Abstract
The ability to introduce transgenes with precise specificity to the desired target cells or tissues is key to a more facile application of genetic therapy. Here, we describe a novel method using nanotechnology to generate lentiviral vectors with altered recognition of host cell receptor specificity. Briefly, the infectivity of the vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral vectors was shielded by a thin polymer shell synthesized in situ onto the viral envelope, and new binding ability was conferred to the shielded virus by introducing acrylamide-tailored cyclic arginine-glycine-aspartic acid (cRGD) peptide to the polymer shell. We termed the resulting virus “targeting nanovirus.” The targeting nanovirus had similar titer with VSV-G pseudotypes and specifically transduced Hela cells with high transduction efficiency. In addition, the encapsulation of the VSV-G pseudotyped lentivirus by the polymer shell did not change the pathway that VSV-G pseudotypes enter and fuse with cells, as well as later events such as reverse transcription and gene expression. Furthermore, the targeting nanovirus possessed enhanced stability in the presence of human serum, indicating protection of the virus by the polymer shell from human serum complement inactivation. This novel use of nanotechnology demonstrates proof of concept for an approach that could be more generally applied for redirecting viral vectors for laboratory and clinical purposes.
Liang and colleagues apply an in situ polymerization method to encapsulate vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral vectors with a crosslinked polymer shell. This approach leads to an enhancement of targeting ability, infectivity, and stability of the vector, even in the presence of human serum complement.
Introduction
Stably integrating retroviral and lentiviral vectors are commonly utilized for gene delivery (Cavazzana-Calvo et al. 2000; Aiuti et al. 2002, 2009; Cartier et al. 2009). Because the current vectors have broad host range, typically due to pseudotyping with VSV-G envelope (Marsh and Helenius, 1989), the vectors are limited in their use of applications in which the desired target cells and tissues can be purified and/or physically isolated for transduction. The creation of retroviral vectors that can target specific cells within mixed populations allows a more general application of genetic therapy. The primary obstacles have been modification of vector envelopes to specifically target, while at the same time maintain, virion stability and titer (Kasahara et al., 1994; Valsesia-Wittmann et al., 1994; Han et al., 1995; Somia et al., 1995; Marin et al., 1996; Nilson et al., 1996; Yu and Schaffer, 2005). In addition, viral envelopes encode a variety of receptor-binding moieties that are nontarget-cell specific, such as binding to heparin sulfate, laminin, integrins, carbohydrates, lipids, etc. (Haywood, 1994).
Several retroviral systems have been reported to redirect vectors to specific cells; yet, few accomplish targeting while maintaining high titers of stable transduction (Kasahara et al., 1994; Valsesia-Wittmann et al., 1994; Han et al., 1995). Modification of lentiviral vectors to achieve specific targeting requires two approaches. First, modifications to vectors must be made so that they can utilize unique cell surface molecules as new receptors to redirect vector binding to the desired target cells. We have successfully accomplished targeted transduction in vitro and in vivo using a modified Sindbis virus envelope pseudotype (Morizono et al., 2001, 2005, 2006, 2009, 2010; Pariente et al., 2007, 2008; Liang et al., 2009b). Our initial construct consisted of a Sindbis virus envelope pseudotype modified by conjugation with affinity reagents such as antibodies directed to cell surface molecules or genetically engineered for covalent incorporation of ligands that bind specific cell surface molecules. We demonstrated that our vectors could be utilized in murine models to target tumors (Morizono et al., 2005; Pariente et al., 2007). The second complementary approach is to reduce off-target binding. We made several specific mutations that ablate native receptor binding of the Sindbis envelope. However, a residual low-level, nonspecific binding complicated the targeted transduction. We recently identified one source of nonspecific binding mediated through virion phosphatidylserine binding to molecules that bridge to receptors on the cell surface (Morizono et al., 2011).
In addition to genetic and metabolic modifications of the virus envelope for targeting, chemical modifications of viral vectors were also reported for adenovirus and VSV-G pseudotyped lentivirus. Until now, chemical modification of adenovirus vectors and VSV-G pseudotyped lentiviral vectors with synthetic polymers, such as polyethylene glycol (PEG), used a “grafting-onto” strategy. This strategy includes two steps, activating linear polymers and conjugating polymers to the surface of the viral vector. Grafting-onto strategy can only conjugate linear polymers onto the viral surface; therefore, the shielding of the viral infectivity is not complete. For example, modification of adenovirus vectors with PEG significantly reduces innate immune responses to adenovirus vectors and evades pre-existing anti-adenovirus antibodies (Muller-Sieburg et al., 2004, 2012; Lee et al., 2005; Giordano et al., 2011). However, in vivo targeting efficiency using PEGylated adenovirus vectors is still not sufficient, and background infectivity still exists in liver cells (Kreppel and Kochanek, 2008). VSV-G envelope protein confers unobtainable robust physical stability on the viruslike particles, which prevents it from being disrupted by shear forces encountered during concentration by ultracentrifugation and multiple freeze–thaw cycles. However, use of VSV-G pseudotyped vectors in vivo continues to be hampered by an innate immune response directed against the virus particles (DePolo et al., 2000). This effect is largely mediated through the classical complement pathway (Beebe and Cooper, 1981). Although PEGylated VSV-G pseudotyped lentiviral vector was reported to be prevented from human serum complement inactivation (Croyle et al., 2004), chemical modification to redirect VSV-G pseudotyped lentiviral vectors to new receptors has not been previously reported.
