Abstract
Cyclin D1 expression is precisely controlled during cell-cycle progression. However, repeated exposure to low-dose fractionated radiation (FR) abrogates cell cycle-dependent cyclin D1 degradation by constitutive activation of AKT survival signaling in normal human fibroblasts. The resulting abnormal nuclear cyclin D1 accumulation induces defects in DNA replication and resulting DNA double-strand breaks, and is associated with induction of genomic instability in low-dose irradiated cells. Here, we investigated the role of DNA damage signaling against such perturbed cell-cycle control of cyclin D1 expression. Nuclear cyclin D1 accumulation was induced within 7 days after low-dose FR (0.01 Gy or 0.05 Gy per fraction) in ATM-deficient cells (AT5BIVA), but appeared later in AT5BIVA cells harboring human ATM cDNA. Thus, ATM prevents abnormal nuclear cyclin D1 accumulation at early time points after low-dose FR. We further demonstrated that ATM-mediated downregulation of protein phosphatase 2A activity caused activation of the AKT/cyclin D1 pathway after long-term FR. Perturbation of cyclin D1 expression induced Rad51 foci that indicate homologous recombination repair (HRR) in control cells, while ATM- and NBS1-deficient cells (GM7166) failed to induce Rad51 foci after long-term low-dose FR. After 21 days of FR, NBS1- and ATM-deficient cells showed a decrease in nuclear cyclin D1-positive cells, and an increase in apoptotic cells. Similarly, inhibition of ATM with KU55933 abrogated nuclear cyclin D1 accumulation by induction of apoptosis in ATM-complemented cells exposed to low-dose FR. In conclusion, we here demonstrate that ATM is involved in controlling cyclin D1 levels after low-dose FR. DNA damage signaling mitigates the harmful effects of low-dose long-term FR by suppression of cell death induced by perturbation of cyclin D1 expression.
Introduction
DNA double-strand breaks (DSBs) are the most critical trigger of genomic instability induced by ionizing radiation. To guard genome stability in irradiated cells, mammalian cells harbor cellular defense systems against radiation-induced DSBs, including activation of cell-cycle checkpoints, apoptosis and DNA repair mechanisms. The DNA damage response (DDR) has been well investigated using acute single radiation (SR) at high doses. However, the effects of long-term exposure to radiation at low doses in humans remain unclear due to lack of sufficient study. To understand the relatively small effects of low-dose radiation, large sample sizes or highly sensitive assays are required. Therefore, we utilized highly radiosensitive human ATM-deficient and NBS1-deficient cells (AT5BIVA and GM7166), which are defective in DDR to elucidate the molecular mechanisms underlying the relatively small effects of low-dose radiation.
Cyclin D1, a regulatory subunit of CDKs (cyclin-dependent kinases), controls cell-cycle progression from G1 phase to S phase.1 Cyclin D1/CDK4 and cyclin D1/CDK6 complexes phosphorylate retinoblastoma, which releases E2F proteins, leading to the transactivation of genes required for the G1/S transition.2, 3 Cyclin D1 levels are regulated at the level of both gene transcription and protein stability. Cyclin D1 gene (CCND1) expression is induced by growth factors through the Ras signaling pathway that involves Ras, Raf, mitogen-activated protein kinase/ERK (extracellular signal-regulated kinase) and ERK.4, 5 Cyclin D1 protein stability is regulated by the v-akt murine thymoma viral oncogene homolog (AKT) pathway. AKT phosphorylates residue 9 of GSK3β (glycogen synthase kinase 3 beta), which prevents GSK3β from phosphorylating Thr286 of cyclin D1 and subsequently promoting nuclear export and proteasomal degradation of cyclin D1.6, 7, 8 Thus, AKT activity results in increased levels of cyclin D1 protein. Cyclin D1 levels vary during cell cycling, with an increase during G1 phase, a peak at G1/S boundary, a decline in S phase and a second increase at G2 phase.9 The cyclin D1 degradation during S phase allows for efficient DNA synthesis.10
ATM is mutated in ataxia-telangiectasia), a disease characterized by high radiosensitivity and neurodegeneration.11 ATM protein has a central role in the DDR to maintain genome stability in response to various stresses. The signal generated by DSB is transduced by ATM to phosphorylate FBXO31, which facilitates ubiquitination and resulting proteasome-mediated degradation of cyclin D1.12 It has been shown that in response to a single 10-Gy dose, cyclin D1 undergoes rapid degradation by the ATM-FBXO31 mediated ubiquitin proteasome pathway, and this degradation results in cell-cycle arrest at the G1/S checkpoint.13, 14 Conversely, we recently showed that cyclin D1 expression is stabilized within the nuclei of human cancer cells after fractionated radiation (FR) for 31 days.14, 15 Constitutive AKT activation following long-term FR exposures downregulates the nuclear export and proteolysis of cyclin D1, which results in the nuclear retention of cyclin D1 during S phase.14 We further reported that this persistent cyclin D1 expression during S phase results in perturbed DNA replication and resulting DSBs.16 Recently, we found that nuclear accumulation of cyclin D1 was induced in normal human fibroblasts cells that were exposed to low doses of FR with 0.01 or 0.05 Gy per fraction, 5 days per week for 31 days (total doses were 0.46 and 2.3 Gy, respectively).17 Furthermore, cells that retained nuclear cyclin D1 were more likely to have micronuclei than non-retaining cells, indicating that the accumulation of nuclear cyclin D1 was associated with induction of genome instability and increased cancer risks.17 This suggests that cyclin D1 may be used as a biomarker of long-term low-dose FR, as cyclin D1 expression is highly radiosensitive and increased expression occurs only after long-term FR exposure but not after SR.17, 18
In this study, human ATM-deficient and NBS1-deficient cell lines and the corresponding cell lines expressing ATM and NBS1 (AT5BIVA, GM7166, AT5BIVA/ATM-wt and GM7166/NBS1-wt) were exposed to 0.01 or 0.05 Gy per fraction of FR for 31 days. Nuclear accumulation of cyclin D1 following long-term FR was investigated in these cells. We found that ATM is associated with cellular response against perturbation of cyclin D1 expression following low-dose FR. Homologous recombination repair (HRR) protects against cell death induced by nuclear cyclin D1 accumulation in irradiated cells.
