Abstract
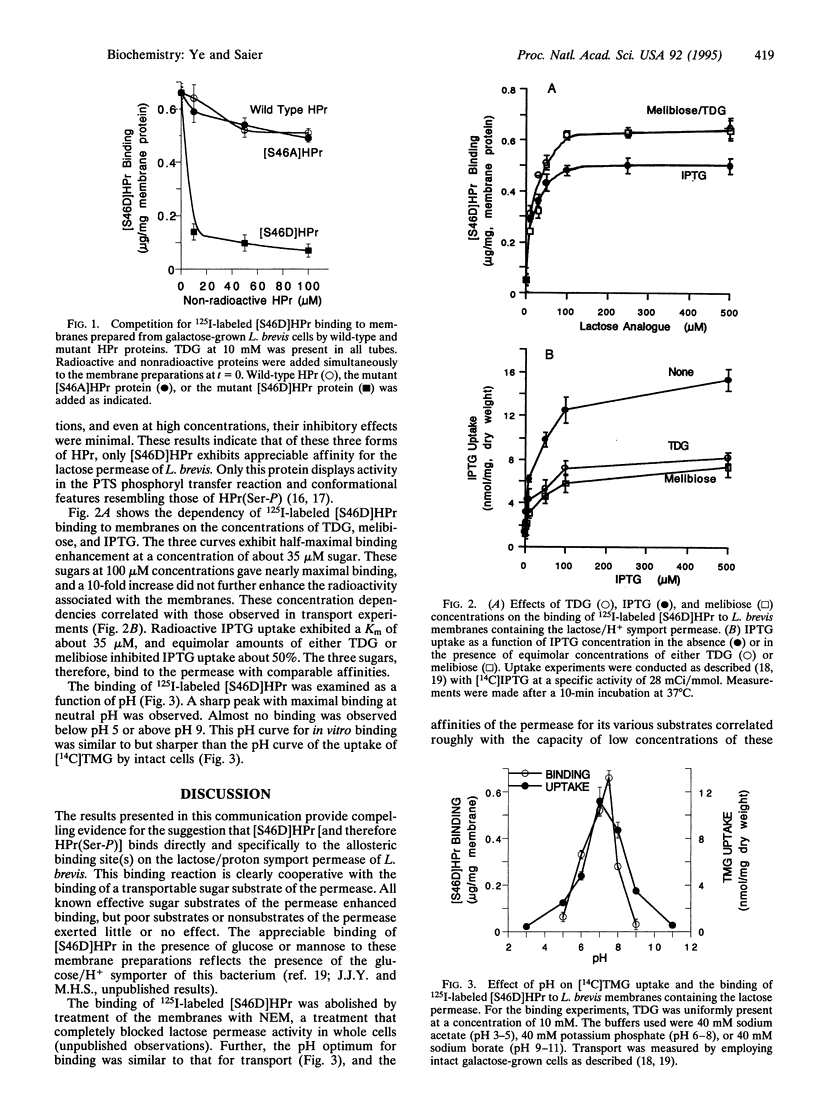
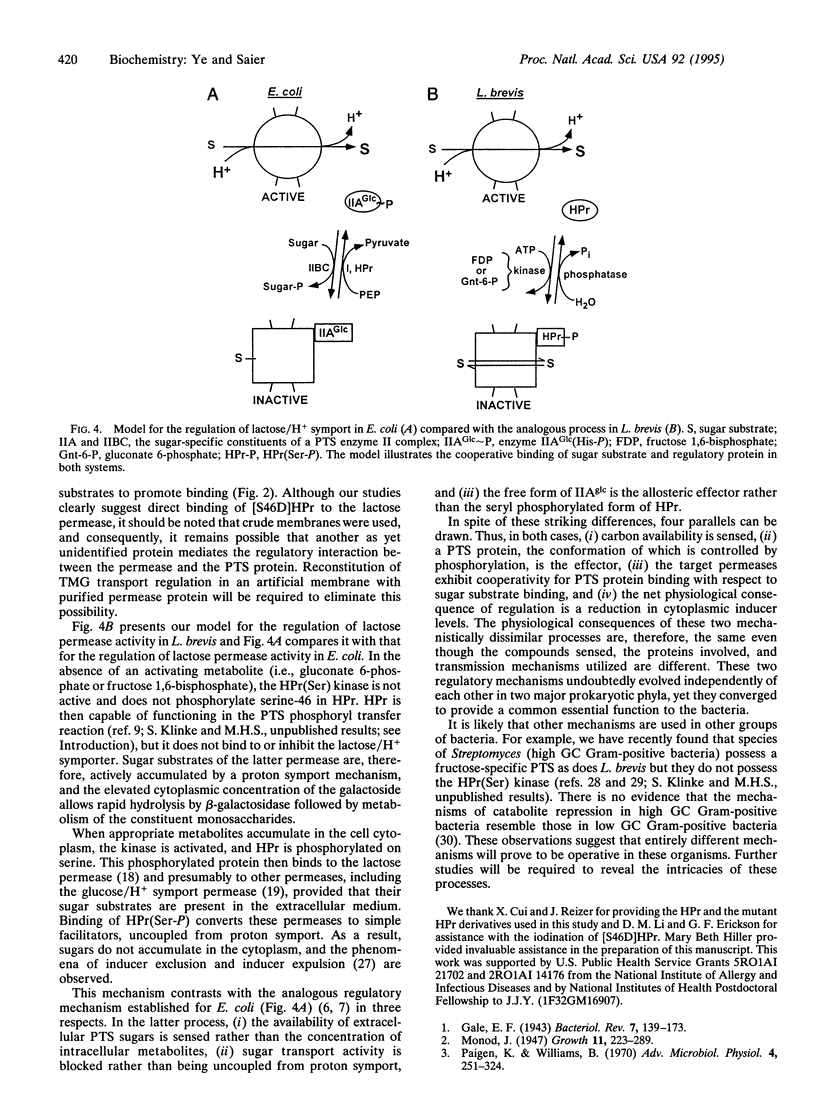
Lactobacillus brevis accumulates lactose and nonmetabolizable lactose analogues via sugar/H+ symport, but addition of glucose to the extracellular medium results in rapid efflux of the free sugar from the cells due to the uncoupling of sugar transport from proton transport. By using vesicles of L. brevis cells, we recently showed that these regulatory/effects could be attributed to the metabolite-activated ATP-dependent protein kinase-catalyzed phosphorylation of serine-46 in the phosphocarrier protein HPr [HPr(Ser-P)] of the phosphotransferase system and that a mutant form of HPr with the serine-46-->aspartate replacement ([S46D]HPr) is apparently locked in the seryl phosphorylated conformation. We here demonstrate that [S46D]HPr binds directly to inside-out membrane vesicles of L. brevis that contain the lactose permease. Sugar substrates of the permease markedly and specifically stimulate binding of [S46D]HPr to the membranes while certain transport inhibitors such as N-ethylmaleimide block binding. The pH dependency for binding follows that for transport. Wild-type HPr and the [S46A]HPr mutant protein did not appreciably compete with [S46D]HPr for binding to the permease. These results provide evidence for the direct interaction of HPr(Ser-P) with an allosteric site on the lactose/proton symporter of L. brevis for the purpose of regulating sugar accumulation in response to the metabolic needs of the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deutscher J., Reizer J., Fischer C., Galinier A., Saier M. H., Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994 Jun;176(11):3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H., Faber H. R., Worthylake D., Meadow N. D., Roseman S., Pettigrew D. W., Remington S. J. Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase. Science. 1993 Jan 29;259(5095):673–677. [PubMed] [Google Scholar]
- Kwakman J. H., Postma P. W. Glucose kinase has a regulatory role in carbon catabolite repression in Streptomyces coelicolor. J Bacteriol. 1994 May;176(9):2694–2698. doi: 10.1128/jb.176.9.2694-2698.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Mechanism of regulation of the lactose permease by the phosphotransferase system in Escherichia coli: evidence for protein-protein interaction. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):269–273. [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Hoischen C., Reizer A., Pham T. N., Saier M. H., Jr Sequence analyses and evolutionary relationships among the energy-coupling proteins Enzyme I and HPr of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Protein Sci. 1993 Apr;2(4):506–521. doi: 10.1002/pro.5560020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Romano A. H., Deutscher J. The role of phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, in the regulation of carbon metabolism in gram-positive bacteria. J Cell Biochem. 