We previously synthesized a family of small nanocapsules in which single protein molecules were encapsulated into an organic polymer nanocapsule with a thin crosslinked network shell (Yan et al., 2006, 2010). Different from the grafting-onto strategy, the crosslinked network shells were synthesized on the protein surface by a two-step “growing-onto” process: (I) First, a polymerizable molecular anchor, N-acryloxysuccinimide (NAS), was used to react with the lysine of the protein to generate polymerizable groups. (II) These polymerizable groups then reacted with the vinyl groups of the monomers, such as acrylamide, to form polymers on the viral surface. Crosslinkers, such as glycerol dimethacrylate (GMA), were included in the reaction to stabilize the polymer structure. These nanocapsules presented uniform size (∼20 nm), high protein activity retention, and outstanding protein stability. Such nanocapsules exhibited two orders of magnitude higher efficiency of intracellular delivery compared with protein transduction through TAT peptide conjugation; moreover, the polymer shell protects the proteins from protease attack and thermal inactivation, greatly increasing the half-life of the protein payload. The in vitro toxicity of nanocapsules was lower than those using TAT peptide conjugation.
In this study, we applied this in situ polymerization method to encapsulate VSV-G pseudotyped lentiviral vectors with crosslinked polymer shell and generated a targeting nanovirus with enhanced targeting ability, infectivity, and stability for gene therapy.
Materials and Methods
Virus production and titer
All lentiviral vectors were produced by calcium phosphate–mediated transient transfection of 293T cells, as previously described (Morizono et al., 2001; Morizono and Chen, 2005). Briefly, 293T cells (1.8×107 cells) were transfected with 12.5 μg of pCMVR8.2_VPR, 12.5 μg of SIN18-RhMLV-E with central polypurine tract (termed cppt2e), and 5 μg VSV-G-expressing plasmids. And 6 ug beta-lactamase-Vpr–fused protein-coding plasmid was included for generating VSV-G pseudotyped virus packaging with beta lactamase. The viral vectors were harvested in AIM V® Medium (Invitrogen) with antibiotics and were concentrated by ultracentrifugation at 28,000 RPM for 90 min at 4°C by SW32 rotor (Beckman). The pellets were resuspended in a 100-fold lower volume of phosphate buffered saline (PBS). The viral titer was measured by anti-p24 Gag enzyme-linked immunosorbent assay (ELISA). Reporter gene expression was monitored by flow cytometry. Data were collected on a Cytomics FC500 (Beckman Coulter) and analyzed using fetal calf serum (FCS) express (De Novo Software).
Synthesis of acrylamide-tailored arginine-glycine-aspartic acid
Cyclic arginine-glycine-aspartic acid, which is marked as cRGD, was ordered from Peptides International, Inc. The preparation of acrylamide-tailored cRGD was achieved by reacting cRGD with acrylic acid and hydroxysuccinimide ester (NAS). Briefly, cRGD (5 mg) was dissolved in 1 mL, pH=8, 50 mM HEPES buffer, and NAS (1.2 mg) were dissolved in 100 uL DMSO. The NAS solution was then added into the cRGD solution gradually at room temperature. After overnight reaction, the mixture was diluted to 0.02% with 1× PBS buffer as stock.
Synthesis and size characterization of nanovirus
In addition, 100x concentrated VSV-G pseudotyped lentiviral vectors were dialyzed in 1× PBS buffer at 4°C overnight. The viral titer was measured by anti-p24 Gag ELISA. Also, 5 mg NAS (Sigma #A8060) was dissolved in 0.1 mL of DMSO and diluted to 0.002% with ice-cold 1× PBS buffer. NAS solution was added to the virus (p24=60 ng) at different molar ratios (m/m) of 1×104, 2×104, 5×104, and 1×105. The reaction was carried out for 1 hr at 4°C. One uL of 100 mM pH=7 TRIS buffer was added to the microtube to stop the surface modification by NAS. A cocktail solution containing 2% (w/v) acrylamide (Sigma #A3553), 2% (w/v) GMA (Sigma #436895), 0.5% (w/v) APS (Sigma #A3678), and 0.1% (w/v) N,N,N',N'-tetramethylethylenediamine (Sigma #T9281) were added to the NAS-modified virus at different weight ratios (w/w) of 125, 250, 500, and 750 with or without acrylamide-tailored cRGD (0.02%) at a molar ratio of 2×104 to initiate the radical polymerization at the surface. The reaction was allowed to proceed at 4°C for another 60 min then the synthesized nanovirus were added to cells right after production. Size distribution of the virus and nanovirus (p24=60 ng) were measured by dynamic light scatter (DLS) with a Malvern particle sizer Nano-ZS in 1× PBS buffer.
