Abstract
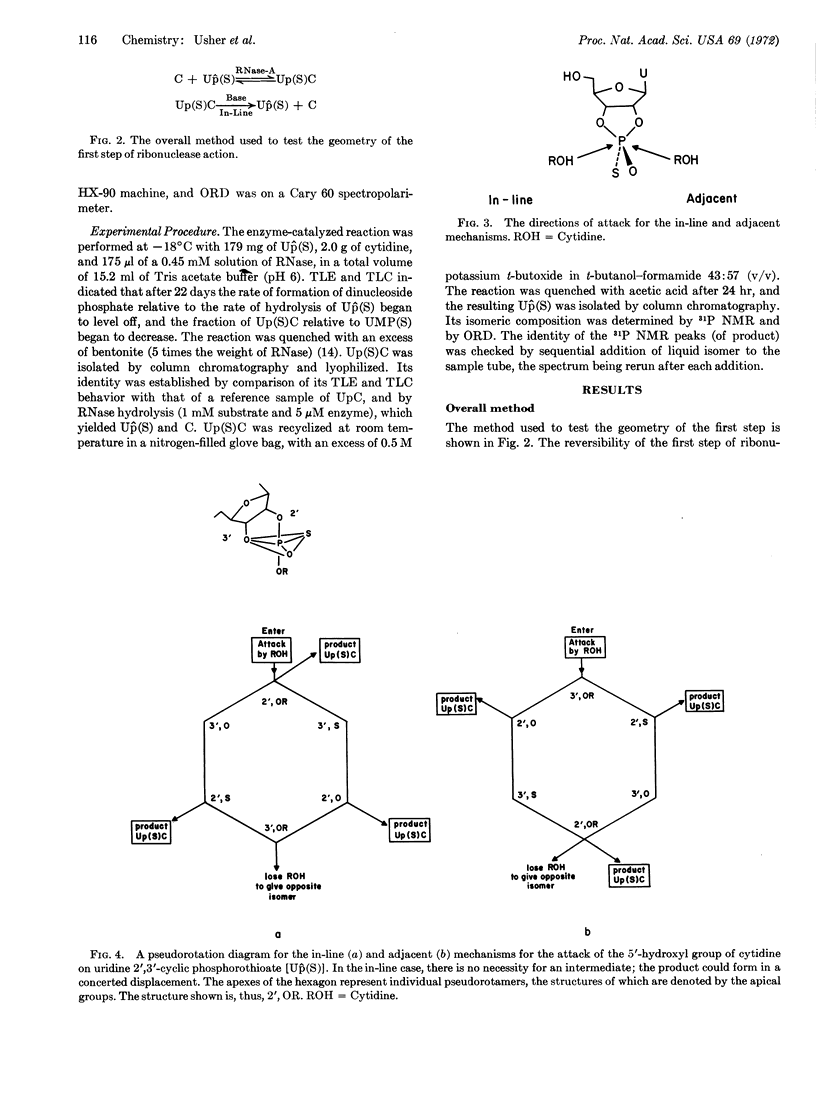
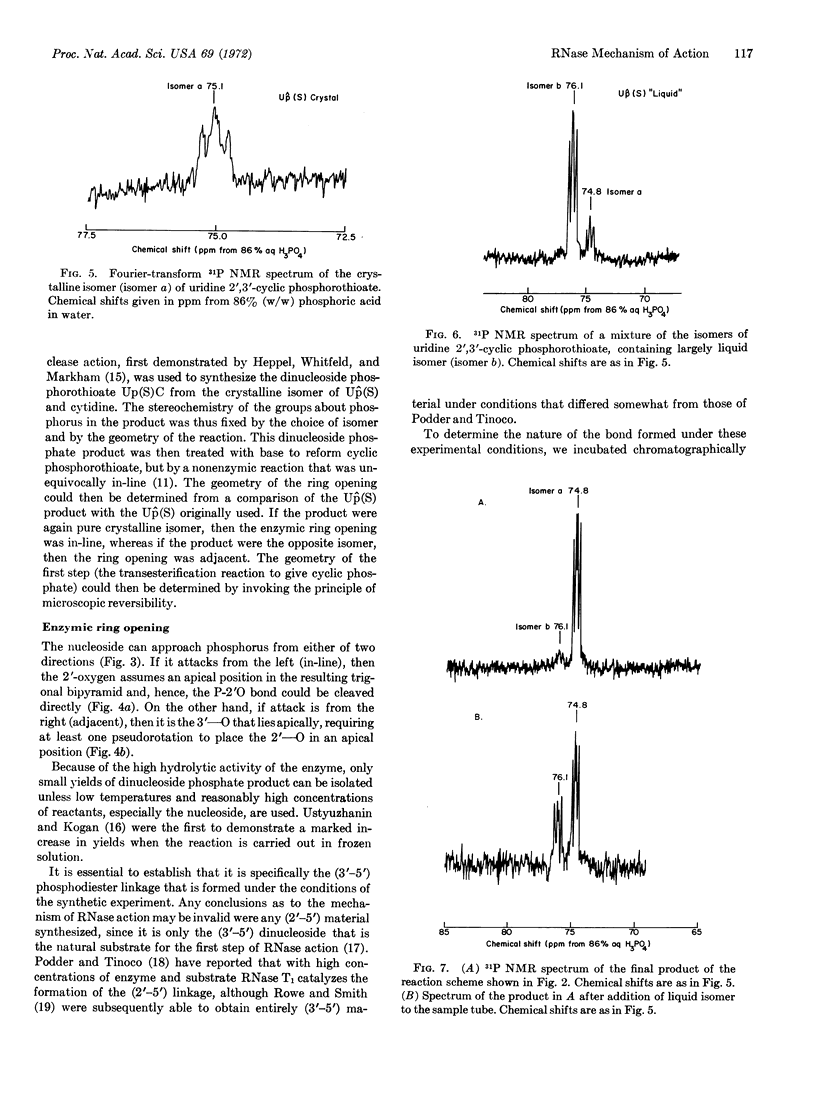
Advantage was taken of the reversibility of the first step of ribonuclease-A action to synthesize the dinucleoside phosphorothioate Up(S)C from the crystalline isomer of uridine 2′,3′-cyclic phosphorothioate [Up̂(S)] and cytidine. Cyclic phosphorothioate was then reformed from Up(S)C by a nonenzymic reaction known to proceed by an in-line mechanism. The geometry of the enzymic reaction was determined to be in-line by a comparison of the Up̂(S) product with the Up̂(S) originally used. By the principle of microscopic reversibility, the geometry of the first step in the action of ribonuclease-A is shown to be in-line.
Keywords: in-line geometry; uridine 2′,3′-cyclic thiophosphate; 31P NMR
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A. Ribonucleases. Annu Rev Biochem. 1969;38:677–732. doi: 10.1146/annurev.bi.38.070169.003333. [DOI] [PubMed] [Google Scholar]
- Deavin A., Mathias A. P., Rabin B. R. Mechanism of action of bovine pancreatic ribonuclease. Nature. 1966 Jul 16;211(5046):252–255. doi: 10.1038/211252a0. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. J Am Chem Soc. 1970 Jul 29;92(15):4718–4723. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Uridine 2',3'-O,O-cyclophosphorothioate as substrate for pancreatic ribonuclease (I). FEBS Lett. 1968 Dec;2(2):85–86. doi: 10.1016/0014-5793(68)80108-5. [DOI] [PubMed] [Google Scholar]
- FINDLAY D., HERRIES D. G., MATHIAS A. P., RABIN B. R., ROSS C. A. The active site and mechanism of action of bovine pancreatic ribonuclease. Nature. 1961 May 27;190:781–784. doi: 10.1038/190781a0. [DOI] [PubMed] [Google Scholar]
