Abstract
Objective
To evaluate the effects of the glucagon-like peptide-1 receptor agonist lixisenatide on elevated liver blood tests in patients with type 2 diabetes.
Design
Systematic review.
Data sources
Electronic and manual searches were combined.
Study selection
Randomised controlled trials (RCTs) on lixisenatide versus placebo or active comparators for type 2 diabetes were included.
Participants
Individual patient data were retrieved to calculate outcomes for patients with elevated liver blood tests.
Main outcome measures
Normalisation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Data synthesis
The results of included trials were combined in meta-analyses. Sequential, subgroup and regression analyses were performed to evaluate heterogeneity and bias.
Results
We included 12 RCTs on lixisenatide versus placebo and 3 RCTs with the active comparators liraglutide, exenatide or sitagliptin. The mean treatment duration was 29 weeks. Lixisenatide increased the proportion of patients with normalisation of ALT (risk difference: 0.07; 95% CI 0.01 to 0.14; number needed to treat: 14 patients, p=0.042). The effect was not confirmed in sequential analysis. No effects of lixisenatide were identified on AST, alkaline phosphatase or bilirubin. No evidence of bias was identified. Mixed effect multilevel meta-regression analyses suggest that the benefit of lixisenatide on ALT was limited to patients who were overweight or obese.
Conclusions
This review suggests that lixisenatide increases the proportion of obese or overweight patients with type 2 diabetes who achieve normalisation of ALT. Additional research is needed to determine if the findings translate to clinical outcome measures.
Trial registration number
PROSPERO; CRD42013005779.
Strengths and limitations of this study.
This systematic review of randomised controlled trials evaluates if lixisenatide has a beneficial effect on liver blood tests associated with non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Based on analyses of individual patient data, lixisenatide increases the proportion of patients with normalisation of alanine aminotransferase (ALT) compared with placebo or active comparators. In subgroup analyses, the effect was verified for patients who were obese or overweight, but not for normal weight patients.
The analyses include data from published and unpublished trials with intention-to-treat analyses of all patients included irrespective of compliance or follow-up. The bias control was classified as adequate in all trials based on four or five of the five components included in the Cochrane bias assessment tool.
Although ALT is the most sensitive biochemical marker of NAFLD and NASH, important effects may be overlooked because patients with severe liver disease were excluded from the trials.
The available data did not allow for assessment of clinical outcome measures such as development of cirrhosis or hepatocellular carcinoma.
Introduction
The incidence of non-alcoholic fatty liver disease (NAFLD) is increasing and the costs are considerable.1–3 About 10% of patients with NAFLD develop non-alcoholic steatohepatitis (NASH), which may progress to cirrhosis and hepatocellular carcinoma. Obesity and decreased insulin sensitivity are associated with NAFLD and NASH, which are common in patients with type 2 diabetes.4–6 NAFLD is generally an asymptomatic disease. Elevated transaminases are independent predictors of NAFLD although the sensitivity is low.7 A large proportion of patients with type 2 diabetes and elevated transaminases have NAFLD or NASH. A systematic review of observational studies found that routinely available biochemical markers may be used in the assessment of NAFLD.8 Elevation of alanine aminotransferase (ALT) is more common than aspartate aminotransferase (AST).9 The gold standard for the assessment of patients with NAFLD is to perform a liver biopsy, but the procedure is associated with risks and potential sampling errors. Biopsy-related complications including bleeding still occur in ultrasonically guided techniques.10 11
Treatment of NAFLD and NASH is important. Antidiabetic interventions have been assessed as a potential treatment option. A systematic review from 2007 found three randomised controlled trials (RCTs) on metformin and pioglitazone in patients with NAFLD or NASH.12 The review found that the interventions increased the proportion of patients with normalisation of ALT. A subsequent health technology assessment on insulin sensitisers for NAFLD reached a similar conclusion.13 However, pioglitazone is associated with a considerable risk of serious adverse events including cardiovascular disease and bladder cancer.14 15 Alternative treatment options are, therefore, needed. Recent studies suggest that glucagon-like peptide-1 receptor agonists (GLP-1RA) improve insulin sensitivity and resistance most likely via their body weight -lowering effect.16 In addition, GLP-1RAs may have a direct effect on hepatocytes reducing hepatic steatosis via glucagon-like peptide-1 receptors in the liver.17 A review on the GLP1-RA liraglutide and exenatide found that the interventions reduce ALT and AST in patients with type 2 diabetes or obesity.18 Unfortunately, analyses of patients with elevated liver blood tests were not available. Lixisenatide is a GLP-1RA that improves glycaemic control and reduces body weight in patients with type 2 diabetes.19 20 There are no RCTs on lixisenatide for patients with NAFLD. However, several trials on lixisenatide included patients who were overweight and allowed inclusion of patients with mildly elevated liver blood tests. The trials were, therefore, likely to include a relatively large proportion of patients with NAFLD. We, therefore, conducted a systematic review with outcomes recalculated based on individual patient data from RCTs to determine the effect of lixisenatide on elevated liver blood tests in patients with type 2 diabetes.
Methods
This review is based on a registered protocol (CRD42013005779). The review methods follow the recommendations described in the Cochrane Handbook for Reviews on Interventions (http://www.cochrane.org). The main objective was to compare the effect of lixisenatide versus placebo or other active interventions on normalisation of liver transaminases. RCTs were included irrespective of blinding, language or publication status. Adult patients with type 2 diabetes and elevated liver blood tests were included irrespective of gender or body weight.