Virus transduction
Both VSV-G pseudotypes and targeting nanovirus (p24=10 ng) were used to transduce 1×105 Hela cells for 4 hr in 24-well tissue culture-treated plate (Corning, 353047), then cells were washed twice with 1× PBS, cultured in 500 ul DMEM, 10% FBS, and 1% GPS for 3 days. Reporter gene expression (EGFP) was monitored by flow cytometry. For blocking assay, cRGD (1 mg/ml), cRAD (1 mg/ml), a mixture of anti-integrin antibodies (20 μg/ml of both anti-integrin αVβ3 and αVβ5 antibody) (Chemicon), or isotype control antibody (40 μg/ml) were incubated with the virus 30 min prior to and during the infection. For fusion assay, bafilomycin-A (Sigma, B1793) was incubated with the virus at a final concentration of 125 nM 30 min prior to and during the transduction. For reverse transcription assay, azidothymidine (AZT) (Sigma, A2169) was incubated with the virus at a final concentration of 25 uM 30 min. prior to and during virus infection. Cells were washed with 1× PBS and continued to be cultured in the presence of AZT for 3 days before flow cytometry analysis.
Entry assay
Both VSV-G pseudotypes and targeting nanovirus incorporating the Vpr-β-lactamase fusion protein (p24=100 ng) were used to transduce 1×105 Hela cells for 5, 15, 30, 45, 60, 90, and 120 min. Cells were washed twice with 1× PBS. Beta lactamase substrate CCF2-AM (Invitrogen, K1039) was incubated with cells for 2 hr at room temperature in the dark following company protocol. Fluorescence was monitored by flow cytometry.
Results
Synthesis of targeting nanovirus
The virus envelope is comprised of proteins, lipids, and carbohydrates. Since proteins are major components of the envelope, we considered our previous method of synthesizing protein nanocapsules could be applied to synthesize virus nanocapsules. We hypothesize that the crosslinked polymer shell synthesized around the virion will ablate the native infectivity of the virus, and new target-binding ability can be conferred through ligands conjugated on the surface of the polymer shell. To synthesize targeting virus nanocapsules, we used a two-step procedure to modify the VSV-G pseudotyped lentiviral vectors expressing EGFP (Fig. 1): (I) A polymerizable molecular anchor, NAS, was used to react with the lysine of the VSV-G envelope protein to generate polymerizable groups. (II) These polymerizable groups then reacted with the vinyl groups of the monomers (acrylamide) to form polymers on the viral surface. Crosslinkers (GMA) were included in the reaction to stabilize the polymer structure. The crosslinkers are degradable at pH <6, which allows release of the virion from the polymer shell at an acidic environment such as endosomes. To target Hela cells, cyclic arginine-glycine-aspartic acid (cRGD), which displays a strong affinity and selectivity to the αVβ3 integrin and is abundantly expressed on tumor endothelial and tumor cells (Mitra et al., 2005), was polymerized to the polymer shell in the form of acrylamide-tailored cRGD to generate targeting nanovirus.
FIG. 1.
Schematic illustration of the synthesis and delivery of vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral nanocapsules: (I) Surface modification of the lysine group of the envelope protein by NAS; (II) in situ polymerization of monomer (acrylamide), crosslinker (GMA), and targeting agent (acrylamide tailored-cRGD) at the surface of the modified virus; and (III) targeting delivery of the nanovirus to cells. GMA, glycerol dimethacrylate; cRGD, cyclic arginine-glycine-aspartic acid. Color images available online at www.liebertpub.com/hgtb
In the synthesis of both protein and virus nanocapsules, the polymerization process starts with reaction of polymerizable molecular anchors (NAS), with lysine of the protein or envelope protein of the virus. Although the mechanisms of polymerization are similar, there are several differences to encapsulate a virus compared to a single protein. First, the size of virus (∼100 nm) is bigger than a single protein (∼10 nm). To fully encapsulate a virion, the amounts of NAS and monomers as well as the reaction time need to be adjusted. Second, the virus is stable at 4°C. To maintain virus stability thus their infectivity, we optimized the reaction temperature to be 4°C instead of 25°C, which was previously used for protein. Third, efficient polymerization requires high concentration of substrate. We concentrated the virus to achieve a high concentration of 100 ug/mL to accelerate the polymerization process.
