Abstract
The postnatal coronary vessels have been viewed as developing through expansion of vessels formed during the fetal period. Using genetic lineage tracing, we found that a substantial portion of postnatal coronary vessels arise de novo in the neonatal mouse heart, rather than expanding from pre-existing embryonic vasculature. Our data show that lineage conversion of neonatal endocardial cells during trabecular compaction generates a distinct compartment of the coronary circulation located within the inner half of the ventricular wall. This lineage conversion occurs within a brief period after birth and provides an efficient means of rapidly augmenting the coronary vasculature. This mechanism of postnatal coronary vascular growth provides avenues for understanding and stimulating cardiovascular regeneration following injury and disease.
Coronary artery disease causes myocardial infarction, the leading cause of death worldwide. How coronary arteries develop is a fundamental biological question with important ramifications for human health and disease (1). Defining the developmental programs that give rise to the coronary arteries will provide critical information for regenerative approaches to congenital and adult heart disease (2–4). Most previous studies of coronary developmental origins have focused on mid-gestation stage when coronary vessels initially form over the heart (5–11). Postnatal coronary vessels were presumed to arise from these embryonic coronary vessels, but few studies have examined postnatal coronary artery growth and this assumption has not been rigorously tested.
Embryonic coronary vessels initially form as a vascular plexus that covers the outer surface of the heart (6, 11). The underlying heart muscle is composed of compact myocardium, the free walls of the ventricular chambers, and trabecular myocardium, the finger-like projections into the chambers (12). Compact myocardium develops intramyocardial coronary vessels, whereas trabecular myocardium largely lacks intramyocardial vessels and exchanges nutrients with the surrounding blood within the ventricular chambers. During rapid postnatal ventricular growth, the trabecular myocardium undergoes “compaction” and coalesces with compact myocardium (12). This new layer of compact myocardium becomes richly supplied with coronary vessels. The developmental program for postnatal vascularization of this newly compacted myocardium was previously unknown.
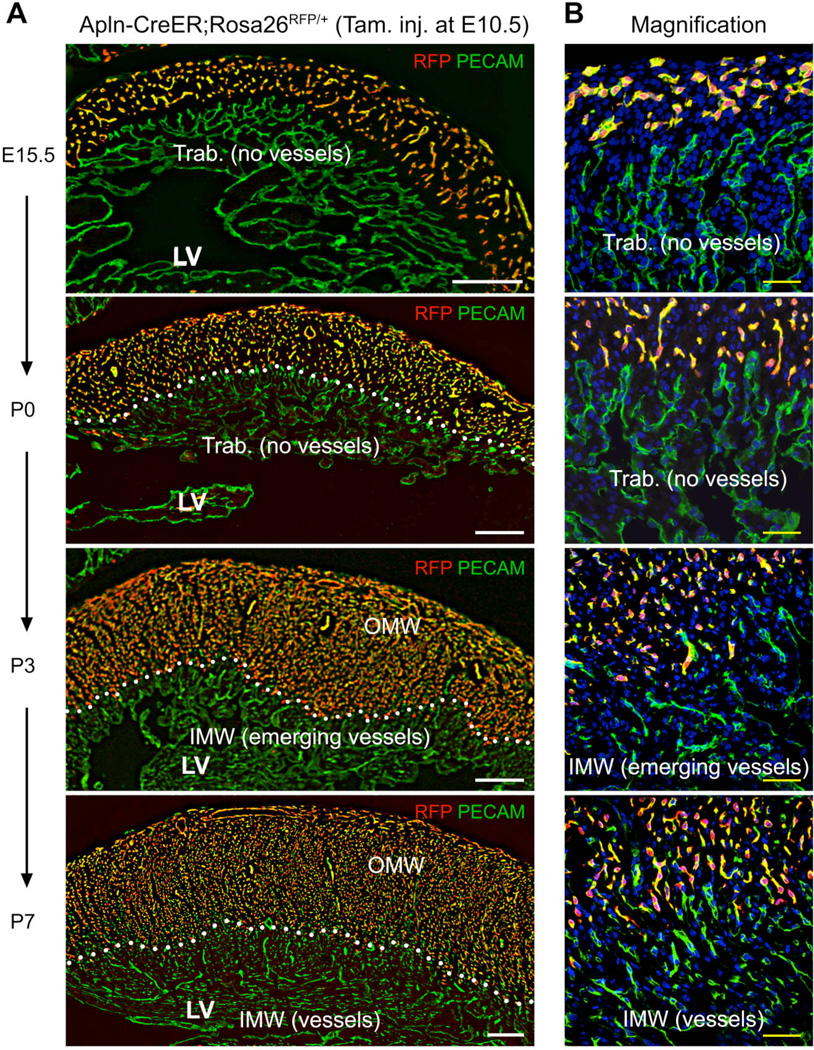
Here we used tamoxifen-inducible Cre recombinase controlled by the Apelin promoter (Apln-CreER) to permanently label embryonic vascular endothelial cells (VECs) of coronary vessels (11). A single tamoxifen injection at mouse embryonic day (E) 10.5 induced recombination of Cre reporter alleles, indelibly labeling early coronary sprouts and their progeny (11). This treatment allowed us to almost completely label coronary vessels in the embryonic ventricle