Results
Growth retardation by exposure to low-dose FR in ATM- and NBS1-deficient cells
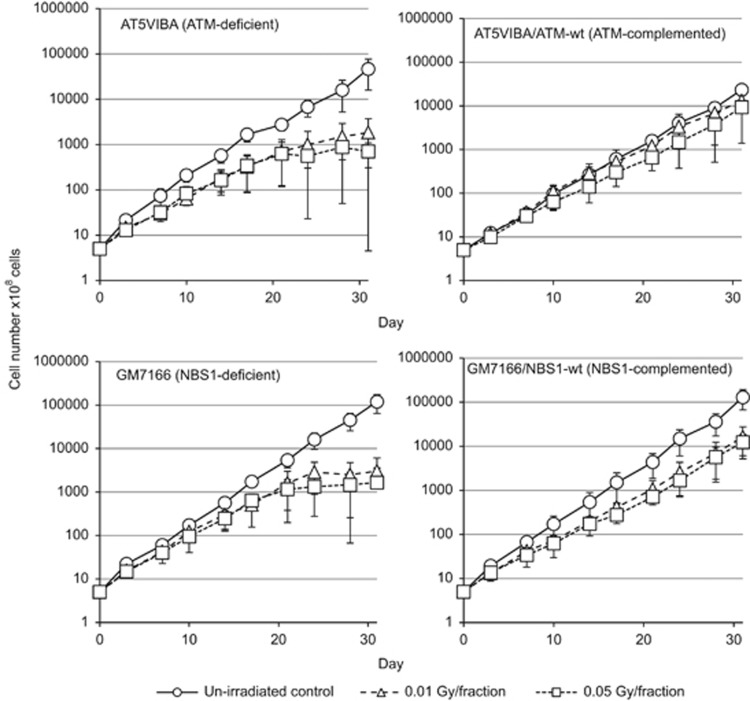
We exposed AT5BIVA, AT5BIVA/ATM-wt, GM7166 and GM7166/NBS1-wt cells to 0.01 or 0.05Gy fractions of X-rays for 31 days. Cell growth was monitored during FR exposure (Figure 1). Exposure to low-dose FR decelerated cell proliferation in all four cell lines (Figure 1). Growth retardation was more evident over 21 days of FR with 0.01 and 0.05 Gy in ATM-deficient AT5BIVA cells and NBS1-deficient GM7166 cells than in corresponding ATM and NBS1-complemented cells (Figure 1). It is likely that exponential growth stopped and cell numbers plateaued at about 20 days for the ATM- and NBS1-deficient cells, but not for the ATM-and NBS1-complemented cells.
Figure 1.
Effect of long-term FR on cell growth in ATM- and NBS1-deficient cells. Cell growth of unirradiated cells (open circles) and cells exposed to 0.01 Gy (open triangles) and 0.05 Gy (open squares) fractions. Growth curves for AT5VIBA (ATM-deficient), AT5VIBA/ATM-wt (ATM-complemented), GM7166 (NBS1-deficient) and GM7166/NBS1-wt (NBS1-complemented) cells are shown.