Optimization of transduction of cRGD conjugated targeting nanovirus in Hela cells
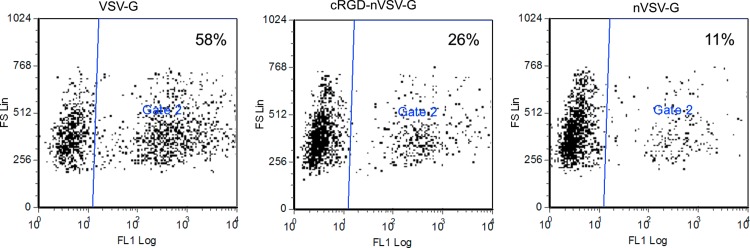
Composition, size, and degradability of the polymer shell of the targeting nanovirus are the essential parameters affecting their delivery efficiency and potency. The amount of NAS determines the amount of surface anchor for subsequent polymerization thus the gap distance between the polymers. The amount of monomers controls the length of the polymer thus the size of the polymer shell. Increased concentration of NAS and monomers resulted in a better shielding of the native viral infectivity; however, overshielding of the viral vectors might also lead to diminished transduction efficiency of the targeting nanovirus. Therefore, a balance of shielding and targeting transduction efficiency is required in the design of the targeting nanovirus. We first tested a ratio of NAS:virion (m:m=1×104) with a ratio of monomer:virion (w:w=250) and achieved transduction efficiency of 26% with cRGD and 11% without cRGD compared to the 58% of VSV-G pseudotypes (Fig. 2), indicating a partial ablation of the virus infectivity by the polymer shell. To achieve optimal targeting transduction efficiency and minimal background infectivity, we further tested a series of combinations of different amounts of NAS and monomers (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/hgtb) with or without cRGD. We examined the transduction efficiency of the targeting nanovirus in HeLa cells. As shown in Supplementary Figure S1, transduction of nanovirus without cRGD is always lower than transduction of nanovirus with cRGD, indicating the polymer shell shielded the native infectivity of the VSV-G pseudotyped lentivirus, and cRGD conferred new binding ability to the virus. In our test, we found that a combination of NAS:virus (m/m=2×104) and monomer:virus (w/w=125) could completely shield the native viral infectivity and resulted in a transduction efficiency of 35% with cRGD, which is similar to the VSV-G pseudotypes (42%) with the same amount of p24 (Supplementary Fig. S1), indicating a high infectivity of targeting nanovirus can be achieved by adjusting the gap distance and size of the polymer shell. Transduction efficiency of nanovirus without cRGD was 0% at this combinational ratio. We also determined the sizes of the nanovirus by dynamic light scattering (DLS). Size of the nanovirus ranged from ∼100 nm to ∼150 nm (Supplementary Fig. S1). As predicted, the size of the nanovirus increased with increased amounts of monomer. The size of nanovirus with or without cRGD was similar (Supplementary Fig. S1). When testing the transduction efficiency of the targeting nanovirus with the optimal ratio of NAS and monomer to the virions in another RGD-expressing cell, human umbilical vein endothelial cells (HUVEC), a transduction efficiency of 8.1% was observed with a transduction efficiency of 11.7% by the VSV-G pseudotypes and a transduction efficiency of 0.2% by the nanovirus without cRGD (Supplementary Fig. S2). This result suggests the possibility of using the targeting nanovirus in a variety of integrin-expressing cells.
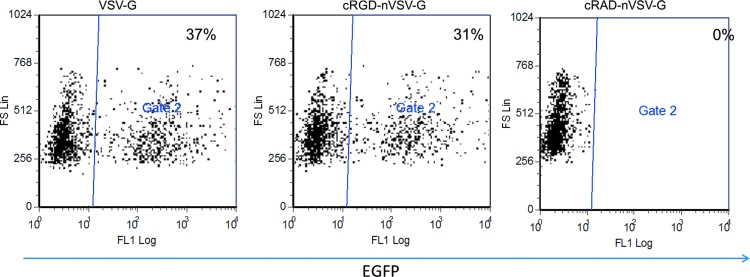
FIG. 2.
Shielding of virus infectivity by polymer shell. 1×105 Hela cells were transduced with equal amounts of VSV-G, cRGD-nVSV-G, and nVSV-G (p24=10 ng). Enhanced green fluorescent protein (EGFP) expression was monitored by flow cytometry 3 days post transduction. Color images available online at www.liebertpub.com/hgtb
Stability of the targeting nanovirus at 4°C and under freeze–thaw cycles
VSV-G pseudotyped lentivirus are stable during short-time storage at 4°C or when frozen at −70°C and thawed for a few times (Burns et al., 1993; Akkina et al., 1996). To examine whether the targeting nanovirus possess similar stability as VSV-G pseudotypes, we tested the transduction efficiency of the targeting nanovirus after keeping it at 4°C for 4 hr, 8 hr, and 24 hr as well as under freeze–thaw cycles. As shown in Supplementary Figure S3, there was no loss of infectivity of both VSV-G pseudotypes and the targeting nanovirus after incubation at 4°C, indicating both VSV-G pseudotypes and the targeting nanovirus are stable at 4°C for at least 24 hr. When under freeze–thaw cycles, the infectivity of VSV-G pseudotypes and the targeting nanovirus were stable after two cycles of freeze–thaw but reduced significantly at the third cycle of freeze–thaw (Supplementary Fig. S4), indicating the targeting nanovirus can be frozen and thawed at least twice without significant loss of infectivity as the VSV-G pseudotypes.