Based on previous evidence,9 the primary outcome measures were normalisation of ALT and AST. Secondary outcome measures included normalisation of alkaline phosphatase and bilirubin as well as the normalisation of the composite outcome measures combining all liver blood tests. The pharmaceutical company producing lixisenatide (Sanofi-Aventis) provided data and additional information on the design of included trials. All outcomes were recalculated based on individual patient data.
All authors participated in the identification and selection of trials. Excluded trials were listed with the reason for exclusion. Eligible trials were identified through electronic and manual searches. Electronic searches were performed without language restrictions in MEDLINE (1946 to February 2014), Cochrane Library (Issue 2, 2014), EMBASE (1974 to February 2014) and Web of Science (1900 to February 2014). The search strategy in the Cochrane Library was lixisenatide, ti,ab,kw (Word variations have been searched). In MEDLINE, the search strategy was (lixisenatide AND ("randomized controlled trial"[Publication Type]) OR ("controlled clinical trial"[Publication Type])). Additional manual searches were performed in reference lists of relevant papers, correspondence with experts, the pharmaceutical company producing lixisenatide and the World Health Organisation Trial Search Database (http://apps.who.int/trialsearch/).
Two authors extracted data in an independent manner (LG and TV). Disagreements were resolved through discussion. The bias risk assessment was based on the Cochrane Collaboration Risk of Bias Assessment tool (http://www.cochrane.org). The assessment included the separate domains random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective bias and other biases. Each domain was classified as having a high, uncertain or low risk of bias.
Random sequence generation: Low risk of bias—The investigators describe a random component in the sequence generation process, such as referring to a random number table or using a computer random number generator. Unclear risk of bias—Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—The investigators describe a non-random component in the sequence generation process.
Allocation concealment: Low risk of bias—Participants and investigators enrolling participants could not foresee risk assignment because one of the following or an equivalent method was used to conceal allocation: Central allocation (including telephone, web based and pharmacy controlled). Unclear risk of bias—Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—Participants or investigators enrolling participants could possibly foresee risk assignments.
Blinding of participants and personnel: Low risk of bias—No blinding or incomplete blinding, but the outcome is not likely to be influenced by lack of blinding (eg, objective outcome measures such as blood tests). Unclear risk of bias—Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment: Low risk of bias—No blinding of outcome assessment, but the outcome measurement is not likely to be influenced by lack of blinding (eg, blood tests). Unclear risk of bias—Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—No blinding of outcome assessment and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data: Low risk of bias—No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; missing data have been imputed using appropriate methods. Unclear risk of bias—Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—Reason for missing outcome data likely to be related to true outcome; the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; ‘as-treated’ analysis done with substantial departure from the assigned intervention; potentially inappropriate application of simple imputation.
Selective reporting: Low risk of bias—The study protocol is available and all of the study's prespecified outcomes (primary and secondary), that are of interest for the review, have been reported in the prespecified way; The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). Unclear risk of bias—Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. High risk of bias—Not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta-analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Other bias: Low risk—The study appears to be free of other sources of bias. Unclear risk of bias—Insufficient information to assess whether an important risk of bias exists. High risk—The study had a potential source of bias related to the specific study design used or has been claimed to have been fraudulent or had some other problem.
Statistical analysis
The analyses were performed in Stata V.13 (STATA Corp, Texas, USA). Random effects meta-analyses were performed due to the expected heterogeneity between studies. The results were expressed as risk differences with 95% CIs; p values and I2 as measures of heterogeneity and with the number needed to treat for statistically significant outcome measures. We defined I2 values below 30% as unimportant, 30–50% as moderate heterogeneity, 50–75% as substantial heterogeneity and >75% as considerable heterogeneity. All patients were included in the analysis irrespective of compliance or follow-up, and with imputation of outcomes for patients with missing outcome data (intention to treat). Mixed effect multilevel meta-regression and subgroup analyses were performed to evaluate heterogeneity. The meta-regression analysis evaluated the influence of the metabolic regulation (glycated haemoglobin (HbA1c) ≤8.5% (69 mmol/mol)), duration of diabetes (≥5 years) and body mass index (BMI) (normal weight ≤25, overweight >25 or obese >30 kg/m2). Post hoc analyses were performed to evaluate the effect of the change in body weight and ALT. The subgroup analyses evaluated the influence of publication status (full paper articles compared with abstracts and unpublished trials), control groups and collateral interventions. Since all trials had a low risk of bias, we did not perform subgroup analyses on bias control. Publication bias and other small study effects were estimated in regression analyses (Harbord's test) and funnel plots. We performed sequential analyses to evaluate the robustness of results from meta-analyses with a statistically significant result. The analysis was performed with α 5%, power 80%, model-based diversity correction 12%, relative risk reduction 8% and control group incidence rate 51%.