Nuclear cyclin D1 accumulation following long-term FR in ATM- and NBS1-deficient cells
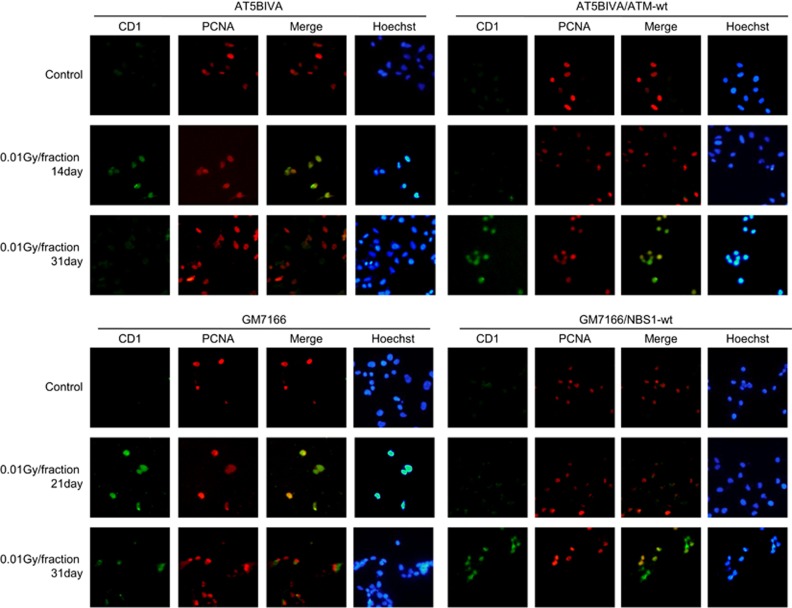
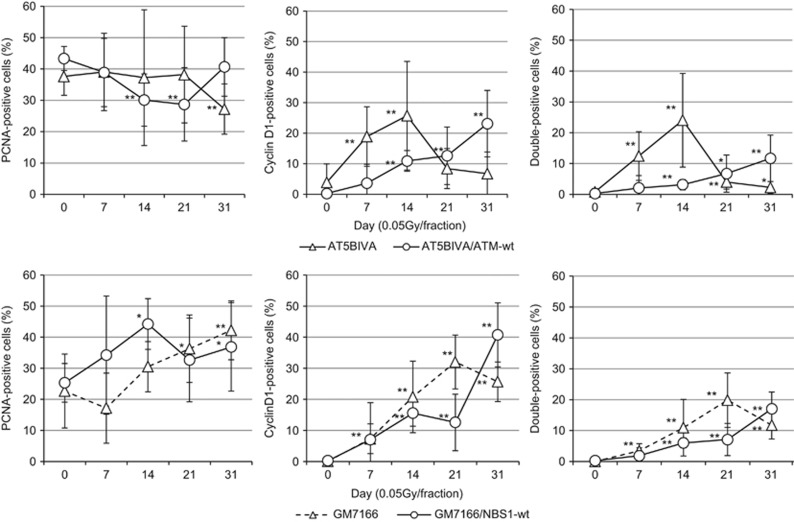
Nuclear cyclin D1 was identified by treatment with a hypotonic buffer containing detergent to remove cytoplasmic cyclin D1. With this approach, we previously found that cyclin D1 accumulated in the nuclei of normal human fibroblasts in S-phase following low-dose FR for 31 days.17 We here tested whether ATM and NBS1 are involved in this FR-induced accumulation of cyclin D1. Proliferating cell nuclear antigen (PCNA) was used to identify cells in S phase. For control cells, PCNA localized to replication sites within nuclei, but cyclin D1 was absent (Figure 2). Detergent-insoluble nuclear cyclin D1 was detected in PCNA-positive nuclei after FR in all four cell lines, but the kinetics of nuclear cyclin D1 expression were different between ATM- or NBS1-deficient cells and the corresponding complemented cells. Percentages of S-phase cells with nuclear PCNA fluctuated around 40% during FR exposure in all four cell lines, indicating cells can enter S phase during FR. The number of cells with PCNA and nuclear cyclin D1 staining increased with the duration of radiation exposure in control AT5BIVA/ATM-wt and GM7166/NBS1-wt cells, as previously reported in normal human fibroblast cell lines exposed to low doses of FR for 31 days (Figures 2 and 3).17 At 7 days of FR, approximately 10% of ATM-deficient cells showed double-positive staining for PCNA and nuclear cyclin D1. Only 2% of control cells expressed nuclear cyclin D1 at this time. However, the number of double-positive ATM-deficient cells declined after 14 days of FR (Figure 3, right panel). Rapid appearance of cells positive for both PCNA and cyclin D1 was also evident in NBS1-deficient cells at 21 days of FR, but this decreased at 31 days of FR. For all cell lines, the kinetics of double-positive expression of PCNA and cyclin D1 were almost the same as the kinetics for positive single staining of cyclin D1 alone (Figure 3, middle panel and right panel).
Figure 2.
Differences in cyclin D1 localization after long-term FR between ATM- or NBS1-deficient cells and their complemented cells. Immunofluorescence localization of cyclin D1 and PCNA is shown for AT5VIBA (upper left panel), AT5VIBA/ATM-wt (upper right panels), GM7166 (lower left panel) and GM7166/NBS1-wt (lower right panel). DNA was stained with Hoechst.
Figure 3.
Kinetics of PCNA and cyclin D1 response following low-dose FR in ATM- and NBS1-deficient cells. Cells positive for PCNA (left panel), cyclin D1 (center panel) and both (right panel) were scored 24 h following the indicated FR exposure. Data for AT5VIBA, AT5VIBA/ATM-wt, GM7166 and GM7166/NBS1-wt cells exposed to 0.05 Gy fractions are shown. Asterisks indicate a significant increase in staining cells compared with control cells.
We further examined nuclear cyclin D1 accumulation by western blotting. A hypotonic buffer was used to isolate nuclear fractions from cells exposed to FR for 31days and amounts of cyclin D1 expression were quantified in the nuclear fraction of ATM-complemented cells. The levels of cyclin D1 in the nuclear fraction increased after 31 days of FR in ATM-complemented cells (Figure 4). Treatment with a specific ATM inhibitor, KU55933, abrogated nuclear cyclin D1 accumulation at 31 days of FR in these cells.
Figure 4.