wall at E15.5 (Fig. 1). We designated this coronary vascular population (CVP) labeled by Apln-CreER activation at E10.5 as the 1st CVP. We followed 1st CVP fate in postnatal day (P) 0 to P7 neonatal hearts and found that labeled coronary vessels were confined to the outer half of the myocardial wall at P3 and P7 (Fig. 1). In neonatal hearts, progressive myocardial compaction was accompanied by accumulation of unlabeled coronary vessels in the inner half of the myocardial wall at P3 and P7 (Fig. 1). These unlabeled PECAM+ cells were bona fide coronary vessels since they were positive for the coronary VEC marker APLN (6) and FABP4 (13) (fig. S1–S3). These data demonstrate that the newly formed coronary vessels in the inner myocardial wall of neonatal heart are not derivatives of the 1st CVP, indicating that they form de novo from an alternative source.
Fig. 1. Derivatives of embryonic coronary vessels do not expand into the inner myocardial wall of the neonatal heart.
(A, B) Embryonic coronary vessels were RFP-labeled by tamoxifen treatment of Apln-CreER;Rosa26RFP/+ embryos at E10.5. In the neonatal heart, these VECs, derived from embryonic coronary vessels, occupied the outer portion of the compact myocardium, defined as the Outer Myocardial Wall (OMW). The remaining, RFP negative portion of the compact myocardium was defined as the Inner Myocardial Wall (IMW). White dotted lines highlight the inner border of left ventricular myocardium occupied by embryonically labeled vessels. From P0 to P7, Trabecular myocardium (Trab.) transformed into compact myocardium where new coronary vessels arise. White bar = 200 µm; yellow bar = 50 µm. LV, left ventricle.
In order to get a holistic view of coronary vascular development of the entire heart, we fate mapped coronary VECs by Apln-CreER labeling at different times throughout heart development. Coronary vessels temporally emerged in three waves (fig. S4). By E10.5, the 1st CVP had emerged in the first wave and formed the coronary vessels of the fetal ventricular free wall (fig. S5A–5B) and postnatal outer myocardial wall (fig. S4C). However, very few VECs of the ventricular septum were labeled by E10.5 tamoxifen induction (fig. S4B and fig. S5B). Tamoxifen administration at E13.5 identified a second wave of coronary vessel development that formed most of the robust vascular supply of the interventricular septum during septal myocardial compaction (fig. S4D and fig. S5C) (12). Labeling of these cells by tamoxifen at E13.5 but not E10.5 therefore indicates de novo formation of these septal vessels from a source other than the 1st CVP. The third wave occurred in the neonatal period, and similar to the second wave, its emergence also accompanied trabecular compaction (fig. S4G). This neonatal wave of de novo vessel formation occurred in the inner myocardial wall and remained spatially segregated from the coronary vessels formed by the first and second wave (fig. S4H).