Results
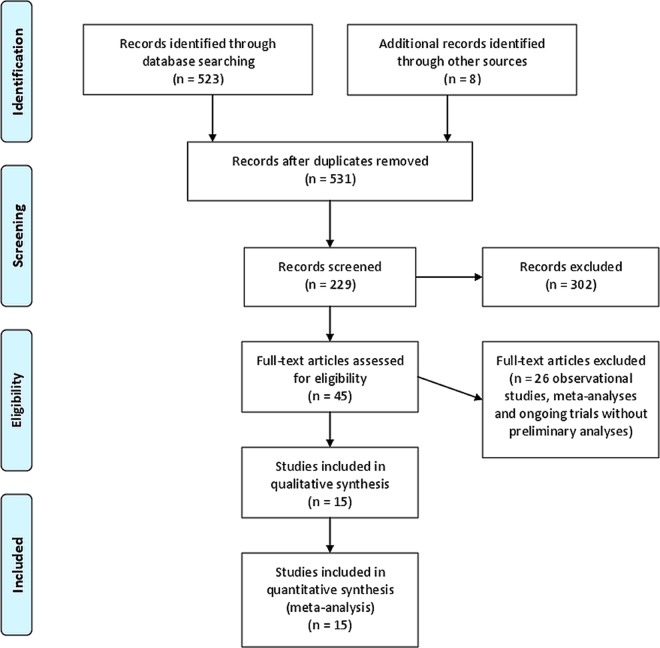
The initial searches identified 531 potentially eligible records (figure 1). After reading the titles and abstracts, duplicates and records that clearly did not describe RCTs on lixisenatide were excluded. One ongoing trial was excluded because data were not yet available. The remaining records referred to 15 multicentre RCTs that were included in the qualitative and quantitative analyses (table 1). Eleven trials were published as full paper articles,21–31 three were published as abstracts32–34 and one was unpublished.
Figure 1.
Trial flow chart.
Table 1.
Characteristics of included trials
| Trial | Publication status | Control | Collateral interventions* | Duration (weeks) |
|---|---|---|---|---|
| Lorenz 2013 ACT6011 QD | Full paper | Placebo | Sulfonulureas±metformin | 4 |
| Ratner 2010 DRI6012 QD | Full paper | Placebo | Metformin | 24 |
| Pan 2012 GetGoal-M-Asia | Abstract | Placebo | Sulfonulureas±metformin | 24 |
| Riddle 2013 GetGoal-Duo1 | Full paper | Placebo | Insulin | 24 |
| Riddle 2013 GetGoal-L | Full paper | Placebo | Insulin | 24 |
| Seino 2012 GetGoal-L-Asia | Full paper | Placebo | Insulin | 24 |
| Ahren 2013 GetGoal-M | Full paper | Placebo | Metformin | 24 |
| Fonseca 2012 GetGoal-Mono | Full paper | Placebo | Diet | 24 |
| Ratner 2012 GetGoal-S | Abstract | Placebo | Sulfonulureas±metformin | 76 |
| PDY6797 QD | Unpublished | Placebo | Sulfonulureas±metformin | 76 |
| Rosenstock 2013 GetGoal-X | Full paper | Exenatide | Metformin | 24 |
| Kapitza 2013 PDY10931 | Full paper | Liraglutide | Metformin | 4 |
| Seino 2012 EFC10780 | Full paper | Sitagliptin | Metformin | 24 |
| Pinget 2013 GetGoal-P | Full paper | Placebo | Pioglitazone±metformin | 24 |
| Bolli 2013 GetGoal-F1 | Full paper | Placebo | Metformin | 24 |
*Collateral interventions were administered equally to the lixisenatide and control groups.
The trials included patients with type 2 diabetes diagnosed based on HbA1c or fasting glucose with inadequate metabolic control on current intervention regimens. The exclusion criteria varied, but overall none of the trials included patients with an ongoing drug or alcohol abuse, or patients with pancreatitis, gastric surgery, inflammatory bowel disease or other severe systemic illnesses such as alcoholic liver disease. All trials were designed to evaluate the effect of lixisenatide on metabolic regulation.
None of the included trials found statistically significant differences between patient characteristics in the lixisenatide and control groups. The proportion of men was 50% and the mean age in the lixisenatide and control group ranged from 43 to 61 years (table 2). For the lixisenatide group, mean BMI ranged from 25.1 to 36.8 kg/m2 and the mean HbA1c from 7.2% to 8.5% (53–69 mmol/mol). For the control group, the mean BMI ranged from 25.2 to 36.8 kg/m2 and the HbA1c 7.4% to 8.9% (56–74 mmol/mol). Table 3 shows the mean baseline liver blood tests in the lixisenatide and control groups. The proportion of patients with elevated ALT ranged from 20% to 77% for the lixisenatide group and from 19% to 75% for the control groups. All trials randomised patients based on computer-generated random numbers with central randomisation. RCTs with a placebo control group were double blind with blinding of participants and personnel. RCTs with an active control group were open. All outcomes were objective (blood tests). We estimated that the outcomes were not likely to be influenced by lack of blinding and therefore, classed all trials as having a low risk of bias in the domains blinding of participants and personnel, and blinding of outcome assessment. All patients were accounted for and included in the analyses, and all clinically relevant outcomes were defined and reported. No discrepancies were detected between the protocol and reported outcomes. No other biases were detected. We, therefore, classified all RCTs as having a low risk of bias.
Table 2.