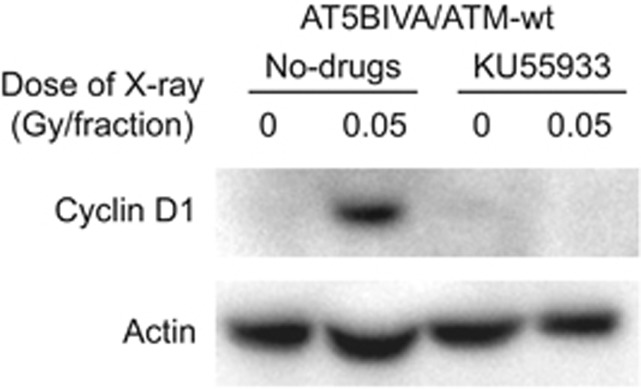
Nuclear accumulation of cyclin D1 in cells exposed to long-term FR. Detergent-insoluble fractions of cell lysates were prepared 24 h after the last radiation exposure, and western blotting was performed. Levels of nuclear cyclin D1 were determined for cells exposed to 31 days of FR. ATM inhibitor KU55933 was used for 72 h and then detergent-insoluble fractions of cell lysates were collected.
Defective constitutive AKT activation after long-term FR in ATM- and NBS1-deficient cells
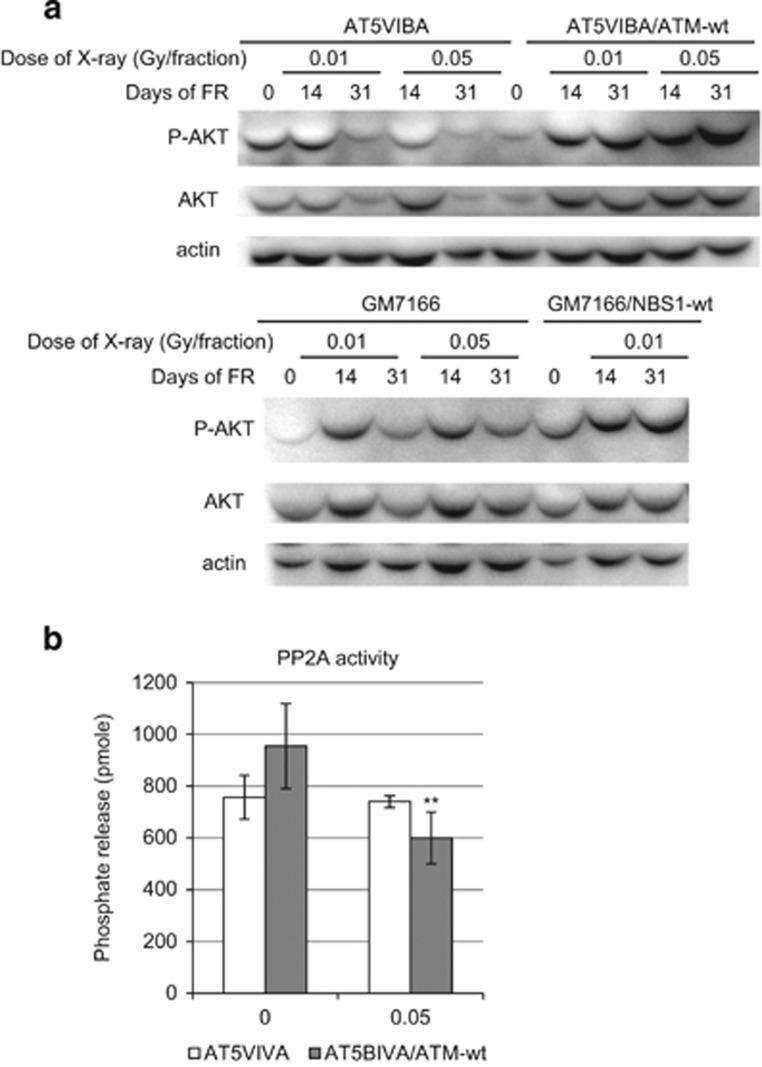
The AKT pathway regulates cyclin D1 stability following long-term FR.18 Therefore, AKT activation was measured by examination of phosphorylation of AKT on serine 473 after FR in ATM- and NBS1-deficient cells. We here confirmed that AKT was transiently activated after 2 Gy of SR in AT5BIVA cells as reported in other cells (Supplementary Figure 1).14, 19 In contrast, AKT was continuously activated for at least 24 h after the last FR treatment in control cells subjected to 14 and 31 days FR (Figure 5a). In ATM- and NBS1-deficient cells, AKT activation was also observed at 14 days of FR, but it was attenuated at 31 days of FR (Figure 5a). The protein phosphatase 2A (PP2A) dephosphorylates and inactivates AKT.20 PP2A activity was examined in ATM-complemented and ATM-deficient cells by using a PP2A immunoprecipitation phosphatase assay kit (Figure 5b). PP2A activity was downregulated after long-term FR in ATM-complemented cells, while the activity was unaffected by long-term FR in ATM-deficient cells. Thus, ATM-mediated downregulation of PP2A activity is associated with the activation of AKT and subsequent nuclear accumulation of cyclin D1 after long-term FR.
Figure 5.
AKT activation at 0, 14 and 31 days after long-term FR. (a) AKT phosphorylation on Ser473 (P-AKTSer473, active AKT) by FR exposure in AT5BIVA and AT5BIVA/ATM-wt cells (upper panel) and in GM7166 and GM7166/NBS1-wt cells (lower panel). (b) PP2A activity of un-irradiated control and 31 days of FR (0.05 Gy/fraction) in AT5BIVA and AT5BIVA/ATM-wt cells. Cell lysates were collected at 24 h after the last FR. Asterisks indicate a significant decrease in PP2A activity compared with un-irradiated control cells.