- Gassen H. G., Witzel H. Zum Mechanismus der Ribonuclease-Reaktion. 1. Die Aufgabe der Pyrimidinbase bei der Reaktion. Eur J Biochem. 1967 Mar;1(1):36–45. doi: 10.1111/j.1432-1033.1967.tb00041.x. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., WHITFELD P. R., MARKHAM R. Nucleotide exchange reactions catalysed by ribonuclease and spleen phosphodiesterase. II. Synthesis of polynucleotides. Biochem J. 1955 May;60(1):8–15. doi: 10.1042/bj0600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G. Relaxation spectrometry of biological systems. Adv Protein Chem. 1968;23:1–57. doi: 10.1016/s0065-3233(08)60399-x. [DOI] [PubMed] [Google Scholar]
- Podder S. K., Tinoco I., Jr Enzymatic synthesis of oligoguanylic acids containing 2'-5' phosphodiester linkages. Biochem Biophys Res Commun. 1969 Mar 10;34(5):569–574. doi: 10.1016/0006-291x(69)90775-x. [DOI] [PubMed] [Google Scholar]
- Roberts G. C., Dennis E. A., Meadows D. H., Cohen J. S., Jardetzky O. The mechanism of action of ribonuclease. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1151–1158. doi: 10.1073/pnas.62.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. J., Smith M. A. Synthesis of 3'-5' dinucleotides with RNase T-1. Biochem Biophys Res Commun. 1970 Feb 6;38(3):393–399. doi: 10.1016/0006-291x(70)90726-6. [DOI] [PubMed] [Google Scholar]
- Usher D. A. On the mechanism of ribonuclease action. Proc Natl Acad Sci U S A. 1969 Mar;62(3):661–667. doi: 10.1073/pnas.62.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. A., Richardson D. I., Jr, Eckstein F. Absolute stereochemistry of the second step of ribonuclease action. Nature. 1970 Nov 14;228(5272):663–665. doi: 10.1038/228663a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H. Facilitated proton transfer in enzyme catalysis. It may have a crucial role in determining the efficiency and specificity of enzymes. Science. 1968 Jul 26;161(3839):328–334. doi: 10.1126/science.161.3839.328. [DOI] [PubMed] [Google Scholar]