Characteristics of included patients (mean and SD)
| Trial | Body mass index (kg/m2) |
Weight (kg) |
Glycated haemoglobin (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide |
Controls |
Lixisenatide |
Controls |
Lixisenatide |
Controls |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Lorenz 2013 ACT6011 QD | 31.38 | 4.05 | 29.77 | 3.76 | 89.40 | 14.66 | 83.80 | 14.68 | 8.54 | 1.07 | 8.87 | 1.07 |
| Ratner 2010 DRI6012 QD | 32.01 | 4.28 | 31.74 | 4.15 | 89.37 | 17.00 | 87.68 | 13.63 | 7.58 | 0.65 | 7.53 | 0.63 |
| Bolli 2013 GetGoal-F1 | 32.53 | 5.36 | 32.37 | 5.45 | 88.81 | 17.98 | 87.87 | 17.37 | 8.05 | 0.88 | 8.03 | 0.82 |
| Riddle 2013 GetGoal-Duo1 | 31.99 | 6.63 | 31.65 | 6.01 | 87.31 | 21.76 | 86.75 | 20.41 | 7.56 | 0.55 | 7.60 | 0.54 |
| Seino 2012 GetGoal-L-Asia | 25.36 | 3.69 | 25.15 | 3.94 | 65.93 | 13.00 | 65.60 | 12.47 | 8.54 | 0.73 | 8.52 | 0.78 |
| Pan 2012 GetGoal-M-Asia | 26.75 | 3.86 | 27.08 | 3.75 | 73.18 | 13.93 | 72.74 | 13.64 | 7.95 | 0.81 | 7.85 | 0.71 |
| Ahren 2013 GetGoal-M | 32.84 | 6.34 | 33.12 | 6.45 | 89.57 | 20.91 | 90.15 | 20.14 | 8.06 | 0.89 | 8.06 | 0.90 |
| Ratner 2012 GetGoal-S | 30.13 | 6.62 | 30.42 | 6.64 | 82.30 | 21.76 | 84.42 | 22.83 | 8.28 | 0.86 | 8.21 | 0.84 |
| Riddle 2013 GetGoal-L | 31.91 | 6.17 | 32.56 | 6.32 | 87.10 | 20.01 | 88.94 | 20.84 | 8.42 | 0.88 | 8.37 | 0.84 |
| Pinget 2013 GetGoal-P | 33.66 | 6.71 | 34.44 | 7.04 | 92.93 | 22.90 | 96.74 | 25.58 | 8.08 | 0.90 | 8.06 | 0.79 |
| Fonseca 2012 GetGoal-Mono | 31.99 | 6.66 | 31.76 | 6.69 | 87.77 | 21.58 | 86.08 | 22.21 | 8.03 | 0.89 | 8.07 | 0.91 |
| PDY6797 | 25.09 | 3.65 | 26.39 | 3.50 | 71.54 | 16.77 | 76.92 | 16.07 | 8.19 | 0.82 | 8.38 | 0.75 |
| Rosenstock 2013 GetGoal-X | 33.68 | 6.27 | 33.51 | 6.54 | 94.01 | 19.63 | 96.09 | 22.52 | 7.95 | 0.81 | 7.97 | 0.78 |
| Seino 2012 EFC10780 | 36.76 | 7.25 | 36.76 | 6.34 | 98.51 | 23.48 | 100.56 | 23.77 | 8.16 | 0.89 | 8.09 | 0.96 |
| Kapitza 2013 PDY10931 | 31.23 | 3.93 | 31.33 | 4.08 | 91.16 | 15.28 | 92.88 | 16.59 | 7.20 | 0.63 | 7.41 | 0.81 |
Table 3.
Baseline liver blood tests expressed as units/litre (mean and SD)
| Trial | Alanine aminotransferase |
Aspartate aminotransferase |
Alkaline phosphatase |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lixisenatide |
Controls |
Lixisenatide |
Controls |
Lixisenatide |
Controls |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Lorenz 2013 ACT6011 QD | 25.48 | 8.18 | 25.91 | 12.50 | 19.43 | 4.95 | 19.23 | 4.62 | 83.52 | 27.00 | 83.55 | 19.43 |
| Ratner 2010 DRI6012 QD | 28.31 | 19.42 | 25.24 | 13.94 | 24.65 | 15.38 | 23.49 | 11.35 | 73.60 | 28.28 | 75.47 | 41.00 |
| Bolli 2013 GetGoal-F1 | 30.69 | 16.34 | 29.34 | 15.41 | 23.93 | 10.30 | 23.62 | 10.68 | 72.83 | 21.58 | 74.54 | 21.23 |
| Riddle 2013 GetGoal-Duo1 | 23.93 | 17.09 | 24.71 | 12.90 | 21.16 | 8.81 | 21.67 | 8.64 | 71.18 | 21.65 | 70.95 | 23.29 |
| Seino 2012 GetGoal-L-Asia | 25.19 | 12.85 | 23.03 | 10.34 | 22.78 | 7.56 | 21.43 | 7.79 | 71.90 | 17.59 | 71.01 | 20.11 |
| Pan 2012 GetGoal-M-Asia | 31.36 | 19.32 | 32.82 | 20.69 | 24.13 | 11.07 | 25.14 | 11.08 | 78.89 | 21.60 | 80.48 | 26.75 |
| Ahren 2013 GetGoal-M | 31.08 | 16.58 | 33.48 | 46.58 | 24.38 | 11.74 | 26.12 | 22.07 | 78.88 | 24.24 | 74.65 | 25.15 |
| Ratner 2012 GetGoal-S | 26.93 | 12.56 | 27.72 | 12.64 | 21.45 | 7.09 | 21.54 | 7.20 | 73.40 | 21.41 | 72.69 | 22.69 |
| Riddle 2013 GetGoal-L | 26.57 | 17.61 | 25.92 | 12.57 | 22.88 | 12.13 | 22.31 | 7.76 | 81.38 | 25.84 | 78.56 | 25.43 |
| Pinget 2013 GetGoal-P | 24.66 | 12.01 | 24.59 | 10.86 | 21.46 | 8.93 | 21.48 | 8.27 | 75.24 | 29.68 | 73.13 | 22.49 |
| Fonseca 2012 GetGoal-Mono | 29.74 | 16.05 | 25.43 | 11.40 | 23.71 | 11.78 | 21.91 | 7.40 | 78.95 | 21.10 | 80.07 | 26.42 |
| PDY6797 | 23.72 | 11.15 | 27.00 | 14.67 | 23.97 | 15.33 | 23.73 | 9.52 | 61.59 | 14.64 | 70.73 | 18.19 |
| Seino 2012 EFC10780 | 35.70 | 20.63 | 39.83 | 23.64 | 25.50 | 13.76 | 28.74 | 20.54 | 81.97 | 25.11 | 83.61 | 28.13 |
| Rosenstock 2013 GetGoal-X | 28.50 | 13.06 | 30.65 | 15.83 | 22.17 | 7.92 | 23.28 | 9.27 | 72.43 | 22.13 | 72.15 | 19.86 |
| Kapitza 2013 PDY10931 | 33.88 | 16.36 | 34.23 | 16.99 | 27.31 | 13.47 | 26.45 | 9.07 | 67.56 | 16.62 | 67.58 | 20.07 |
The duration of therapy ranged from 4 to 76 weeks (mean 29 weeks). Two trials (one unpublished)21 were designed to evaluate dose titration. The dose of lixisenatide was 20 µg once daily in the remaining trials. Twelve trials compared lixisenatide versus placebo and three trials compared lixisenatide versus active controls administered once daily (liraglutide and sitagliptin) or twice daily (exenatide). The collateral interventions (background therapy) were metformin (five trials), metformin and sulfonylurea (four trials), metformin and pioglitazone (one trial), insulin (three trials) or diet (one trial). The collateral interventions were administered equally to the lixisenatide and control groups.