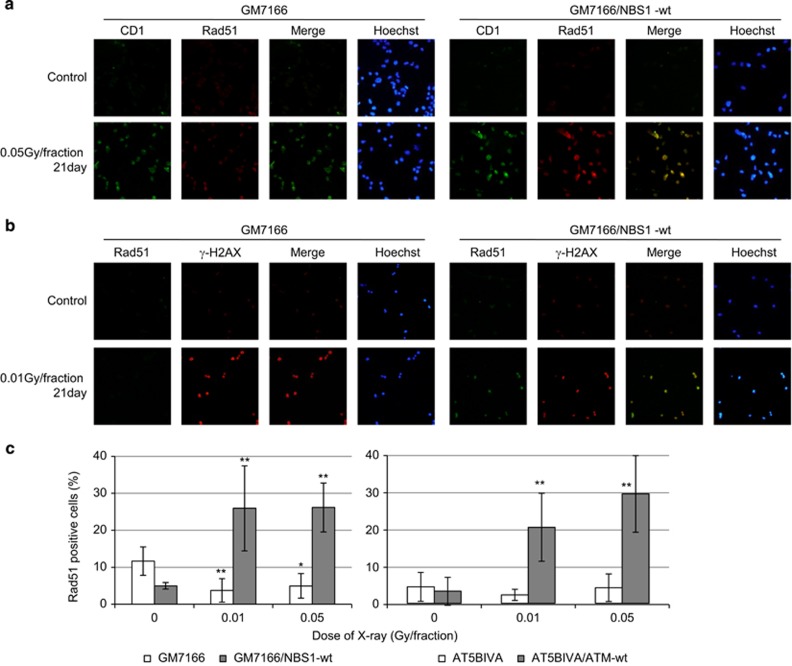
Induction of γ-H2AX and Rad 51 foci in cells with nuclear cyclin D1
Since HRR is predominant in S phase, replication-associated DSBs induced by perturbation of cyclin D1 expression are thought to be repaired by HRR to resume cell proliferation. Immunostaining with cyclin D1 and Rad51 revealed that Rad51 foci were induced in nuclear cyclin D1-positive NBS1-complemented cells (Figure 6a). We further investigated by immunostaining for γ-H2AX and Rad51 (Figure 6b). Rad51 foci were observed in control cells exposed to FR for 21 days. In contrast, γ-H2AX-positive cells did not contain Rad51 foci in NBS1-deficient FR cells exposed to FR for 21 days. The number of cells with Rad51 foci increased in control cells, while no foci were induced after low-dose FR in ATM- and NBS1-deficient cells (Figures 6b and c).
Figure 6.
Rad51 focus formation in FR cells. (a) Images of Cyclin D1 (green) and Rad51 (red)-positive cells. DNA was stained with Hoechst. (b) Images of Rad51 (green) and γ-H2AX (red)-positive cells. DNA was stained with Hoechst. (c) The percentage of cells with a Rad51 foci is shown for GM7166 and GM7166/NBS1-wt cells (left panel) and in AT5BIVA and AT5BIVA/ATM-wt cells (right panel). Asterisks indicate a significant Rad51-positive cells compared with control cells.
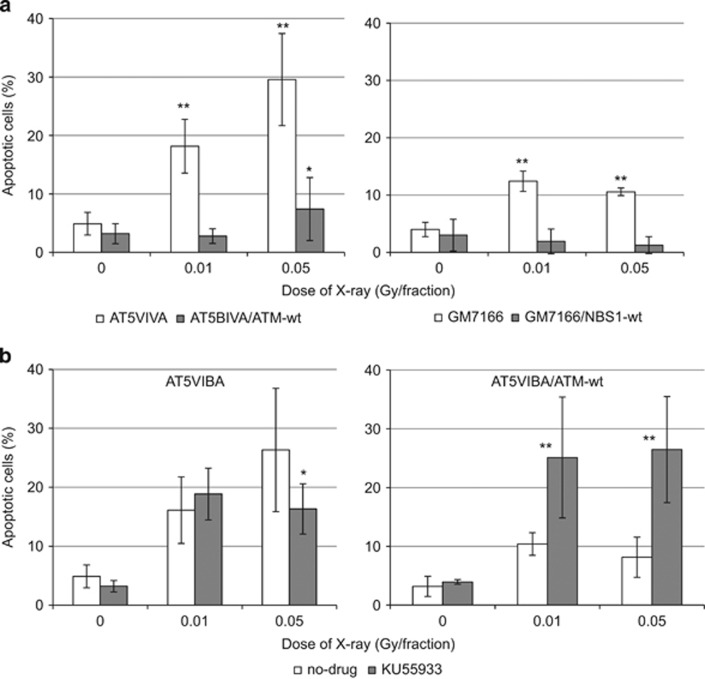
Induction of apoptosis following long-term FR in ATM- and NBS1-deficient cells
Defects in HRR may induce cell death in ATM- and NBS1-deficient cells following long-term exposure to low-dose FR. Apoptosis was examined using an apoptosis detection kit in control and ATM- or NBS1-deficient cells. As we expected, incidence of apoptosis was clearly higher in the ATM- and NBS1-deficient cell lines after 21 days of FR compared with the control complemented cells (Figure 7a). Treatment with KU55933 induced apoptosis in ATM complemented cells after 31 days of FR but this treatment did not affect incidence of apoptosis in ATM-deficient cells after 31 days of FR (Figure 7b).
Figure 7.
