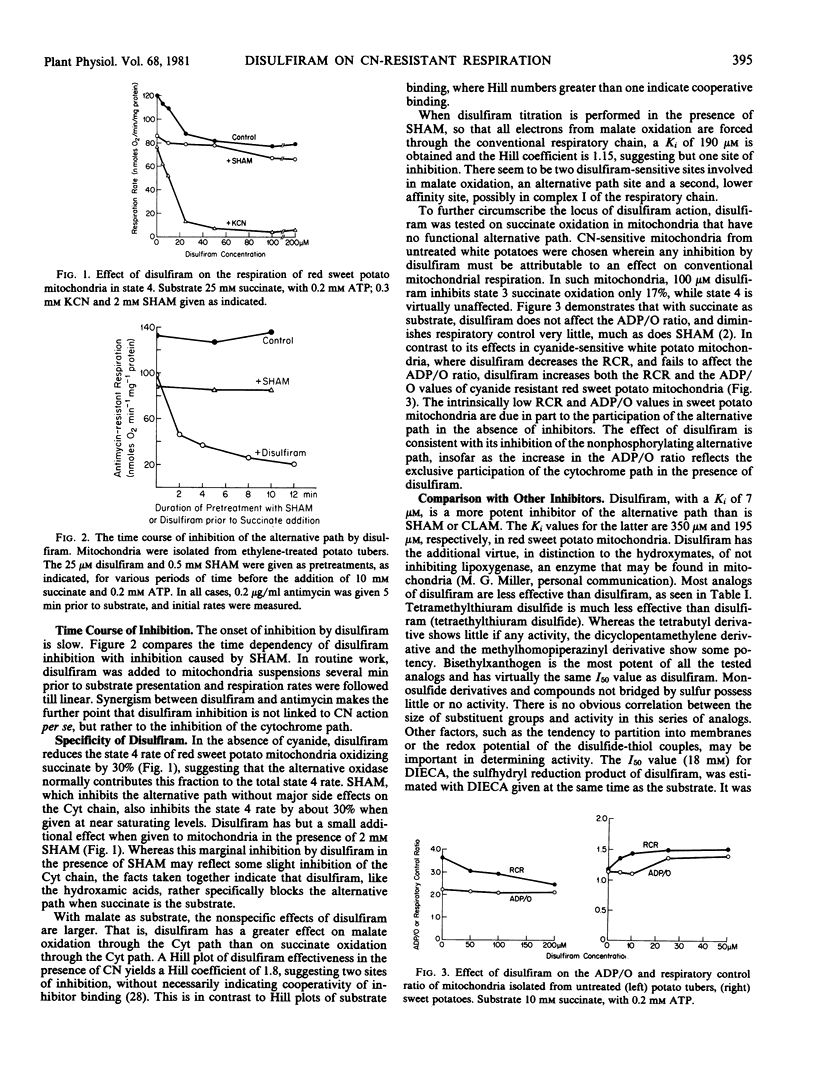
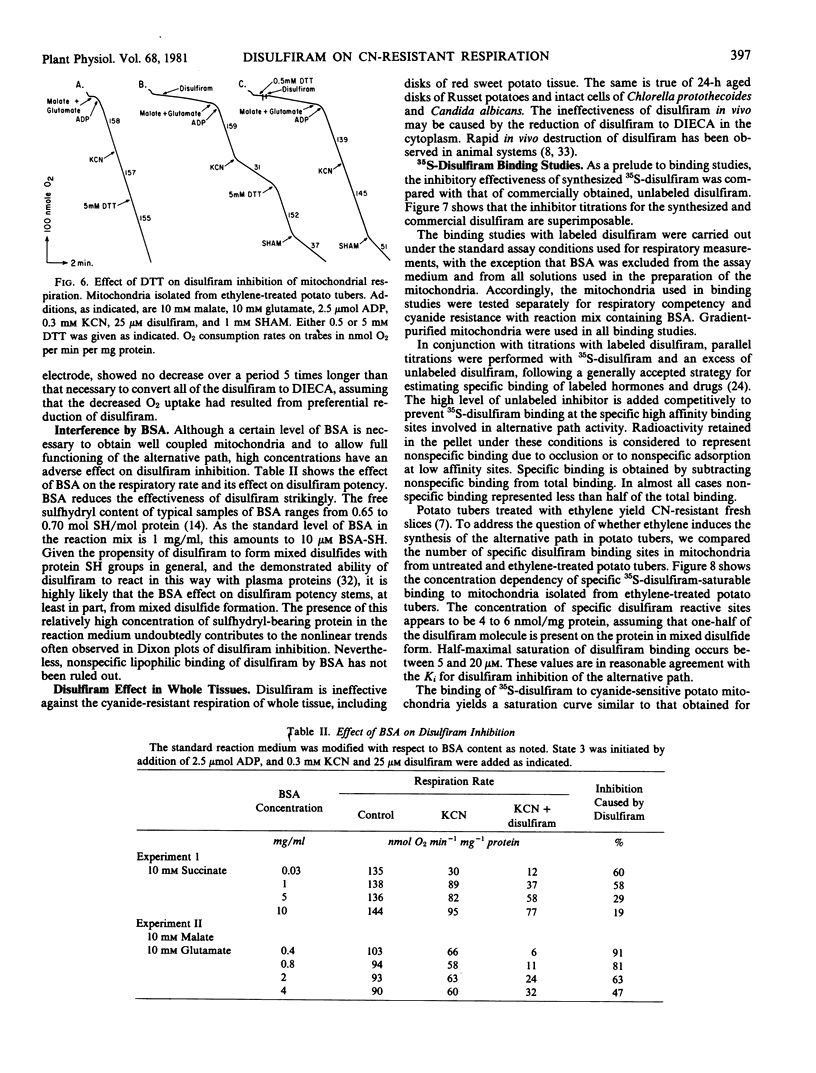
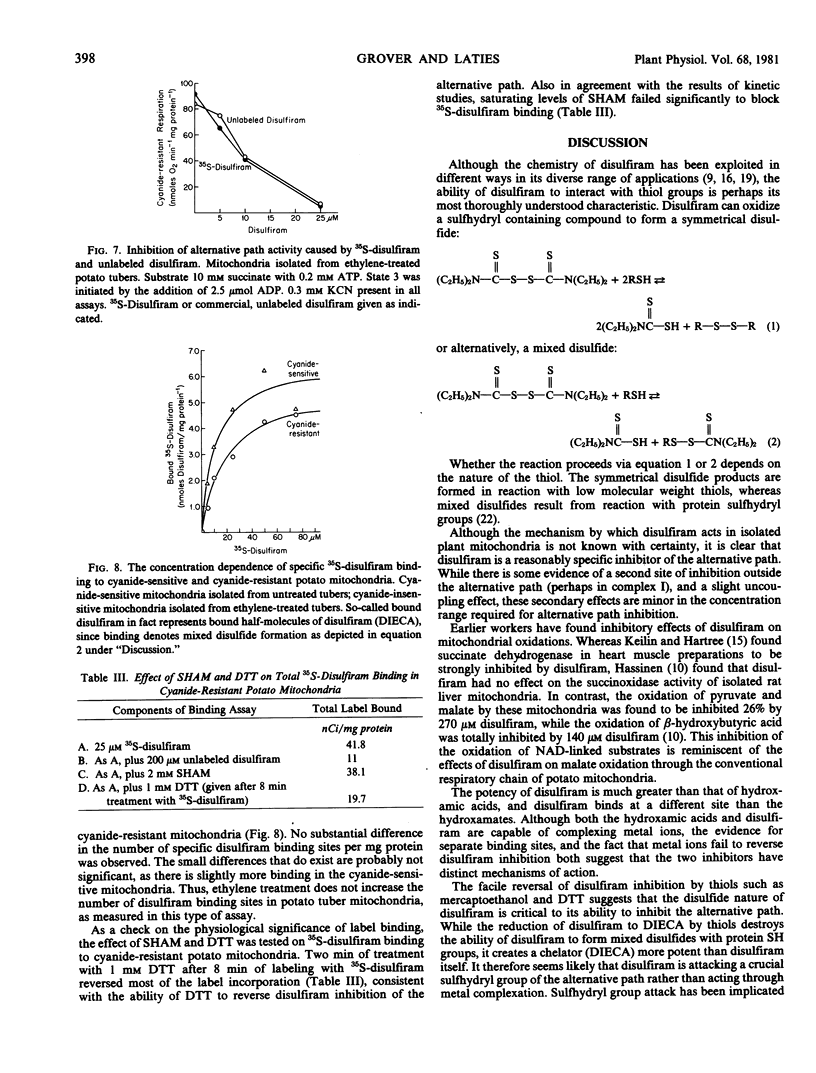
Abstract
Disulfiram (tetraethylthiuram disulfide) was found to be a potent and selective inhibitor of the alternative respiratory path of plant mitochondria. The onset of inhibition by disulfiram takes several minutes and the inhibition is not readily reversed by washing, nor by metal ions. By contrast, thiols such as dithiothreitol not only reverse, but also prevent, disulfiram inhibition. Inhibition by disulfiram and by hydroxamic acids are not mutually exclusive. Structural analogs of disulfiram are far less potent inhibitors, with the exception of bisethyl xanthogen. Inhibition is due to disulfiram, per se, and not to its reduction product, diethyldithiocarbamate, a powerful chelator. Accordingly, the inhibitory effect of disulfiram is considered to involve the formation of mixed disulfides with one or more sulfhydryl groups in the alternative path. Disulfiram does not act as an electron sink diverting electron flow from oxygen.