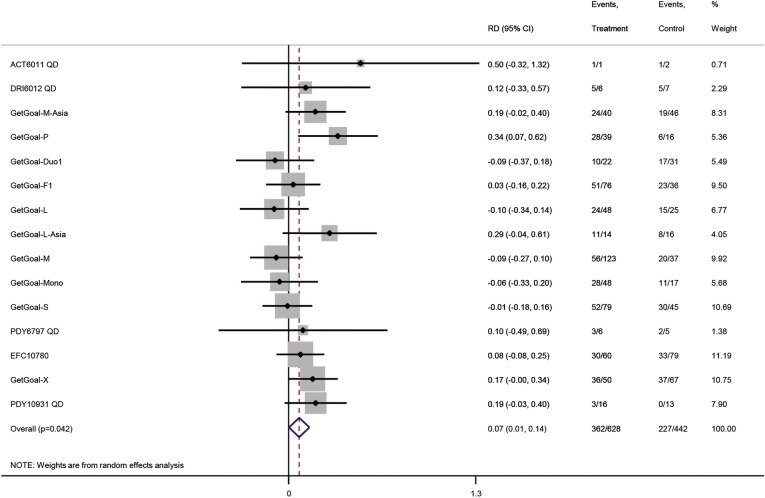
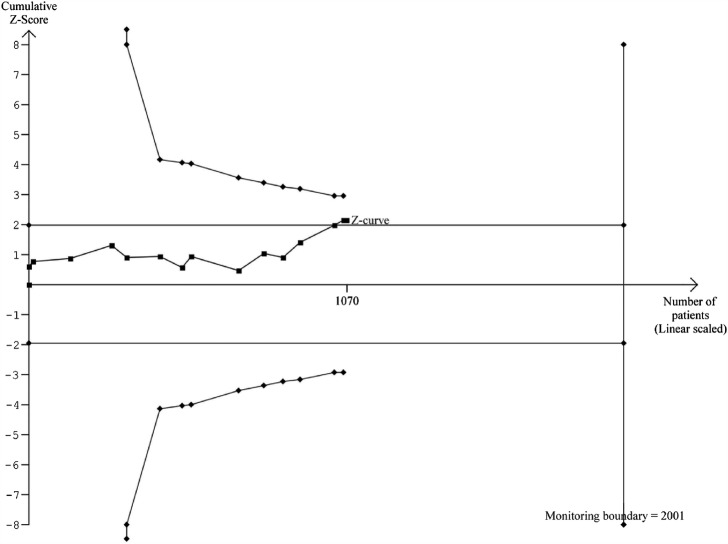
In total, 1070 patients had elevated ALT at baseline (figure 2). Lixisenatide had a beneficial effect on normalisation of ALT (risk difference 0.07; 95% CI 0.01 to 0.14; I2=23%; number needed to treat 14 patients, p=0.042). The sequential analysis confirmed the primary meta-analysis when using the traditional 5% level of statistical significance (figure 3), but not after adjusting for multiple testing (the trial monitoring boundary was not crossed). Mixed effect multilevel meta-regression analyses of double blind trials (figure 4) found no effect of the metabolic regulation, duration of diabetes or BMI on the overall result (p>0.05 for all analyses). There was no difference between patients with BMI<25 or ≥25. When the analyses were repeated for RCTs with an active control group, lixisenatide had a beneficial effect on normalisation of ALT among patients who were obese (p=0.01) or overweight (p=0.004), but not among normal weight patients (p=0.98). Random effects subgroup meta-analyses of RCTs with an active comparator found a beneficial effect of lixisenatide in patients with BMI≥25 (0.07; 0.02 to 0.12; p=0.004), but not in patients with BMI<25 (−0.04; −0.36 to 0.27; p=0.28). There was a moderate correlation between change in body weight and change in ALT (regression coefficient=0.38). The baseline metabolic regulation and duration of diabetes did not predict the intervention effect. No evidence of small study effects was seen in regression analysis (Harbord's test, p=0.26) or funnel plots (figure 5). Subgroup analyses showed no differences between trials stratified by the publication status, control groups or collateral interventions.
Figure 2.
Random effects meta-analysis of randomised controlled trials on normalisation of alanine aminotransferase (ALT). The result of the analysis is expressed as the risk difference (RD) with 95% CIs and level of significance (p value). The intervention comparisons are lixisenatide versus placebo or active interventions. The included patients have type 2 diabetes and elevated ALT at baseline. The outcome measure is normalisation of ALT.
