Abstract
Purpose/Objective(s)
Stereotactic body radiotherapy (SBRT) in central lung tumors has been associated with higher rates of severe toxicity. We sought to evaluate toxicity and local control in a large cohort, and to identify predictive dosimetric parameters.
Methods and Materials
We identified patients who received SBRT for central tumors according to either of two definitions. Local failure (LF) was estimated using a competing-risks model, and multivariate analysis (MVA) was used to assess factors associated with LF. We reviewed patient toxicity, and applied Cox proportional hazard analysis and logrank tests to assess whether dose-volume metrics of normal structures correlated with pulmonary toxicity.
Results
One hundred twenty-five patients received SBRT for NSCLC (n=103) or metastatic (n=22) lesions using IMRT. The most common dose was 45Gy in 5 fractions. Median followup was 17.4 months. Incidence of grade ≥ 3 toxicity was 8.0%, including 5.6% pulmonary toxicity. Sixteen patients (12.8%) experienced grade ≥2 esophageal toxicity, including 50% of patients where PTV overlapped the esophagus. There were two treatment-related deaths. Among patients receiving biologically effective dose (BED) ≥ 80 Gy (n=108), 2-year LF was 21%. On MVA, gross tumor volume (GTV) was significantly associated with LF. None of the studied dose-volume metrics of the lungs, heart, proximal bronchial tree (PBT), or 2 cm expansion of the PBT (“no-fly-zone” or NFZ) correlated with grade ≥ 2 pulmonary toxicity. There was no difference in pulmonary toxicity between central tumors located inside the NFZ, and those outside the NFZ but with planning target volume (PTV) intersecting the mediastinum.
Conclusion
Using moderate doses, SBRT for central lung tumors achieves acceptable local control with low rates of severe toxicity. Dosimetric analysis showed no significant correlation between dose to the lungs, heart, or NFZ and severe pulmonary toxicity. Esophageal toxicity may be an underappreciated risk, particularly when PTV overlaps the esophagus.
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is now a well-established treatment for medically inoperable early-stage non-small cell lung cancer (NSCLC), with 2-year local control rates ranging from 80-97%. [1,2] However, an early prospective trial indicated that patients with centrally located lung tumors were at increased risk for severe pulmonary toxicity when treated with SBRT. [3] As a result, tumors within a 2 cm radius of the proximal bronchial tree, often described as the “no-fly zone (NFZ),” were excluded from the landmark RTOG 0236 trial [2] and are now being studied separately in a phase I/II trial (RTOG 0813) [4], which aims to determine the maximum tolerated dose (MTD) for SBRT in central lung tumors.
Until data from RTOG 0813 are available, the optimal dose for SBRT in central lung tumors will remain uncertain. Most institutions, including ours, have adopted more conservative fractionation schemes for central lung tumors in the absence of prospective data establishing the MTD, but substantial data on local control and toxicity with these schemes is also lacking. For this reason, we retrospectively assessed local control and toxicity in a large cohort of patients treated with SBRT for central lung tumors at our institution, where a variety of fractionation schemes have been used in an effort to balance efficacy and toxicity.
It is unclear whether the NFZ as defined by Timmerman et al. is itself the appropriate structure to evaluate for risk of excessive pulmonary toxicity, or whether this region is simply an arbitrary surrogate for the true at-risk structure or structures. This uncertainty is reflected in the diverging definitions of central lung tumors in RTOG 0236 and RTOG 0813. We therefore also undertook dose-volume histogram (DVH) analysis to determine whether dose to the NFZ was predictive of pulmonary toxicity, and whether dose to heart, esophagus, ipsilateral or bilateral lungs might also be predictive of pulmonary toxicity.
MATERIALS AND METHODS
Inclusion Criteria
The Institutional Review and Privacy Boards approved this study, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. We reviewed treatment plans of all patients in our institutional lung SBRT database to identify treated lung tumors within a 2 cm radius of the proximal bronchial tree, as per the RTOG 0236 definition of the NFZ. We also included patients whose planning target volume (PTV) intersected mediastinal structures (including the heart, great vessels, vertebral bodies, esophagus, and trachea), as per the RTOG 0813 inclusion criteria for central lung tumors. Patients with prior thoracic radiotherapy or treated synchronously to multiple tumors were excluded. Because we wished to assess toxicity across a wide variety of fractionation schedules, we included all patients who received at least 600cGy per fraction and five or fewer fractions in the toxicity analysis.