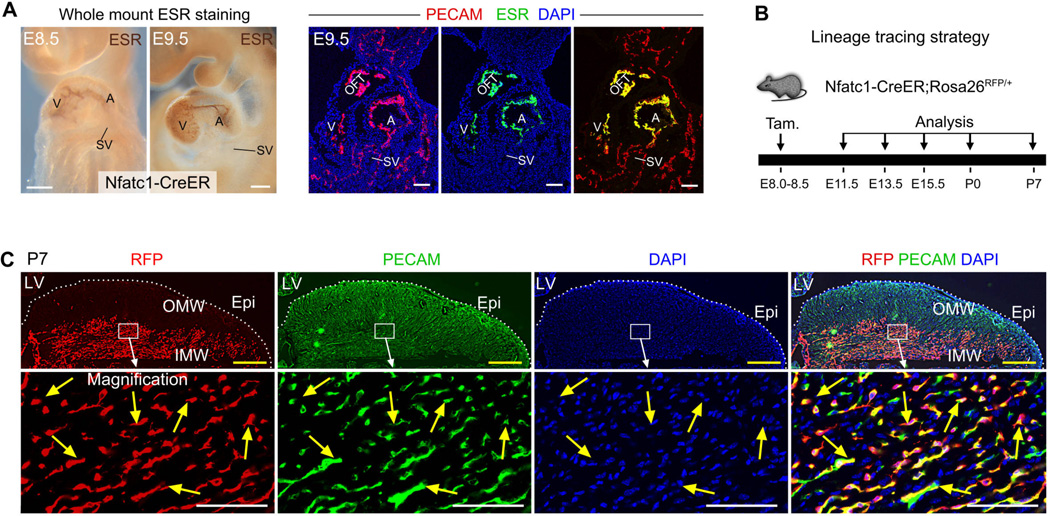
We next sought to define the developmental origin for the de novo-formed vessels that did not originate from the 1st CVP. We generated inducible Nfatc1-CreER;Rosa26RFP/+ mice (fig. S6A and S6B), in which Nfatc1 regulatory elements direct selective expression of CreER to the endocardium (14). Staining of estrogen receptor (ESR) showed expression of CreER in atrial and ventricular endocardial cells in early developing heart (Fig. 2A and fig. S6C, S6D). We administered tamoxifen at E8.0–E8.5 to label E8.5–E9.5 endocardial cells prior to the formation of coronary vessels (Fig. 2B), and analyzed embryos at multiple subsequent time points. Sinus venosus endocardium did not express CreER and was not labeled (SV, Fig. 2A and fig. S7A). Nfatc1-CreER specifically labeled endocardial cells but not coronary VECs in the myocardial free wall (fig. S7B–S7C). This result of inducible genetic labeling driven by Nfatc1-CreER differed from a previous report using constitutively active Nfatc1-Cre (see Supplementary text). At P0, Nfatc1-CreER labeled 72.2% of neonatal ventricular endocardial cells and did not significantly label free wall VECs (fig. S7D and S7E). In P7 hearts, 76.7 ± 5.4% of VECs in the inner myocardial wall expressed the RFP lineage tracer (Fig. 2C). A subset of non-labeled VECs in the inner myocardial wall may arise from embryonic vessels or epicardium. In contrast to the inner myocardial wall, fewer VECs of the outer myocardial wall were labeled by Nfatc1-CreER (Fig. 2C). Together, these data indicate that inner myocardial wall VECs arise by lineage conversion of neonatal endocardial cells during trabecular compaction.
Fig. 2. Neonatal endocardial cells contribute to the de novo-formed coronary vessels in the inner myocardial wall.