Transduction by targeting nanovirus to Hela cells is specifically mediated by RGD and integrin interaction
To confirm the specificity of the targeting transduction, we tested transduction of targeting nanovirus conjugated with a nonspecific peptide, cyclic RAD (cRAD), in Hela cells and observed no transduction, indicating the transduction of the nanovirus in Hela cells was conferred by cRGD not cRAD (Fig. 3). We also blocked HeLa cells by soluble cRGD or cRAD then transduced the cells with either VSV-G pseudotyped lentivirus or the cRGD-contained targeting nanovirus (cRGD-nVS-VG). Blocking by either cRGD or cRAD had no effect on the transduction efficiency by VSV-G pseudotyped lentivirus (Table 1). Blocking by cRGD but not cRAD inhibited the transduction by cRGD-nVSV-G to 40% of the transduction in the absence of blocking molecules (Table 1). These results further support that transduction by cRGD-nVSV-G in Hela cells is cRGD specific.
FIG. 3.
Targeted transduction of Hela cells by the nanovirus is cRGD dependent. 1×105 Hela cells were transduced with equal amount of VSV-G, cRGD-nVSV-G, and cRAD-nVSV-G (p24=10 ng) using the best ratio of N-acryloxysuccinimide (NAS) and monomer obtained from Figure S1. EGFP expression was monitored by flow cytometry 3 days post transduction. Color images available online at www.liebertpub.com/hgtb
Table 1.
Targeted Transduction of Hela Cells by Nanovirus is cRGD and Integrin Dependent
| Relative transduction efficiency (%) | ||
|---|---|---|
| VSV-G | No block | 100 |
| cRGD peptide | 96 | |
| cRAD peptide | 92 | |
| Anti-integrins | 101 | |
| Isotype control | 98 | |
| cRGD-nVSV-G | No block | 100 |
| cRGD peptide | 40 | |
| cRAD peptide | 97 | |
| Anti-integrins | 70 | |
| Isotype control | 96 |
The 1×105 Hela cells were preincubated with cRGD (1 mg/ml), cRAD (1mg/ml), anti-integrins antibodies (20 ug/ml), and isotype control (40 ug/ml) for 30 min, then transduced with equal amount of VSV-G and cRGD-nVSV-G (p24=10 ng).
Integrin is the receptor for RGD peptide on cell surface. Therefore, we further tested whether the transduction by cRGD-nVSV-G is integrin-dependent. We blocked the Hela cells by anti-integrin antibodies followed by transduction of VSV-G pseudoetypes or cRGD-nVSV-G. Anti-integrin antibodies suppressed transduction by cRGD-nVSV-G but not VSV-G pseudotypes (Table 1), indicating binding of cRGD on the cell surface is integrin-dependent. Isotype antibodies had no effect on either the VSV-G pseudotypes or the cRGD-nVSV-G. The incomplete blocking by soluble cRGD peptide or anti-integrin antibody is probably due to the competition with the multivalent binding of the targeting nanovirus to the cell-surface integrins.
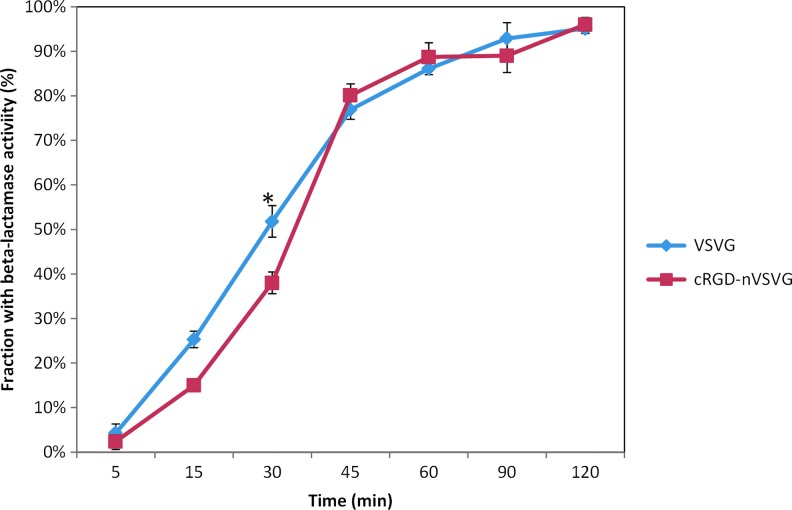
Entry kinetics of targeting nanovirus
We examined the virological properties of the targeting nanovirus compared to the classical standard VSV-G pseudotypes. First, we accessed the entry kinetics of the virus by β-lactamase (BlaM) assay, which has been used previously as a measure of viral entry into cells. BlaM-Vpr fusion protein was incorporated into both VSV-G pseudotypes and targeting nanovirus. Cytosolic BlaM activity was subsequently detected by loading cells with CCF2-AM, which is converted to a BlaM substrate by endogenous cytoplasmic esterases and retains in the cytosol. CCF2-AM exhibits a shift from green to blue fluorescence upon BlaM cleavage. BlaM activity was examined at 5, 15, 30, 45, 60, 90, and 120 min after incubating the virus with cells at 37°C. As shown in Figure 4, at 5, 15, and 30 min, BlaM activity was slightly higher in VSV-G pseudotype-transduced cells compared to the targeting nanovirus-transduced cells. After 30 min incubation, no significant difference was observed for BlaM activity in both virus-transduced cells (Fig. 4). These results indicated that the VSV-G pseudotypes entered cells and released BlaM slightly faster than the targeting nanovirus within the first 30 min. The delayed release of BlaM from the targeting nanovirus may be due to the degradation of the crosslinker, thus the polymer shell, before fusion of the viral membrane occurs.