Apoptosis in ATM- and NBS1-deficient cells following FR. (a) Annexin V staining was performed for untreated control cells and cells treated with FR for 21 days. Asterisks indicate a significant increase in annexin V-positive cells compared with control cells. (b) Treatment with KU55933 induced apoptosis in ATM-complemented cells exposed to FR for 31 days. Cells were treated with KU55933 for 48 h. Asterisks indicate a significant increase in annexin V-positive cells compared with cells without KU55933 treatment.
Discussion
The role of ATM in response to nuclear cyclin D1 accumulation induced by long-term low-dose FR
Upon SR, ATM is known to be involved in the G1/S checkpoint by facilitating cyclin D1 degradation. ATM enhances GSK-3β-mediated cyclin D1 phosphorylation and FBXO31 mediated cyclin D1 ubiquitination, both of which correlate with cyclin D1 degradation after irradiation.12, 21 Here, we investigated the role of ATM in response to long-term low-dose FR. We found that ATM suppressed nuclear cyclin D1 accumulation in ATM-complemented control cells at early treatment time points until 14 days after low-dose FR. Thus, it appears that ATM may protect against low-dose FR-induced nuclear cyclin D1 expression by facilitating its degradation as a checkpoint response. ATM is also associated with protection against cell death induced by nuclear cyclin D1 accumulation in irradiated cells. Our data demonstrated that after 21 days of low-dose FR, ATM-deficient cells showed greater levels of apoptosis than cells expressing ATM.
Crosstalk between the AKT/cyclin D1 pathway and DDR
The AKT pathway is a key to stabilizing cyclin D1 expression after irradiation. SR does not induce nuclear cyclin D1 accumulation, because AKT is only transiently activated after SR.14 AKT inactivation is associated with induction of apoptosis after irradiation.22 AKT is dephosphorylated and inactivated by several phosphatases, including PH domain leucine-rich repeat protein phosphatase, PP2A and phosphatase with tensin homology.20, 23, 24, 25 ATM is shown to be involved in regulating PP2A activity after irradiation.26, 27 In the present study, we demonstrated that ATM is associated with constitutive activation of the AKT pathway after long-term FR by inactivation of PP2A. Nuclear cyclin D1 accumulation was induced when AKT was constitutively activated after long-term FR. Our data demonstrated that continuous AKT activation was observed 24 h after 14 days of FR but not at 31 days of FR in ATM- and NBS1-deficient cells. The absence of AKT activation is correlated with induction of apoptosis in these cells. Indeed, pro-survival signaling via the AKT/ERK pathway is activated at lower DSB levels (<2 Gy) but not at higher DSB levels (>2 Gy).28 Thus, cell death signaling in NBS1- and ATM-deficient cells prevented AKT activation and subsequent nuclear cyclin D1 accumulation following long-term FR. In contrast, AKT was constitutively activated for cell survival when control cells were exposed to FR for over 14 days. ATM is involved in AKT activation after irradiation by stimulating phosphorylation of AKT.29 DNA-activated protein kinase (DNA-PK) also activates AKT in response to various genotoxic stresses, including low doses of radiation.30 DNA-PK acts upstream of AKT in the AKT-mediated pathway upregulating cyclin D1 levels after FR.14
HRR for cyclin D1-mediated DSBs
The MRN (MRE11–RAD50–NBS1) complex is important in DSB recognition and promotes DSB repair through various pathways, including HRR.19 The MRN complex also has a role in the resolution of stalled replication forks in response to replication stresses.31, 32 Thus, the MRN complex is thought to be involved in the stress response to abnormal cyclin D1 expression in S phase. In our current study, Rad51 foci were observed in nuclear-cyclin D1-positive control cells following long-term FR. It is notable that cyclin D1 has been shown to be recruited to sites of DNA damage and to bind Rad51 to enhance HRR.33 On the other hand, Rad51 foci were induced to a lesser extent in ATM- and NBS1-deficient cells after FR, although phosphorylation of the histone H2AX (gamma-H2AX) remained 24 h after last FR. Defects in HRR resulted in induction of apoptosis in ATM and NBS1-deficient cells with nuclear cyclin D1.
Perturbation of cell-cycle control is an early feature of tumorigenesis
Activated oncogenes such as CDC25, cyclin E and cyclin D1 induce DSBs and DDR, which contribute to tumorigenesis in non-malignant cells.34, 35, 36 Furthermore, perturbation of cyclin D1 expression is shown to be implicated in neural degeneration due to inappropriate cell-cycle entry.37 Reducing cyclin D1 levels, therefore, may promote genomic stability and mitigate the harmful effects of long-term low-dose FR. Thus, cyclin D1 may be considered as a target to reduce radiation risks of low-dose long-term FR. We have previously reported that API-2, which is an AKT inhibitor, abrogated nuclear cyclin D1 accumulation induced by low-dose long-term FR exposure by facilitating cyclin D1 degradation in normal human fibroblasts.17 Data from this current study indicated that treatment with KU-55933, a specific ATM inhibitor, efficiently reduced the amounts of cyclin D1 expression in the nuclear fractions of ATM- and NBS1-deficient cells at 31 days of FR and increased the number of apoptotic cells. KU-55933 has been shown to induce apoptosis in cells with abnormal AKT activation.38 Thus, inhibition of ATM is effective in eliminating cells with active AKT and nuclear cyclin D1 by induction of apoptosis. Complete eradication of cyclin D1-positive cells after radiation treatment may be important, for such cells that escape from apoptosis may be associated with radiation-induced cancer. Indeed, cyclin D1-mediated genomic instability and lymphomagenesis was increased in the absence of ATM.39
In conclusion, we have demonstrated that nuclear cyclin D1 accumulation occurred rapidly in the absence of intact DNA repair or DNA damage signaling pathways. Abnormal cyclin D1 expression resulted in induction of apoptosis in these cells. Thus, cellular defense systems guard against perturbation of cell-cycle control to mitigate the effects of radiation and to maintain genome stability.
