Abstract
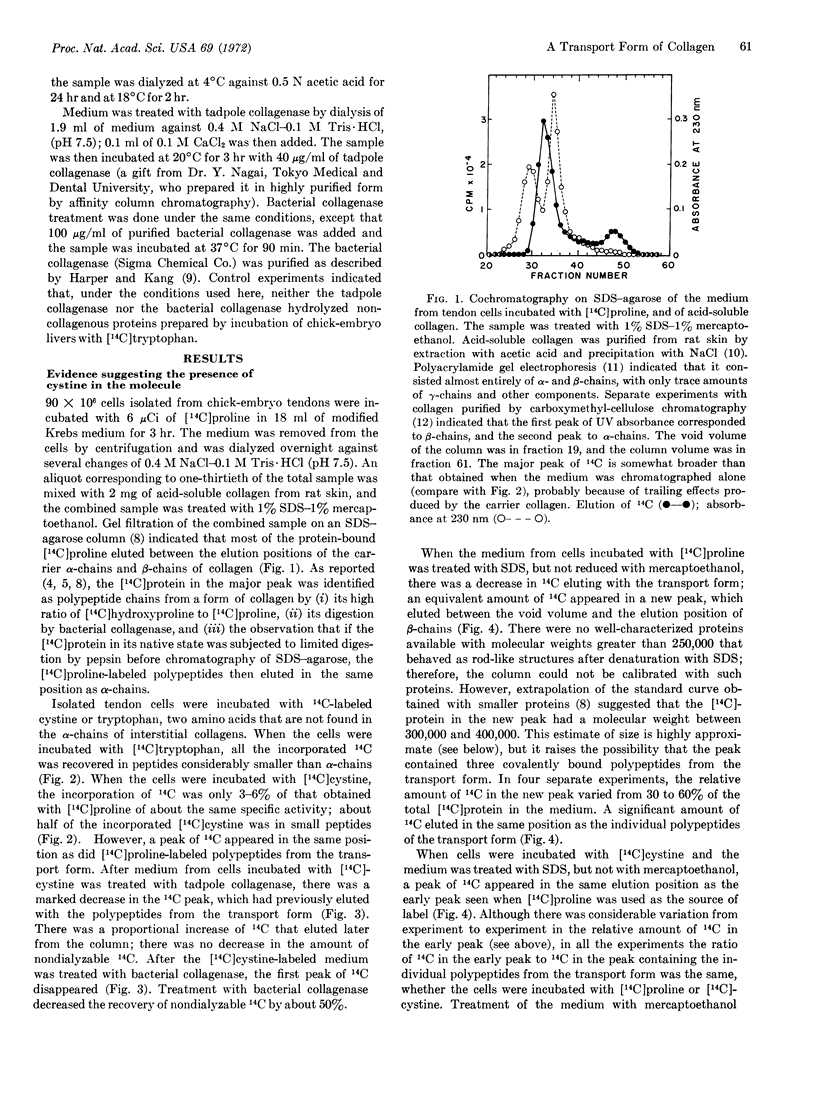
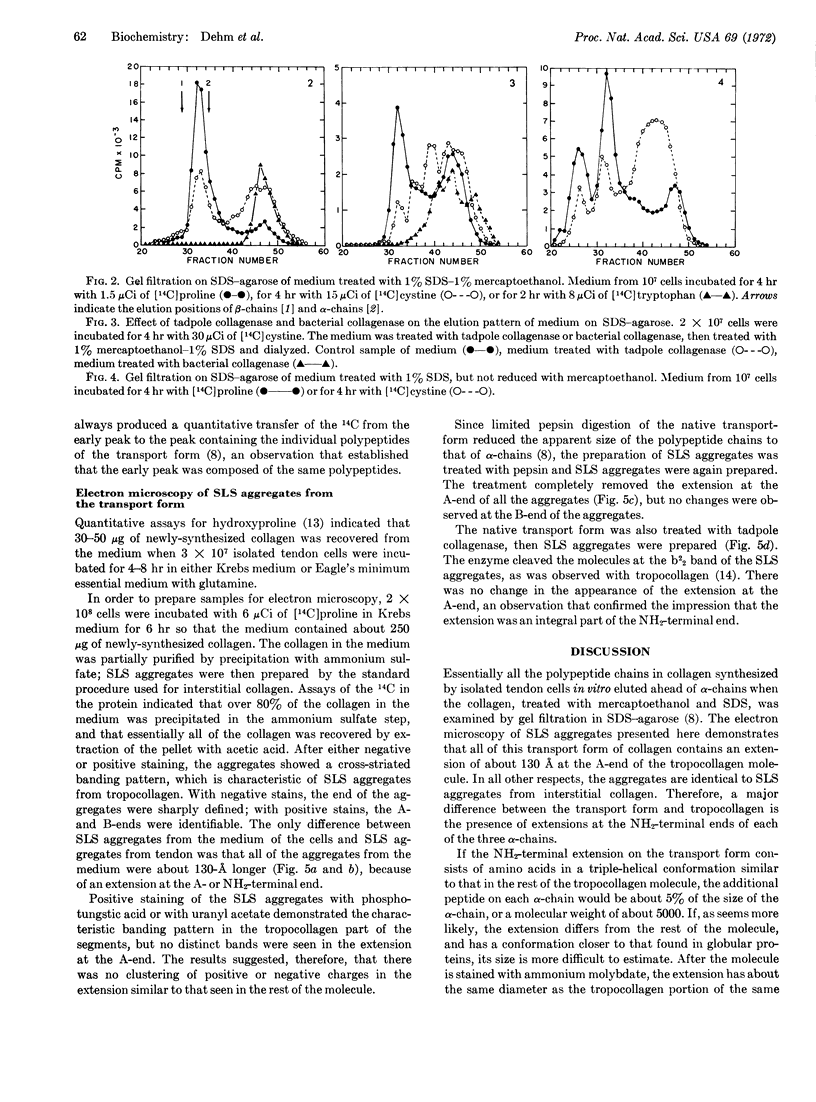
When cells were isolated from chickembryo tendons and incubated in vitro for 2-6 hr, essentially all the newly-synthesized collagen was recovered from the incubation medium as a transport form larger than tropocollagen. Experiments in which cells were incubated with [14C]cystine suggested that the transport form contained cystine and that it was, in part, stabilized by disulfide bonds. Electron microscopy of segment-long-spacing aggregates prepared from the transport form of collagen showed that the native molecule differed from tropocollagen in that it had an extension of about 13 nm (130 Å) at the NH2-terminal end.
Keywords: chick embryo, tropocollagen, gel filtration
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Kang A. H. Studies on the specificity of bacterial collagenase. Biochem Biophys Res Commun. 1970 Oct 23;41(2):482–487. doi: 10.1016/0006-291x(70)90531-0. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Prockop D. J. Further evidence for a transport form of collagen. Its extrusion and extracellular conversion to tropocollagen in embryonic tendon. FEBS Lett. 1971 Oct 1;17(2):245–248. doi: 10.1016/0014-5793(71)80156-4. [DOI] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P. K., McGoodwin E., Martin G. R. Studies on protocollagen: identification of a precursor of proto alpha 1. Biochem Biophys Res Commun. 1971 Jul 2;44(1):110–117. doi: 10.1016/s0006-291x(71)80165-1. [DOI] [PubMed] [Google Scholar]
- Nelson C. A. The binding of detergents to proteins. I. The maximum amount of dodecyl sulfate bound to proteins and the resistance to binding of several proteins. J Biol Chem. 1971 Jun 25;246(12):3895–3901. [PubMed] [Google Scholar]
- Ramaley P. B., Rosenbloom J. Inhibition of proline and lysine hydroxylation prevents normal extrusion of collagen by 3T6 fibroblasts in culture. FEBS Lett. 1971 Jun 2;15(1):59–64. doi: 10.1016/0014-5793(71)80079-0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