FIG. 4.
Entry kinetics of the targeting nanovirus by β-lactamase assay. Both VSV-G pseudotypes and targeting nanovirus incorporating the BlaM-Vpr fusion protein (p24=100 ng) were used to transduce 1×105 Hela cells for 5, 15, 30, 45, 60, 90, and 120 min. The percentage of cells with β-lactamase activity was measured by flow cytometry (*p<0.05). Color images available online at www.liebertpub.com/hgtb
Fusion of targeting nanovirus
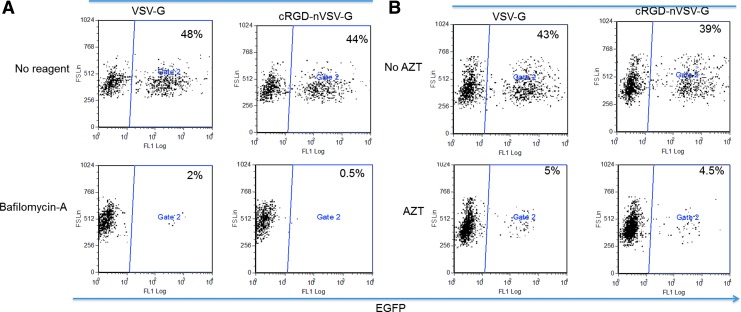
Native VSV-G virus enters cells via endocytosis and fuses at the endosome. Bafilomycin-A, an inhibitor of the vacuolar-type H+-ATPase, has been used to neutralize the pH in endosome thus inhibiting pH-dependent virus fusion and the following transduction. The crosslinker of the polymer shell of the targeting nanovirus degrades at low-pH, which allows release of the virion. Therefore, we hypothesized that the targeting nanovirus might also enter cells via endocytosis and the crosslinker degrades at the endosome to expose VSV-G protein for fusion. To confirm whether the endosome is the fusion site of the targeting nanovirus, VSV-G pseudotypes and targeting nanovirus were used to transduce HeLa cells with or without bafilomycin-A. In the presence of bafilomycin A, both transduction of VSV-G pseudotypes and targeting nanovirus were inhibited (Fig. 5A), indicating both viruses entered and fused through the endosome, and the shielding by polymer shell only ablated the binding but not fusion of the virus.
FIG. 5.
Fusion and reverse transcription of the targeting nanovirus. (A) 1×105 Hela cells were treated or not treated with vacuolar-type H+-ATPase inhibitor bafilomycin-A for 30 min then transduced with VSV-G or cRGD-nVSV-G (p24=10 ng) with or without bafilomycin-A for 4 hr. (B) Transduction by the nanovirus is inhibited by reverse transcriptase AZT. 1×105 Hela cells were treated or not treated with AZT for 30 min then transduced with VSV-G or cRGD-nVSV-G (p24=10 ng) for 4 hr with or without AZT. Color images available online at www.liebertpub.com/hgtb
Transduction of targeting nanovirus can be inhibited by reverse transcription inhibitor
To confirm that reverse transcription occurs during the transduction of targeting nanovirus, AZT, a reverse transcription inhibitor, was used to block transduction. VSV-G pseudotypes and targeting nanovirus were used to transduce HeLa cells with or without AZT. In the absence of AZT, both viruses transduced HeLa cells with transduction efficiency of 43% and 39% respectively (Fig. 5B). In the presence of AZT, both transductions were blocked (Fig. 5B), demonstrating reverse transcription is required for the infectivity by the targeting nanovirus.
Stability of the targeting nanovirus in the presence of human serum
It has been reported that VSV-G pseudotyped HIV vectors produced in human cells can be inactivated by human serum complement, suggesting higher stability of the envelope is required for therapeutic vector in clinical applications. We further examined whether the polymer shell of the targeting nanovirus can provide a protection to the virus from the inactivation by human serum complement. VSV-G pseudotypes or targeting nanovirus were incubated with PBS, heat-inactivated human serum (Sigma, S1764), or noninactivated human serum at a 1:1 ratio for 30 min prior to the infection and throughout the infection. As shown in Table 2, after human serum treatment, transduction efficiency of VSV-G pseudotypes was reduced, which is consistent with the published data. In the presence of human serum, transduction by the targeting nanovirus was 5-fold higher compared to VSV-G pseudotypes (Table 2). The transduction efficiency of VSV-G pseudotypes and targeting nanovirus were similar with PBS or heat-inactivated human serum treatment. These data demonstrated that VSV-G pseudotypes can be inactivated by human serum complement, and the polymer shell can protect the targeting nanovirus from inactivation by human serum.
Table 2.
Enhanced Stability of the Targeting Nanovirus in the Presence of Human Serum
| Relative transduction efficiency (%) | ||
|---|---|---|
| VSV-G | PBS | 100 |
| HI HS | 92 | |
| HS | 7 | |
| cRGD-nVSV-G | PBS | 100 |
| HI HS | 105 | |
| HS | 37 |
VSV-G or cRGD-nVSV-G were preincubated with phosphate buffered saline (PBS), heat-inactivated (HI) human serum (HS), or HS (v:v=1:1) for 1 hr. The pretreated viruses (p24=10 ng) were then used to transduce 1×105 Hela cells.