Figure 3.
Sequential analysis of risk ratios (random effects) in randomised controlled trials on lixisenatide versus placebo or active interventions for patients with type 2 diabetes and elevated alanine aminotransferase (ALT) at baseline. The analysis was performed with α 5%, power 80%, model-based diversity correction 12%, relative risk reduction 8% and control group incidence rate 51%. The outcome measure is normalisation of ALT. The analysis shows that lixisenatide has a beneficial effect on normalisation of ALT when assessed using the traditional 5% level of significance (the horizontal line), but not after adjusting for cumulative assessment (the trial monitoring boundary).
Figure 4.
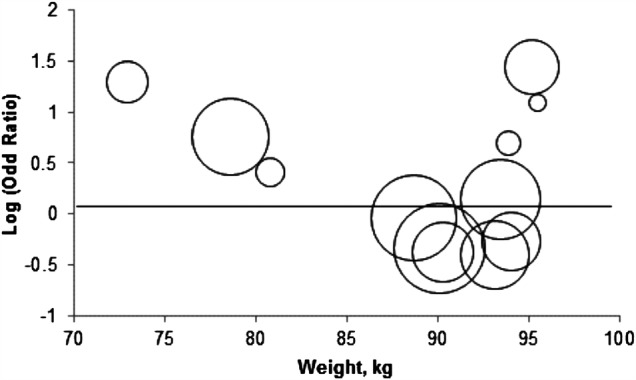
Mixed model meta-regression analysis of the effect of lixisenatide versus placebo on normalisation of alanine aminotransferase (ALT). Included patients have type 2 diabetes and elevated ALT at baseline. The outcome measure is normalisation of ALT. The figure shows the estimated intervention effect (normalisation of ALT) on the log-OR scale in relation to the baseline body weight of included patients from 12 randomised placebo-controlled trials. The size (area) of each circle is inversely proportional to the variance of the log OR (the larger the circle the lesser the variance).
Figure 5.
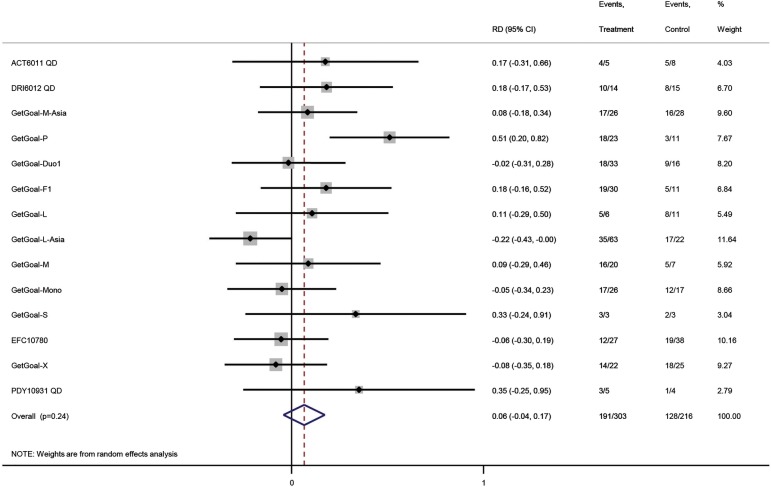
Random effects meta-analysis of randomised controlled trials on normalisation of aspartate aminotransferase (AST). The result of the analysis is expressed as the risk difference (RD) with 95% CIs and level of significance (p value). The intervention comparisons are lixisenatide versus placebo or active interventions. The included patients have type 2 diabetes and elevated ALT at baseline. The outcome measure is normalisation of ALT.
In total, 191 of 303 (37%) patients randomised for lixisenatide and 128 of 216 (41%) for controls achieved normalisation of AST after treatment (figure 5). Random effects meta-analysis found no effect of lixisenatide on AST (0.06; −0.04 to 0.17), alkaline phosphatase (−0.10; −0.23 to 0.03), bilirubin (−0.12, −0.30 to 0.07) or normalisation of all liver blood tests (0.01, −0.01 to 0.03). No differences between subgroups were identified.
Discussion
This systematic review evaluated the effects of lixisenatide on elevated transaminases among patients with type 2 diabetes and a high risk of NAFLD. Analyses of outcome measures recalculated based on individual patient data showed that lixisenatide increased the proportion of patients who achieved normal ALT levels compared with placebo or other glucose-lowering agents. The number needed to treat was 14 patients suggesting that the size of the potential benefit is clinically relevant. Our subgroup analyses suggested that lixisenatide was more effective in obese patients. We also found that lixisenatide was more effective than other active controls, which included liraglutide, sitagliptin and exenatide. However, the number of patients in the subgroup analyses was small and the findings therefore uncertain. Further, ALT is used in the diagnostic evaluations and follow-up of patients with NAFLD and NASH in clinical practice, but previous evidence suggests that the sensitivity is low. The low sensitivity suggests that we may overlook intervention benefits. Additional information about outcomes such as histological changes is needed.