(A) CreER was expressed in atrial and ventricular endocardium but not sinus venosus at E8.5 and E9.5. CreER was visualized by staining for the estrogen receptor (ESR) portion of the CreER fusion protein. V, ventricle; A, atrium; OFT, outflow tract; SV, sinus venosus. (B) Lineage tracing strategy using Nfatc1-CreER;Rosa26RFP/+ line. Tamoxifen was injected at E8.0 to E8.5, and hearts were collected at later stages. (C) Immunostaining of genetic marker RFP and endothelial cell marker PECAM on P7 heart sections. Nfatc1-CreER labeled endocardial cells contribute to the majority of VECs (76.7 ± 5.4%, yellow arrows) in the inner myocardial wall (IMW), but not VECs in the outer myocardial wall (OMW). White bar = 100 µm, yellow bar = 500 µm. 3 – 5 embryos were examined at each time point.
We also investigated the contribution of endocardial cells labeled by Nfatc1-CreER (pulse activated by tamoxifen at E8.0–E8.5) to VECs of the interventricular septum. In both embryonic and neonatal hearts, Nfatc1-CreER labeled the majority of coronary VECs within the interventricular septum (fig. S6E). These data indicate that the interventricular septum, like the inner myocardial wall, is vascularized by lineage conversion of endocardial cells to coronary VECs.
The inverse and nearly exclusive relationship between the Apln-CreER and Nfatc1-CreER fate maps was striking (fig. S8A). This result indicates that the postnatal coronary vasculature originates from two distinct coronary vascular populations (CVPs) that arise through different developmental mechanisms and remain spatially segregated in their location. The 1st CVP, labeled by Apln-CreER induced with tamoxifen at E10.5, derives from sub-epicardial precursors that generate the initial coronary vascular plexus of the fetal hearts (6, 11) and ultimately gives rise to the VECs of the outer myocardial wall of the neonatal hearts (Fig. 1). Nfatc1-CreER labels ventricular endocardial cells, which give rise to a 2nd CVP that ultimately irrigates the interventricular septum and VECs of the inner myocardial wall of the neonatal hearts (fig. S6E and Fig. 2C).
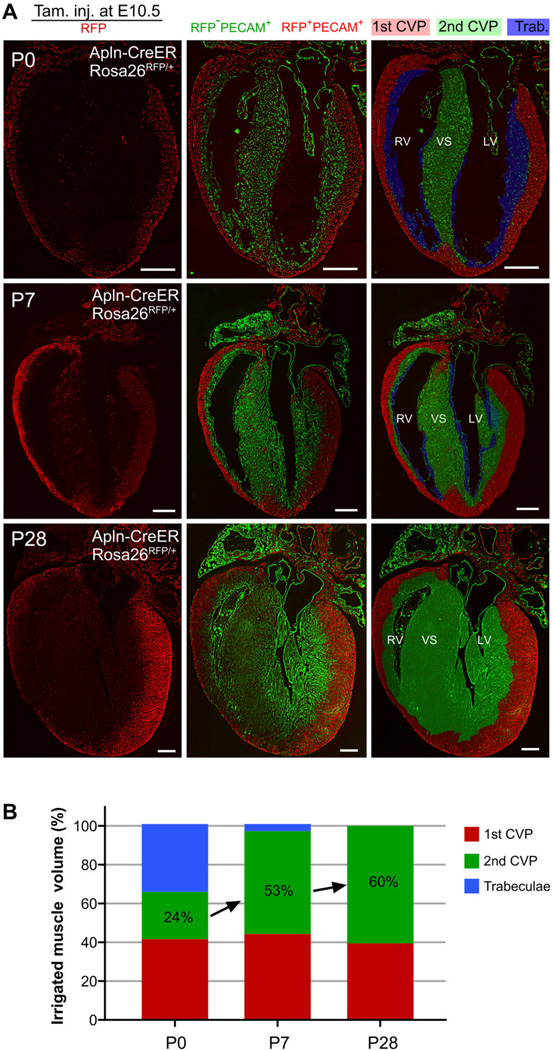
We next measured the relative vascular territory occupied by 1st and 2nd CVPs in adult heart ventricle. Since labeling efficiency was higher using Apln-CreER than Nfatc1-CreER, we used Apln-CreER labeling for quantification of the relative myocardial volume irrigated by 1st CVP (RFP-labeled) and 2nd CVP (unlabeled; fig. S8B and S8C). We examined serial sections spanning the entire heart at P0, P3, P7, P28 and P63 (Fig. 3A and Fig. S8–S9). At P0, the 1st CVP existed in 42% of the ventricular myocardium, whereas the 2nd CVP was confined to the ventricular septum and supplied 24% of the ventricle myocardium (Fig. 3B). Trabeculae, lacking intramyocardial vessels, were still present along the luminal surface of the ventricles, and accounted for the remaining 34% of the ventricular myocardium (Fig. 3B). By P7, trabecular myocardium transformed into compact myocardium, which was populated by the 2nd CVP (Fig. 3B). In the P28 and P63 adult heart, a strong boundary was maintained at the interface of the 1st and 2nd CVPs throughout entire heart (Fig. 3A, fig. S9 and S8E). These data indicate that the vascular territory supplied by these developmentally distinct CVPs remain spatially segregated in the adult heart, and that expansion of the 2nd CVP accounts disproportionately for postnatal coronary vascular expansion (fig. S10).