Discussion
Successful application of gene therapy for treatment of human disease requires the efficient and safe delivery of therapeutic genes to the desired sites of expression. The most effective approach would be to develop vectors that are home to and transduce specific cells and tissues. Numerous previous efforts have been made to develop retroviral vectors that can target specific cells and tissues. Typically, this involved modification of the native envelope and/or pseudotyping with other viral envelopes. These approaches have not been generally applicable because modifications in native envelope lead to large reductions in viral titer, and pseudotypes with other viral envelopes are not generally applicable to targeting many different types of cells and tissues (Kasahara et al., 1994; Valsesia-Wittmann et al., 1994; Han et al., 1995; Somia et al., 1995; Marin et al., 1996; Nilson et al., 1996; Yu and Schaffer, 2005).
We previously developed and described a targeting lentiviral vector pseudotyped with a modified version of the Sindbis virus envelope proteins that can target human leukocyte antigen (HLA) class I, CD4, CD19, CD20, CD45, CD146, CD34, P-glycoprotein of melanoma cells, and prostate stem cell antigen either in vitro or in vivo (Morizono et al., 2001, 2005, 2006; Morizono and Chen, 2005; Pariente et al., 2007, 2008; Liang et al., 2009a, 2009b). The distinguishing properties of this vector relative to past retroviral targeting vectors were that it could be produced in high titers and home to specific cells and tissues after systemic administration via the bloodstream. However, despite our extensive analysis of the Sindbis virus envelope and genetic ablation of envelope domains that confer native binding, we still observed residual off-target infectivity in some cell types, most notably endothelial cells. We discovered that this infectivity is conferred by bovine protein S in fetal calf serum, or Gas6, its human homolog (Morizono et al., 2011). Gas6 enhances native infectivity of pseudotypes of multiple viral envelope proteins. Gas6 mediates binding of the virus to target cells, bridging virion envelope phosphatidylserine to Axl, a Tyro-3, Axl, and Mer (TAM) receptor tyrosine kinase on target cells. The interactions between native virion envelope proteins as well as between novel interactions such as those through virion phosphatidylserine let us to consider other means to ablate binding between virions and cells and to confer novel specificities.
We designed a novel nanotechnology that encapsulates the virion by a polymer shell to reduce nonspecific targeting by preventing interactions between virion components and the target cells. Indeed, our results showed that the infectivity of VSV-G pseudotypes could be ablated completely by the polymer shell. For targeting delivery, we further introduced the acrylamide tailored-cRGD peptide to the polymer shell to direct targeted transduction to Hela cells. RGD specifically target αVβ3 integrin and has been considered as a ligand to deliver anticancer drugs to inhibit tumor angiogenesis and tumor growth (Shuhendler et al., 2012). We selected the cRGD peptide, which confer greater stability and selectivity over the linear RGD, to target Hela cells as a proof of concept of the targeting delivery of the targeting nanovirus. Unlike genetic modification for desired retargeting properties, a two-step chemical approach was used to transform a lentiviral vector into a novel targeting nanovirus: (1) Anchor molecules are conjugated to the specific amino acid (lysine) of envelope proteins; and (2) a thin degradable polymer network grows through in situ polymerization from those anchors, and the ligands (cRGD peptides) are embeded in the polymer shell of the nanovirus to redirect binding to the desired receptor. We previously observed that the protein nanocapsules entered cells via endocytosis (Yan et al., 2010). Since the nanovirus was synthesized via a similar process as protein nanocapsules, we assume that the nanovirus will also enter cells via endocytosis. Once the nanovirus is internalized via endocytosis, the acid-degradable linkages of the polymer react to release the virion and allow fusion and entry of the virion into the cytoplasm.
This nano-engineering approach has several advantages. First, the polymer shell shields the virion envelope from interaction with cells, preventing both specific and off-target binding due to interactions between envelope proteins as well as N-glycans and lipids, which we reported. In addition, we expect that there are as yet uncharacterized envelope-cell interactions through other carbohydrates, proteins, and lipids that would contribute to off-target transduction. We optimized the polymerization of the nanovirus shell to prevent all such interactions without affecting subsequent steps involved in entry, fusion, and reverse transcription. Conjugation of PEG has been proven to dramatically blunt the immune response against adenovirus and vesicular stomatitis virus G (VSV-G) pseudotyped lentiviral vector (Croyle et al., 2000, 2004). To further reduce potential nonspecific interactions between the nanovirus and the immune system, we can replace the monomer, acrylamide, with PEG.
In addition to confer properties of targeting and reduced off-target infectivity, the targeting nanovirus also presents distinctive properties such as high titer and enhanced stability in human serum. Although VSV-G envelope possesses robust physical stability, which allows it to be concentrated and achieve high titer, there are no successful attempts to transform it for targeting. Our targeting nanovirus successfully combines the advantages of both VSV-G envelope and a polymer shell to achieve targeting with high transduction efficiency and enhanced stability.