Individual patient data meta-analyses are based on original research data instead of data extracted from published reports. The benefits of this approach include a reduced risk of errors as well as the ability to perform the relevant subgroup and sensitivity analyses. The quality of such analyses is high and individual patient data meta-analyses are considered as a ‘gold standard’ of systematic reviews.35 36 The main limitation of our review is related to the number of events and patients. As demonstrated in our sequential analyses, the available evidence cannot support or refute clinically relevant intervention effects. None of the included trials were specifically designed to evaluate the effect of lixisenatide on patients with NAFLD or NASH. However, one of the specific strengths of the meta-analytic approach is that it allows an assessment of questions not posed by the individual studies. In general, analyses of specific subgroups may be difficult in systematic reviews of RCTs that are based on published data. By contrast, such subsets of participants can be analysed when individual patient data are collected.37 Lixisenatide only appeared to have an effect on ALT, which is the most sensitive biochemical marker of NAFLD. The objective nature of this outcome measure strengthens the validity of our findings. Theoretically, our analyses would have been more sensitive if we had analysed the change in ALT as a continuous outcome. However, there is no clear evidence between quantitative changes in ALT and intervention effects. We found no beneficial or detrimental effects when analysing the remaining liver blood tests.
Incretin-based therapies such as lixisenatide and other GLP-1RAs are an important part of the pharmacological treatment of patients with type 2 diabetes. Experimental studies suggest that activation of GLP-1 receptors may prevent diabetes-related comorbidity including obesity and NASH,38 and that GLP-1RAs may improve hepatic steatosis.39 The beneficial effects of GLP-1RA include improved glycaemic control as well as beneficial effects on body weight, blood pressure, cholesterol and cardiovascular biomarkers. Lixisenatide is a once-daily GLP-1RA. The included RCTs found beneficial effects of lixisenatide used as monotherapy or in combination with metformin, sulfonylureas, thiazolidinediones or basal insulin glargine. The improved glycaemic control was mainly demonstrated in RCTs with a placebo control. Conversely, we found that the benefit of lixisenatide on liver blood tests was more pronounced in RCTs with an active control group. This result suggests that improved metabolic regulation and reductions in body weight may not be the only reason for the potentially beneficial effect on lixisenatide on NAFLD. Some experimental studies suggest that hepatocytes have specific GLP-1 receptors.17 40 The findings are controversial. In theory, different GLP-1RA may vary in their receptor affinity. The differences between the intervention effects in RCTs with an active comparator and placebo-controlled trials could also reflect the proportion of patients who were overweight. However, at present there is no clear evidence to support or refute this theory. Although we found a potential difference between lixisenatide and other GLP-1RA, the number of trials and the number of patients were too small to make any definite conclusions.
It may be argued that the beneficial effect of lixisenatide reflects changes to the daily intake of alcohol due to gastrointestinal adverse effects. However, none of the primary RCTs included patients with an ongoing alcohol abuse or alcoholic liver disease. None of the RCTs collected data on the exact daily intake of alcohol during the trial. We were therefore unable to determine the potential influence of alcohol.
In conclusion, the risk of bias in this systematic review was small supporting the validity of our findings. The use of lixisenatide seems to have beneficial effects on elevated levels of ALT in patients with type 2 diabetes and could possibly have a role in the treatment of patients with type 2 diabetes and NAFLD. We found potential differences between patients who were obese or normal weight, and in trials with a placebo control or active comparator. However, additional trials are clearly needed to assess our findings. The evidence does not allow definite treatment recommendations.
Supplementary Material
Acknowledgments
The authors thank Elena Nikonova and Edward Wang from Sanofi-Aventis for their statistical help and for retrieving data from included trials.
Footnotes
Contributors: LLG wrote the initial draft for the paper, extracted data and performed the statistical analyses. TV extracted data. LLG, TV and FKK participated in the selection of trials and revised the paper for important intellectual comments.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Sanofi-Aventis funded the BMJ Open publication fee.
Competing interests: LLG has participated as an investigator in a trial funded by MSD. FKK has received research funding from Sanofi-Aventis and lecture fees from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Gilead Sciences, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceuticals, Sanofi-Aventis and Zealand Pharma; is part of the Advisory Boards of Eli Lilly, Bristol-Myers Squibb/AstraZeneca, Novo Nordisk and Zealand Pharma; and has consulted AstraZeneca, Gilead Sciences, Ono Pharmaceuticals, Novo Nordisk, Sanofi-Aventis and Zealand Pharma. TV has received fees for being part of an advisory board from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, GI Dynamics, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis and Takeda, and received fees for speaking from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Novartis, Sanofi-Aventis, Takeda and Zealand Pharma, and received research support from Novo Nordisk.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extra data is available by emailing LLG (liselottegluud@yahoo.dk).