Materials and methods
Cell culture condition and drugs
SV40-transformed ATM-defective human fibroblasts (AT5BIVA), ATM-wt reconstituted cells (AT5BIVA /ATM-wt), SV40-transformed NBS1-defective human fibroblasts (GM7166) and NBS1-wt reconstituted cells (GM7166/NBS1-wt) were obtained from the Radiation Biology Center of Kyoto University. These cells were grown in RPMI-1640 medium (NacalaiTesque, Kyoto, Japan) supplemented with 10% heat-inactivated fetal calf serum. An ATM inhibitor (KU55933) was purchased from Calbiochem (San Diego, CA, USA). Cells were treated for 48 or 72 h with KU55933 at a final concentration of 5 μM. Non-treated cells were used as controls.
Irradiation experiments
Cells were irradiated using a 150-kVp X-ray generator (Model MBR-1505R2, Hitachi-Medico CO., Hitachi, Japan) with a 0.5-mm Cu and 0.1-mm Al filter at a dose of 0.7 Gy/min. Fractionation schedules were described before.17 Total doses delivered over 31 days were 0.46 and 2.3 Gy for cells exposed to FR of 0.01 and 0.05 Gy, respectively.
Cell growth assay
Cells (5 × 105) were seeded into 25-cm2 flasks (Thermo Fisher Scientific, San Jose, CA, USA), incubated overnight, and irradiated daily. Growth rates were monitored by counting cell numbers twice a week. When the total cell number exceeded 5 × 105, cells were subcultured to 5 × 105 cells in a new flask.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously.40 Cells (3 × 105) were seeded onto 18 mm × 18 mm coverslips placed in 10 mm tissue culture dishes. The coverslips were fixed with ice-cold acetone (5 min), ice-cold methanol (5 min) following treatment with a hypotonic buffer (10 mM Tris-HCl pH 7.4, 2.5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride and 0.5% Nonidet P-40) for 5 min, and then washed twice with phosphate-buffered saline (PBS). The cells were blocked for 15 min at room temperature in 5% BSA (bovine serum albumin) in PBS. Anti-PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-cyclin D1 (Nichirei Bioscience, Tokyo, Japan), anti-Rad51 (Millipore, Temecula, CA, USA) and anti-γ-H2AX (Upstate, Charlottesville, VA, USA) were diluted in PBS with 0.5% BSA and incubated with the coverslips for 1 h. The coverslips were then washed three times with 0.1% Triton X-100 in PBS, incubated for 1 h with secondary antibodies conjugated with Alex 488 (Molecular Probes, Eugene, OR, USA) or Cy-3 (Jackson Immuno Research Laboratories, West Grove, PA, USA). The coverslips were washed three times with 0.1% Triton X-100 in PBS, counterstained for DNA with Hochest33258 (4 μg/ml prepared in Vectashield mounting medium; Vector Laboratories, Burlingame, CA, USA). Images were captured with a CCD camera attached to a fluorescence microscope (Keyence, Osaka, Japan). More than 100 cells were counted to quantify immunofluorescence data with at least three independent samples.
Western blot analyses
Western blotting was performed as described previously.40 To isolate nuclear fractions, cells were treated with a hypotonic buffer for 5 min on ice. Proteins were separated by sodium-lauryl-sulfate-polyacrylamide gel electrophoresis and transferred electro-phoretically onto PVDF membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked with 5% (W/v) phospho-blocker (Cell Biolabs. Inc., San Diego, CA, USA) for 1 h and incubated with primary antibodies such as anti-β-actin (A2066, Sigma, St Louis, MO, USA), anti-phospho-AKT-Ser473 (4060, Cell Signaling, Beverly, MA, USA), anti-AKT (4685, Cell Signaling), anti-cyclin D1 (Nichirei Bioscience), for 1 h at room temperature or overnight at 4 °C. Membranes were then incubated for 1 h at room temperature with the secondary antibody of HRP-conjugated goat anti-rabbit IgG (GE Healthcare, Buckinghamshire, UK) or HRP-conjugated goat anti-mouse IgG (R&D Systems, Minneapolis, MN, USA). The protein bands were visualized with Chemi-Lumi One L Western blotting substrate (NacalaiTesque). Band intensity was measured by densitometry using the Image Lab Software (Bio-Rad).
Measurement of PP2A phosphatase activity
PP2A phosphatase activity was measured with cell lysates (100 μg) using a PP2A immunoprecipitation phosphatase assay kit according to the manufacturer's instructions (Millipore). Samples and phosphate standard with assay buffer (25 μl) were prepared to a 96-well plate. Add 100 μl of the phosphate detection solution to each well. Incubate the reaction for 10 min at room temperature. OD values at 650 nm were measured with a microplate reader (Model Sunrise, Tecan Group Ltd., Maennedorf, Switzerland).