Fig. 3. 1st and 2nd CVP irrigate mutually exclusive regions of the myocardium of the adult heart.
(A) Long axis sections of mouse heart delineating the temporal evolution of 1st CVP (red), 2nd CVP (green), and trabecular regions (blue) during the neonatal to adult heart transition. The 1st CVP was defined as the vessels genetically labeled (PECAM+;RFP+, pseudo color red) by tamoxifen treatment of Apln-CreER;Rosa26RFP/+ embryos at E10.5. The 2nd CVP was defined as the remaining unlabeled intramyocardial vessels (PECAM+;RFP−, pseudo coloar green). Trabecular myocardium was identified as the myocardium lacking intramyocardial vessels (PECAM−;RFP−). Shading in the right column indicates regions annotated as occupied by 1st CVP, 2nd CVP or trabecular myocardium based on staining patterns. Images are representative of 3 - 6 hearts for each time point. White bar = 0.5 mm. (B) Relative ventricular myocardial volume occupied by 1st CVP, 2nd CVP or trabecular myocardium during postnatal heart growth. Note the rapid expansion of the myocardium irrigated by 2nd CVP and diminution of the trabecular myocardium between P0 – P7. n = 3 – 6 for each time point.
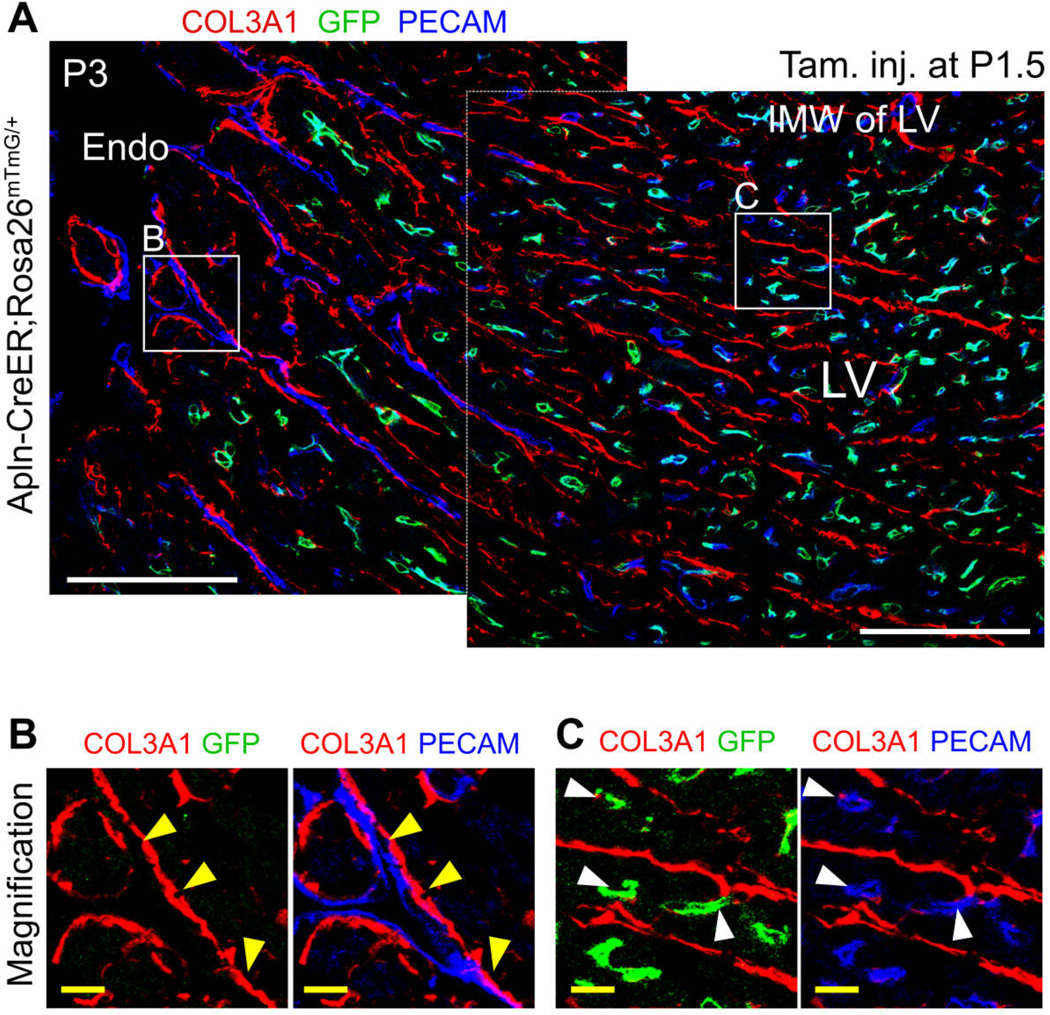
To begin to understand how neonatal endocardial cells transform from a single sheet lining the heart lumen into many tube-shaped blood vessels, we observed their association with the basement membrane of trabecular myocardium during myocardial compaction. COL3A1, an extracellular matrix protein, is part of the trabecular basement membrane located just beneath the endocardial layer at P0. During subsequent myocardial compaction, COL3A1 was not degraded, leaving a historical mark of the remodeled trabecular protrusions (fig. S11A). Endocardial cells in the trabecular protrusions resided on the basement membrane at P0 (fig. S11B–C). However, at P7, endocardial cells and