References
- 1.Verbeek J, Cassiman D, Lannoo M et al. . Treatment of non-alcoholic fatty liver disease: can we already face the epidemic? Acta Gastroenterol Belg 2013;76:200–9. [PubMed] [Google Scholar]
- 2.Parkash O, Hamid S. Are we ready for a new epidemic of under recognized liver disease in South Asia especially in Pakistan? Non alcoholic fatty liver disease. J Pak Med Assoc 2013;63:95–9. [PubMed] [Google Scholar]
- 3.Blachier M, Leleu H, Peck-Radosavljevic M et al. . The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013;58:593–608. 10.1016/j.jhep.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Szczepaniak LS, Nurenberg P, Leonard D et al. . Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–8. 10.1152/ajpendo.00064.2004 [DOI] [PubMed] [Google Scholar]
- 5.Bedogni G, Miglioli L, Masutti F et al. . Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005;42:44–52. 10.1002/hep.20734 [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Bertolini L, Padovani R et al. . Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–18. 10.2337/dc06-2247 [DOI] [PubMed] [Google Scholar]
- 7.Estep JM, Birerdinc A, Younossi Z. Non-invasive diagnostic tests for non-alcoholic fatty liver disease. Curr Mol Med 2010;10:166–72. 10.2174/156652410790963321 [DOI] [PubMed] [Google Scholar]
- 8.Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int 2011;31:461–73. 10.1111/j.1478-3231.2011.02451.x [DOI] [PubMed] [Google Scholar]
- 9.Barsic N, Lerotic I, Smircic-Duvnjak L et al. . Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:3945–54. 10.3748/wjg.v18.i30.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terjung B, Lemnitzer I, Dumoulin FL et al. . Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion 2003;67:138–45. 10.1159/000071293 [DOI] [PubMed] [Google Scholar]
- 11.Copel L, Sosna J, Kruskal JB et al. . Ultrasound-guided percutaneous liver biopsy: indications, risks, and technique. Surg Technol Int 2003;11:154–60. [PubMed] [Google Scholar]
- 12.Angelico F, Burattin M, Alessandri C et al. . Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev 2007;(1):CD005166. [DOI] [PubMed] [Google Scholar]
- 13.Shyangdan D, Clar C, Ghouri N et al. . Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review. Health Technol Assess 2011;15:1–110. 10.3310/hta15380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009;32:187–202. 10.2165/00002018-200932030-00002 [DOI] [PubMed] [Google Scholar]
- 15.Lewis JD, Habel L, Quesenberry C et al. . Proteinuria testing among patients with diabetes mellitus is associated with bladder cancer diagnosis: potential for unmeasured confounding in studies of pioglitazone and bladder cancer. Pharmacoepidemiol Drug Saf 2014;23:636–45. 10.1002/pds.3619 [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Soto G, de Luis DA, Conde-Vicente R et al. . Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with type 2 diabetes: a prospective study. Diabetes Res Clin Pract 2014;104:92–6. 10.1016/j.diabres.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 17.Gupta NA, Mells J, Dunham RM et al. . Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010;51:1584–92. 10.1002/hep.23569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilsboll T, Christensen M, Junker AE et al. . Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771 10.1136/bmj.d7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott LJ. Lixisenatide: a review of its use in patients with type 2 diabetes mellitus. BioDrugs 2013;27:509–23. 10.1007/s40259-013-0057-y [DOI] [PubMed] [Google Scholar]
- 20.Christensen M, Knop FK, Vilsboll T et al. . Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs 2011;20:549–57. 10.1517/13543784.2011.562191 [DOI] [PubMed] [Google Scholar]
- 21.Lorenz M, Pfeiffer C, Steinstrasser A et al. . Effects of lixisenatide once daily on gastric emptying in type 2 diabetes—relationship to postprandial glycemia. Regul Pept 2013;185C:1–8. 10.1016/j.regpep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Ratner RE, Rosenstock J, Boka G. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–32. 10.1111/j.1464-5491.2010.03020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddle MC, Aronson R, Home P et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489–96. 10.2337/dc12-2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddle MC, Forst T, Aronson R et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013;36:2497–503. 10.2337/dc12-2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seino Y, Min KW, Niemoeller E et al. . Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910–17. 10.1111/j.1463-1326.2012.01618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahren B, Dimas AL, Miossec P et al. . Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013;36:2543–50. 10.2337/dc12-2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca VA, Alvarado-Ruiz R, Raccah D et al. . Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–31. 10.2337/dc11-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapitza C, Forst T, Coester HV et al. . Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013;15:642–9. 10.1111/dom.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstock J, Raccah D, Koranyi L et al. . Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013;36:2945–51. 10.2337/dc12-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinget M, Goldenberg R, Niemoeller E et al. . Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab 2013;15:1000–7. 10.1111/dom.12121 [DOI] [PubMed] [Google Scholar]
- 31.Bolli GB, Munteanu M, Dotsenko S et al. . Efficacy and safety of lixisenatide once daily vs. placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med 2014;31:176–84. 10.1111/dme.12328 [DOI] [PubMed] [Google Scholar]
- 32.Ratner RE, Hanefeld M, Shamanna P, et al. Efficacy and safety of lixisenatide once-daily versus placebo in patients with type 2 diabetes mellitus insufficiently controlled on sulfonylurea ± metformin (GetGoal-S). Diabetologia 2011;54:785. [Google Scholar]
- 33. doi: 10.1016/j.jdiacomp.2015.07.003. Seino Y, Akane T, Niemoeller E, et al.; on behalf of the GetGoal-Mono Study Group. Long-term Safety of Once-daily (QD) Lixisenatide in Japanese Patients with Type 2 Diabetes Mellitus (T2DM): GetGoal-Mono Japan. 2012. http://www.aa-sd.org/ [DOI] [PubMed] [Google Scholar]
- 34. Pan CY, Shang S, Niemoeller E, et al.; on behalf of the GetGoal-M Asia Study Investigators. Lixisenatide in Asian Patients with Type 2 Diabetes (T2DM) Uncontrolled on Metformin±Sulfonylurea (SU): GetGoal-M Asia. 2012. http://www.aa-sd.org/ [Google Scholar]
- 35.http://handbook.cochrane.org/. Cochrane Handbook for Systematic Reviews of Interventions. Secondary Cochrane Handbook for Systematic Reviews of Interventions.
- 36.Stewart GB, Altman DG, Askie LM et al. . Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS ONE 2012;7:e46042 10.1371/journal.pone.0046042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deeks JJ, Higgins JPT, Altman DG, eds. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane-handbook.org. [Google Scholar]
- 38.Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J Diabetes Investig 2013;4:108–30. 10.1111/jdi.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabetes Complications 2013;27:401–6. 10.1016/j.jdiacomp.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 40.Ding X, Saxena NK, Lin S et al. . Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006;43:173–81. 10.1002/hep.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.