Disulfiram inhibition was observed only with isolated mitochondria or submitochondrial particles. In intact cells or tissues either a failure to absorb disulfiram, or its dissipation in the cytosol, precludes inhibition. In vitro, bovine serum albumin reduces disulfiram inhibition by complexing free inhibitor.
The binding of 35S-disulfiram by cyanide-resistant mitochondria displays the same kinetics as disulfiram inhibition. A comparison was made of 35S-disulfiram binding by cyanide-sensitive and cyanide-resistant potato mitochondria. Cyanide-resistant mitochondria were obtained from ethylene-treated potato tubers. Incorporation of label proved essentially the same in both types of mitochondria, suggesting that the disulfiram-sensitive component of the alternative path is present in untreated potato tubers, and is not induced by ethylene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer R. E., Peters G. A., Ikuma H. Oxido-Reduction States and Natural Homologue of Ubiquinone (Coenzyme Q) in Submitochondrial Particles From Etiolated Mung Bean (Phaseolus aureus) Seedlings. Plant Physiol. 1968 Sep;43(9):1395–1400. doi: 10.1104/pp.43.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Day D. A., Arron G. P., Christoffersen R. E., Laties G. G. Effect of ethylene and carbon dioxide on potato metabolism: stimulation of tuber and mitochondrial respiration, and inducement of the alternative path. Plant Physiol. 1978 Nov;62(5):820–825. doi: 10.1104/pp.62.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman M. D., Dodd D. E., Nolan R. J., Artman L., Hanzlik R. E. A rapid and simple radioactive method for the determination of disulfiram and its metabolites from a single sample of biological fluid or tissue. Res Commun Chem Pathol Pharmacol. 1977 Jul;17(3):481–496. [PubMed] [Google Scholar]
- GOLDSTEIN M., ANAGNOSTE B., LAUBER E., MCKEREGHAM M. R. INHIBITION OF DOPAMINE-BETA-HYDROXYLASE BY DISULFIRAM. Life Sci. 1964 Jul;3:763–767. doi: 10.1016/0024-3205(64)90031-1. [DOI] [PubMed] [Google Scholar]
- HELLERMAN L., COFFEY D. S., NEIMS A. H. STUDIES ON CRYSTALLINE D-AMINO ACID OXIDASE. I. SELECTIVE INHIBITION IN THE ACTION OF SULFHYDRYL-BINDING REAGENTS. J Biol Chem. 1965 Jan;240:290–298. [PubMed] [Google Scholar]
- Hassinen I. Effect of disulfiram (tetraethylthiuram disulfide) on mitochondrial oxidations. Biochem Pharmacol. 1966 Aug;15(8):1147–1153. doi: 10.1016/0006-2952(66)90280-2. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., De Troostember J. C., Nyns E. J. Reversal by Fe3+ of the inhibition by benzhydroxamic acid of the cyanide-insensitive respiration of Candida lipolytica. Arch Int Physiol Biochim. 1973 Dec;81(5):971–971. [PubMed] [Google Scholar]
- Janatova J., Fuller J. K., Hunter M. J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968 Jul 10;243(13):3612–3622. [PubMed] [Google Scholar]
- Kitson T. M. The effect of disulfiram on the aldehyde dehydrogenases of sheep liver. Biochem J. 1975 Nov;151(2):407–412. doi: 10.1042/bj1510407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Neims A. H., Coffey D. S., Hellerman L. A sensitive radioassay for sulfhydryl groups with tetraethylthiuram disulfide. J Biol Chem. 1966 Jul 10;241(13):3036–3040. [PubMed] [Google Scholar]
- Neims A. H., Coffey D. S., Hellerman L. Interaction between tetraethylthiuram disulfide and the sulfhydryl groups of D-amino acid oxidase and of hemoglobin. J Biol Chem. 1966 Dec 25;241(24):5941–5948. [PubMed] [Google Scholar]
- Posner B. I. Polypeptide hormone receptors: characteristics and applications. Can J Physiol Pharmacol. 1975 Oct;53(5):689–703. doi: 10.1139/y75-097. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Wiegand N. K., Blum H., Moore A. L., Bonner W. D., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978 Aug 7;525(2):325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- Solomos T., Laties G. G. The mechanism of ethylene and cyanide action in triggering the rise in respiration in potato tubers. Plant Physiol. 1975 Jan;55(1):73–78. doi: 10.1104/pp.55.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Antimycin-insensitive Cytochrome-mediated Respiration in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):238–242. doi: 10.1104/pp.62.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Membrane Lipid Breakdown in Relation to the Wound-induced and Cyanide-resistant Respiration in Tissue Slices: A COMPARATIVE STUDY. Plant Physiol. 1980 Nov;66(5):890–896. doi: 10.1104/pp.66.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]