Annexin V staining
Apoptotic cells were identified and quantified using an annexin V-FITC apoptosis detection kit (Bio Vision, Mountain View, CA, USA) following the manufacturer's protocol. Cells were stained with annexin V-FITC and propidium iodide 48 h after treatment with radiation. Annexin V-positive apoptotic cells were analyzed by the fluorescence activated cell sorting (FACScan) (Becton Dickinson, Mountainview, CA, USA).
Statistical analysis
Error bars represent standard deviations; in some cases the standard deviations were too small to be visible on the histogram. All the experiments were repeated at least three times with independent samples. Student's t-test was used for the analysis. A single asterisk and double asterisks indicate significance of P<0.01 and P<0.05, respectively.
Acknowledgments
We thank the staff of the National Institute of Public Health for assistance with the research. This research was supported by a grant from the Japanese Ministry of Education and Science Kiban C (24510063). This work was performed at the Joint Usage/Research Center (Radiation Biology Center), Kyoto University.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
Supplementary Material
References
- Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- DeGregori J. The Rb network. J Cell Sci. 2004;117:3411–3413. doi: 10.1242/jcs.01189. [DOI] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yang K, Harwalkar J, Nye JM, Mason DR, Garrett MD, et al. Phosphorylation of cyclin D1 at Thr 286 during S phase leads to its proteasomal degradation and allows efficient DNA synthesis. Oncogene. 2005;24:2599–2612. doi: 10.1038/sj.onc.1208326. [DOI] [PubMed] [Google Scholar]
- McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra MK, Wajapeyee N, Green MR. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature. 2009;459:722–725. doi: 10.1038/nature08011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- Shimura T, Kakuda S, Ochiai Y, Nakagawa H, Kuwahara Y, Takai Y, et al. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene. 2010;29:4826–4837. doi: 10.1038/onc.2010.238. [DOI] [PubMed] [Google Scholar]
- Shimura T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J Radiat Res. 2011;52:539–544. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- Shimura T, Ochiai Y, Noma N, Oikawa T, Sano Y, Fukumoto M. Cyclin D1 overexpression perturbs DNA replication and induces replication-associated DNA double-strand breaks in acquired radioresistant cells. Cell Cycle. 2013;12:773–782. doi: 10.4161/cc.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Hamada N, Sasatani M, Kamiya K, Kunugita N. Nuclear accumulation of cyclin D1 following long-term fractionated exposures to low-dose ionizing radiation in normal human diploid cells. Cell Cycle. 2014;13:1248–1255. doi: 10.4161/cc.28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Fukumoto M, Kunugita N. The role of cyclin D1 in response to long-term exposure to ionizing radiation. Cell Cycle. 2013;12:2738–2743. doi: 10.4161/cc.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, Kato M, et al. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol Cell Biol. 2007;27:3187–3198. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CY, Brautigan DL, Larner JM. ATM-dependent dissociation of B55 regulatory subunit from nuclear PP2A in response to ionizing radiation. J Biol Chem. 2002;277:4839–4844. doi: 10.1074/jbc.M110092200. [DOI] [PubMed] [Google Scholar]
- Shouse GP, Nobumori Y, Panowicz MJ, Liu X. ATM-mediated phosphorylation activates the tumor-suppressive function of B56gamma-PP2A. Oncogene. 2011;30:3755–3765. doi: 10.1038/onc.2011.95. [DOI] [PubMed] [Google Scholar]
- Hawkins AJ, Golding SE, Khalil A, Valerie K. DNA double-strand break - induced pro-survival signaling. Radiother Oncol. 2011;101:13–17. doi: 10.1016/j.radonc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viniegra JG, Martinez N, Modirassari P, Hernandez Losa J, Parada Cobo C, Sanchez-Arevalo Lobo VJ, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Bruhn C, Zhou ZW, Ai H, Wang ZQ. The essential function of the MRN complex in the resolution of endogenous replication intermediates. Cell Rep. 2014;6:182–195. doi: 10.1016/j.celrep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Carr AM, Lambert S. Replication stress-induced genome instability: the dark side of replication maintenance by homologous recombination. J Mol Biol. 2013;425:4733–4744. doi: 10.1016/j.jmb.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen K, Jiao X, Wang C, Willmarth NE, Casimiro MC, et al. Cyclin D1 integrates estrogen-mediated DNA damage repair signaling. Cancer Res. 74:3959–3970. doi: 10.1158/0008-5472.CAN-13-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiao X, Wang C, Shirley LA, Elsaleh H, Dahl O, et al. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010;70:8802–8811. doi: 10.1158/0008-5472.CAN-10-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi M, Stacey DW. The checkpoint kinase ATM protects against stress-induced elevation of cyclin D1 and potential cell death in neurons. Cytometry A. 2010;77:524–533. doi: 10.1002/cyto.a.20885. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang DQ. The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther. 2010;9:113–125. doi: 10.1158/1535-7163.MCT-08-1189. [DOI] [PubMed] [Google Scholar]
- Vaites LP, Lian Z, Lee EK, Yin B, DeMicco A, Bassing CH, et al. ATM deficiency augments constitutively nuclear cyclin D1-driven genomic instability and lymphomagenesis. Oncogene. 2014;33:129–133. doi: 10.1038/onc.2012.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Toyoshima M, Adiga SK, Kunoh T, Nagai H, Shimizu N, et al. Suppression of replication fork progression in low-dose-specific p53-dependent S-phase DNA damage checkpoint. Oncogene. 2006;25:5921–5932. doi: 10.1038/sj.onc.1209624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.