their derivatives (labeled by Tie2-Cre or VE Cad-CreER) were no longer aligned with COL3A1 strands but rather took up intramyocardial positions (P7, fig. S11B–S11C). To better visualize the process by which P0 endocardium transforms into intramyocardial VECs at P7, we studied an intermediary stage (P3) when hearts contain both un-remodeled trabeculae (Fig. 4B) and actively compacting regions (Fig. 4C). We labeled developmental intermediates by treating Apln-CreER;Rosa26mTmG/+ mice with tamoxifen at P1.5 (Fig. 4A). Trapped endothelial cells remote from the basement membrane adopted the morphology of individual capillary-like cells and were marked by the VEC genetic lineage tracer (GFP+; Fig. 4C), whereas endothelial cells facing the ventricular lumen and residing on the basement membrane retained sheet-like morphology and did not express VEC lineage tracer, consistent with endocardial identity (Fig. 4B). These observations suggest that myocardial compaction traps sheets of endocardial cells, which convert to the VEC lineage and translocate to an intramyocardial location.
Fig. 4. Neonatal endocardial cells were trapped in the inner myocardial wall.
(A) Coronary vessels forming in the myocardial wall were labeled by tamoxifen treatment of Apln-CreER;Rosa26mTmG/+ at P1.5 and analyzed at P3. Region (B) represents the luminal surface and contains un-remodeled trabecular myocardium that persists at this stage. Yellow arrowheads indicate PECAM+ endothelial cells that are not labeled by Apln-CreER and that reside on the basement membrane, indicative of endocardial cells. Region (C) represents the inner myocardial wall, containing PECAM+ endothelial cells that are labeled by Apln-CreER and that reside within myocardium rather than on COL3A1 basement membrane, identifying them as tube-like coronary VECs (white arrowheads). White bar = 100 µm, yellow bar = 10 µm.
