Abstract
The localization and activity of polyamine oxidase (PAO; EC 1.5.3.3), was investigated in leaves and protoplasts of oat seedlings. Activity of the enzyme is highest with spermine as substrate; spermidine is also oxidized, but putrescine and cadaverine are unaffected by the enzyme. Protoplasts isolated following digestion of leaves with cellulase in hypertonic osmoticum showed no PAO activity, and about 80% of the total leaf PAO activity could be accounted for in the cell wall debris. Histochemical localization experiments showed intense PAO activity in guard cells and in vascular elements whose walls are not digested by cellulase. When protoplasts were cultured in a medium suitable for regeneration of cell wall, PAO activity could be detected as the cellulose wall developed. Thus, PAO appears to be localized in cell walls.
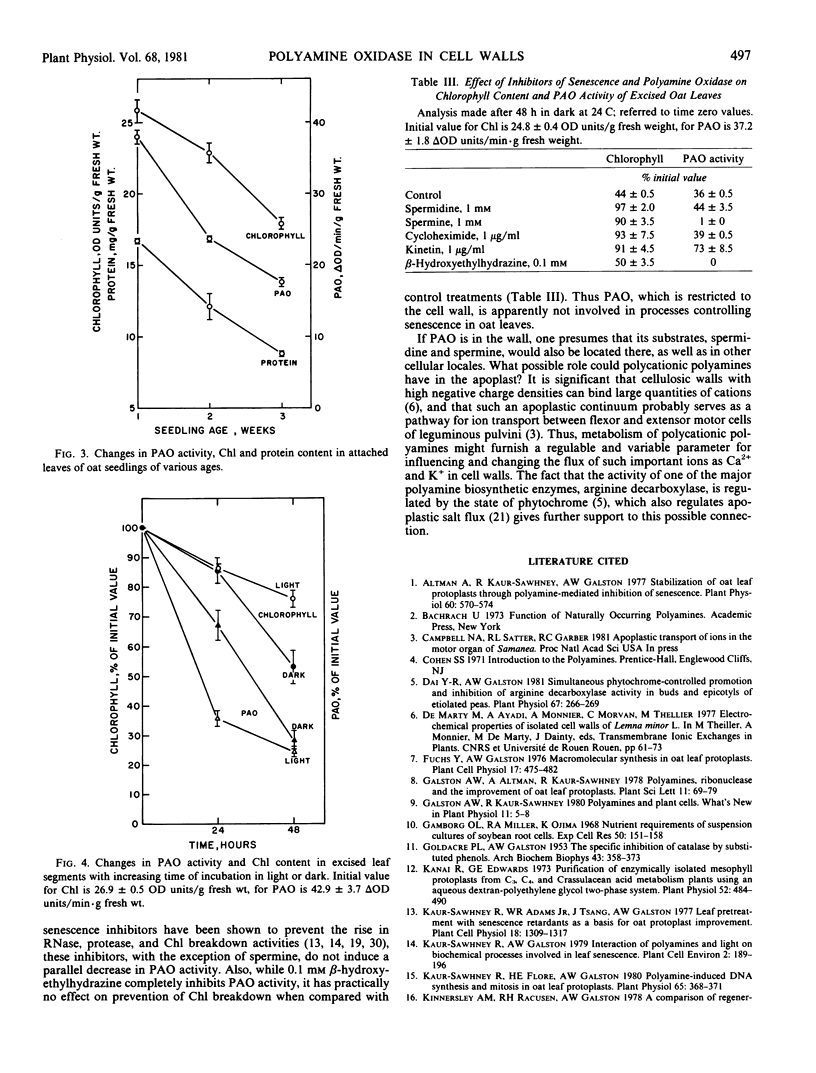
Since applied spermine and spermidine prevent senescence of detached leaves, PAO activity was investigated during leaf senescence. The specific activity of PAO declines with increasing age of attached leaves and with increasing senescence of excised leaves incubated in darkness. This decline in enzyme activity, which parallels the decreases in chlorophyll and protein content used as measures of leaf senescence, suggests that the enzyme is not involved in the control of senescence of oat leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. R., Galston A. W. Simultaneous Phytochrome-controlled Promotion and Inhibition of Arginine Decarboxylase Activity in Buds and Epicotyls of Etiolated Peas. Plant Physiol. 1981 Feb;67(2):266–269. doi: 10.1104/pp.67.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDACRE P. L., GALSTON A. W., WEINTRAUB R. L. The effect of substituted phenols on the activity of the indoleacetic acid oxidase of peas. Arch Biochem Biophys. 1953 Apr;43(2):358–373. doi: 10.1016/0003-9861(53)90130-1. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Galston A. W. Potassium flux: a common feature of albizzia leaflet movement controlled by phytochrome or endogenous rhythm. Science. 1971 Oct 29;174(4008):518–520. doi: 10.1126/science.174.4008.518. [DOI] [PubMed] [Google Scholar]
- Smith T. A. Polyamine oxidase in higher plants. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1452–1456. doi: 10.1016/0006-291x(70)90549-8. [DOI] [PubMed] [Google Scholar]