1993 Jan;51(1):19–24. doi: 10.1002/jcb.240510105. [DOI] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Wu L. F., Deutscher J., Reddy P., Saier M. H., Jr Functional interactions between proteins of the phosphoenolpyruvate:sugar phosphotransferase systems of Bacillus subtilis and Escherichia coli. J Biol Chem. 1992 May 5;267(13):9158–9169. [PubMed] [Google Scholar]
- Romano A. H., Brino G., Peterkofsky A., Reizer J. Regulation of beta-galactoside transport and accumulation in heterofermentative lactic acid bacteria. J Bacteriol. 1987 Dec;169(12):5589–5596. doi: 10.1128/jb.169.12.5589-5596.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Regulatory interactions involving the proteins of the phosphotransferase system in enteric bacteria. J Cell Biochem. 1993 Jan;51(1):62–68. doi: 10.1002/jcb.240510112. [DOI] [PubMed] [Google Scholar]
- Titgemeyer F., Mason R. E., Saier M. H., Jr Regulation of the raffinose permease of Escherichia coli by the glucose-specific enzyme IIA of the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1994 Jan;176(2):543–546. doi: 10.1128/jb.176.2.543-546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titgemeyer F., Walkenhorst J., Cui X., Reizer J., Saier M. H., Jr Proteins of the phosphoenolpyruvate:sugar phosphotransferase system in Streptomyces: possible involvement in the regulation of antibiotic production. Res Microbiol. 1994 Feb;145(2):89–92. doi: 10.1016/0923-2508(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Wittekind M., Reizer J., Klevit R. E. Sequence-specific 1H NMR resonance assignments of Bacillus subtilis HPr: use of spectra obtained from mutants to resolve spectral overlap. Biochemistry. 1990 Aug 7;29(31):7191–7200. doi: 10.1021/bi00483a006. [DOI] [PubMed] [Google Scholar]
- Ye J. J., Neal J. W., Cui X., Reizer J., Saier M. H., Jr Regulation of the glucose:H+ symporter by metabolite-activated ATP-dependent phosphorylation of HPr in Lactobacillus brevis. J Bacteriol. 1994 Jun;176(12):3484–3492. doi: 10.1128/jb.176.12.3484-3492.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. J., Reizer J., Cui X., Saier M. H., Jr ATP-dependent phosphorylation of serine-46 in the phosphocarrier protein HPr regulates lactose/H+ symport in Lactobacillus brevis. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3102–3106. doi: 10.1073/pnas.91.8.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. J., Reizer J., Cui X., Saier M. H., Jr Inhibition of the phosphoenolpyruvate:lactose phosphotransferase system and activation of a cytoplasmic sugar-phosphate phosphatase in Lactococcus lactis by ATP-dependent metabolite-activated phosphorylation of serine 46 in the phosphocarrier protein HPr. J Biol Chem. 1994 Apr 22;269(16):11837–11844. [PubMed] [Google Scholar]