We investigated conditions that might promote endocardium to VEC transition in the neonatal heart. Hypoxyprobe, a hypoxia-sensitive chemical probe, indicated that rapid expansion of the compact myocardium by trabecular coalescence in the first several postnatal days of life creates a hypoxic environment within the inner myocardial wall (fig. S12). Expression of HIF1α and VEGFa, genes known to be upregulated by hypoxia, increased in the inner myocardial wall of the P1 and P3 neonatal hearts (fig. S12B–C). This corresponds to the region in which we observed endocardial to VEC lineage conversion, suggesting that hypoxia and its resulting upregulation of the key angiogenic factor VEGFa contribute to this process.
Our work reveals a mechanism by which trabecular coalescence and endocardial to VEC lineage conversion drive vascular expansion in the postnatal heart. Why does coronary vascular growth in this setting rely on this alternative mechanism, rather than occurring through more typical angiogenic sprouting from pre-existing vessels? The transition from fetal to postnatal circulation acutely increases the hemodynamic burden on the left ventricle. To accommodate this increased workload, we reason that mammals developed trabecular myocardium as a reservoir of new cardiomyocytes that is quickly recruited following birth through myocardial compaction to increase neonatal left ventricular mass. In addition to cardiomyocytes, this myocardial reservoir also contains coronary vessel precursors in the form of endocardial cells. Trabeculae coalesce during neonatal myocardial compaction causes regional hypoxia that stimulates the trapped neonatal endocardial cells to form the vascular supply for the newly compacted myocardium. This mechanism likely allows more rapid vascular and myocardial growth than angiogenic sprouting of the 1st CVP from the periphery. Understanding this endogenous mechanism for rapidly developing a functional vascular supply has important implications for cardiac diseases and cardiac regenerative medicine (4).
Supplementary Material
Acknowledgments
We thank K. Red-horse and N. Jin for insightful discussions and R. Adams, H. Zeng, Z. Yang, T. Quertermous, J. Rossant and A. Nagy for mouse strains. This work was supported by National Basic Research Program of China (2012CB945102 and 2013CB945302), National Natural Science Foundation of China (91339104, 31271552, 31222038, 1301188), Chinese Academy of Sciences (No.874 and KSCX2-EW-R-09), Shanghai Pu Jiang Grant (11PJ1411400), AstraZeneca, SA-SIBS Scholarship, Postdoc Fund (SIBS-2013KIP311, China-2013M541561), NIH (2 R01 HL094683) and American Heart Association Established Investigator Award to WTP.
Footnotes
Supplementary Materials:
Materials and Methods
Figs. S1 to S12
References (15–25)
References and Notes
- 1.Riley P, Smart N. Cardiovasc Res. 2011;91:260. doi: 10.1093/cvr/cvr035. [DOI] [PubMed] [Google Scholar]
- 2.Yi B, Wernet O, Chien K. J Clin Invest. 2010;120:20. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smart N, et al. Nature. 2007;445:177. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 4.Porrello E, et al. Science. 2011;331:1078. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett HS. Am. J. Anat. 1936;60:27–53. [Google Scholar]
- 6.Red-Horse K, Ueno H, Weissman I, Krasnow M. Nature. 2010;464:549. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz Tamar C, et al. Developmental Cell. 2012;22:639. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu B, et al. Cell. 2012;151:1083. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley P. Nature. 2010;464:498. doi: 10.1038/464498a. [DOI] [PubMed] [Google Scholar]
- 10.del Monte G, Harvey Richard P. Cell. 2012;151:932. doi: 10.1016/j.cell.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Tian X, et al. Cell Res. 2013;23:1075. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedmera D, et al. Anat Rec. 2000;258:319. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Elmasri H, et al. FASEB J. 2009;23:3865. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B, et al. Development. 2005;132:1137. doi: 10.1242/dev.01640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.