This is the first demonstration of encapsulating VSV-G pseudotyped lentivirus for efficient targeting delivery by polymer nanotechnology. Further development of this technology should allow for targeted delivery using other ligands.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- Aiuti A. Slavin S. Aker M., et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Aiuti A. Cattaneo F. Galimberti S., et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. New Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Akkina R.K. Walton R.M. Chen M.L., et al. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J. Virol. 1996;70:2581–5. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D.P. Cooper N.R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J. Immunol. 1981;126:1562–8. [PubMed] [Google Scholar]
- Burns J.C. Friedmann T. Driever W., et al. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N. Hacein-Bey-Abina S. Bartholomae C., et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Hacein-Bey S. De Saint Basile G., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Croyle M.A. Yu Q.C. Wilson J.M. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 2000;11:1713–22. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- Croyle M.A. Callahan S.M. Auricchio A., et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004;78:912–21. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depolo N.J. Reed J.D. Sheridan P.L., et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2:218–22. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Giordano F. Sorg U. Appelt J., et al. Clonal inventory screens uncover monoclonality following serial transplantation of MGMT P140K-transduced stem cells and dose-intense chemotherapy. Hum. Gene Ther. 2011;22:697–710. doi: 10.1089/hum.2010.088. [DOI] [PubMed] [Google Scholar]
- Han X. Kasahara N. Kan Y.W. Ligand-directed retroviral targeting of human breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A.M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J. Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara N. Dozy A. Kan Y. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- Kreppel F. Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol. Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- Lee G.K. Maheshri N. Kaspar B. Schaffer D.V. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol. Bioeng. 2005;92:24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- Liang M. Morizono K. Pariente N. Kamata M., et al. Targeted transduction via CD4 by a lentiviral vector uses a clathrin-mediated entry pathway. J. Virol. 2009a;83:13026–31. doi: 10.1128/JVI.01530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M. Pariente N. Morizono K. Chen I. Targeted transduction of CD34+ hematopoietic progenitor cells in nonpurified human mobilized peripheral blood mononuclear cells. Journal of Gene Medicine. 2009b;11:185–96. doi: 10.1002/jgm.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M. Noel D. Valsesia-Wittman S., et al. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J. Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. Helenius A. Virus entry into animal cells. Adv. Virus Res. 1989;36:107–51. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A. Mulholland J. Nan A., et al. Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J. Control Release. 2005;102:191–201. doi: 10.1016/j.jconrel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Morizono K. Chen I. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle. 2005;4:854–6. doi: 10.4161/cc.4.7.1789. [DOI] [PubMed] [Google Scholar]
- Morizono K. Bristol G. Xie Y., et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Xie Y. Ringpis G., et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Morizono K. Ringpis G. Pariente N., et al. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virol. 2006;10:71–81. doi: 10.1016/j.virol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Morizono K. Pariente N. Xie Y. Chen I. Redirecting lentiviral vectors by insertion of integrin-tageting peptides into envelope proteins. J. Gene Med. 2009;11:549–58. doi: 10.1002/jgm.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Ku A. Xie Y., et al. Redirecting lentiviral vectors pseudotyped with Sindbis virus-derived envelope proteins to DC-SIGN by modification of N-linked glycans of envelope proteins. J. Virol. 2010;84:6923–34. doi: 10.1128/JVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Xie Y. Olafsen T., et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9:286–98. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg C. Cho R. Karlsson L., et al. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–8. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg C. Sieburg H. Bernitz J. Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–7. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson B. Morling F. Cosset F. Russell S. Targeting of retroviral vectors through protease-substrate interactions. Gene Ther. 1996;3:280–286. [PubMed] [Google Scholar]
- Pariente N. Morizono K. Virk M., et al. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol. Ther. 2007;15:1973–1981. doi: 10.1038/sj.mt.6300271. [DOI] [PubMed] [Google Scholar]
- Pariente N. Mao S. Morizono K. Chen I. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 2008;10:242–8. doi: 10.1002/jgm.1151. [DOI] [PubMed] [Google Scholar]
- Shuhendler A.J. Prasad P. Leung M., et al. A novel solid lipid nanoparticle formulation for active targeting to tumor alpha(v) beta(3) integrin receptors reveals cyclic RGD as a double-edged sword. Adv. Healthc. Mater. 2012;1:600–8. doi: 10.1002/adhm.201200006. [DOI] [PubMed] [Google Scholar]
- Somia N.V. Zoppe M. Verma I.M. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsesia-Wittmann S. Drynda A. Deleage G., et al. Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. J. Virol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M. Ge J. Liu Z. Ouyang P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 2006;128:11008–9. doi: 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- Yan M. Du J. Gu Z., et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotech. 2010;5:48–53. doi: 10.1038/nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- Yu J.H. Schaffer D.V. Advanced targeting strategies for murine retroviral and adeno-associated viral vectors. Adv. Biochem. Eng. Biotechnol. 2005;99:147–67. doi: 10.1007/10_006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.