Treatment
All patients were assessed by a multidisciplinary team and were considered either to be medically inoperable, or opted for SBRT over surgery after consideration of the risks and benefits. No specific tumor locations were excluded from consideration of SBRT, and prescription doses were generally chosen to maintain normal tissue constraints. Patients underwent simulation with a four-dimensional CT scan (4DCT) and immobilization with an alpha cradle or other customized immobilization device. The gross tumor volume (GTV) was contoured and expanded to generate an internal target volume (ITV) based on respiratory excursion. A clinical target volume (CTV) was generated with a 2-3mm expansion of the ITV, and the CTV was expanded 5mm in all directions to generate the PTV. All patients were treated with IMRT, and treatment plans were generated using our in-house treatment planning system. The planning system uses a pencil beam algorithm with radiological path length correction along the central ray of each pencil beam. [5,6] Dose was prescribed with the objective of achieving a D95 to the PTV equal to or greater than the prescription dose; if this was not achievable due to normal tissue constraints, a lower prescription dose was selected. PTV coverage was kept as homogeneous as possible, with tolerance of a hotspot up to 110% of the prescription dose. The proximal bronchial tree (PBT) was defined by contouring the bilateral mainstem bronchi and lobar bronchi up to the branching of the segmental bronchi, as per RTOG 0236 criteria. The NFZ was a 2cm expansion of the PBT in all directions. The lungs were defined as the entire lung parenchyma excluding the GTV. The heart was contoured by including the entire pericardial sac below the level where the pulmonary trunk turns across the mediastinum.
Normal tissue constraints included a 55Gy maximum point dose for the NFZ, and 55Gy maximum point dose for the PBT when treating with 5 fractions (all tumors near the PBT were treated with 5 fractions). Maximum point dose to the spinal cord was 25Gy in 5 fractions, or 24 Gy for 3-4 fractions. We attempted to limit the maximal esophageal dose to 30Gy, but in cases where this was not realistic due to proximity of the PTV to the esophagus, a maximum point dose of 45Gy in 5 fractions was allowed, with the exception of 4 cases where the tumor approached the esophagus and the physician allowed the maximum esophageal dose to be slightly higher in order to maintain adequate target coverage. During the time that most of the patients were treated, no standardized heart constraint was in place, other than to keep hotspots out. Lung constraints were V20 ≤ 12% for both lungs, and V20 ≤ 25% for the ipsilateral lung, which was not varied for different fraction numbers given the lack of data that fraction number affects the V20 threshold for lung toxicity in SBRT.
Generally, patients with NSCLC within the NFZ were treated with five fractions of 8-10Gy each. However, a variety of other schedules were also used at the discretion of the treating physician, such as for tumors outside the NFZ but approaching mediastinal structures. Patients were treated with 4-7 coplanar, intensity-modulated 6MV beams. After each initial setup using skin and immobilization marks, a kilovoltage (kV) cone-beam CT (CBCT) was acquired and reviewed to refine patient setup so that the visualized tumor was no more than 2mm from the ITV contour. Just before each treatment, orthogonal kV images were acquired to ensure that the patient had not shifted, and intrafraction motion was also monitored with infrared beacons placed on the patient surface. Treatment was given every other weekday.
Follow-up
Follow-up data was collected through April 25, 2013 from institutional records, records from referring facilities, or direct patient or family contact. Follow-up visits and imaging were obtained according to standard guidelines,[7] and included a follow-up visit 1 month after treatment, and, starting at 3 months after treatment, a CT scan and follow-up visit every 3 months for the first two years, and every 6 to 12 months thereafter.
Local failure was defined as disease progression or recurrence in the originally radiated lesion, as defined by CT, PET-CT, or biopsy. Toxicity was scored using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.4.0. The highest score was recorded for each patient in the following organ toxicity categories: pulmonary (including dyspnea, cough, radiation pneumonitis, and pneumonia), gastrointestinal, and cardiac.
Statistical Analysis
All endpoints were calculated from the completion of SBRT. For local failure (LF), death without an event was treated as a competing risk. Patients receiving BED10 <80 Gy (n=17) were excluded from the local failure analysis because we wished to report disease control results that are generalizable to contemporary practice, in which there is increasing consensus that higher-BED regimens are necessary for durable local control. However, all 125 patients were retained in the toxicity analysis, since significant toxicity events occurred in lower-BED patients as well, and such data may provide valuable information on the dose-volume dependence of those toxicity endpoints, which are dependent upon dose distribution and not directly on prescription dose. Gray (for categorical variables) and Fine-Gray competing risks method (for continuous variables) were used for univariate analysis (UVA), and the latter was used for stepwise multivariate analysis (MVA). The Kaplan-Meier method was used to estimate overall survival (OS). Log-rank test was used to analyze categorical variables and Cox proportional hazards regression model was used for continuous variables for UVA. Stepwise selection was also used to construct multivariate Cox models. All variables with a p-value <0.1 on UVA were candidates for the stepwise multivariate analysis. The SAS 9.2 (SAS institute Inc., Cary, NC) and R version 3.0.1 package “cmprsk” were used for statistical analysis.
For DVH analysis of pulmonary toxicity, doses were converted to linear-quadratic (LQ) equivalent doses delivered in 2-Gy fractions using α/β = 3 Gy for each dose bin in the DVH.[8] Unless otherwise stated, all following normal tissue dose-volume and generalized Equivalent Uniform Dose (gEUD) metrics are such LQ corrected quantities. Cox proportional hazard analysis and logrank tests were used to determine whether Dv (dose to a volume v of the given structure) or Vd (volume of the given structure receiving the dose d) were predictive of pulmonary toxicity. Structures analyzed in this fashion included the heart, lungs, esophagus, PBT, and NFZ. Additionally, logistic models were tested using generalized equivalent uniform dose (gEUD) for a range of the volume parameter a between log10(a) = -1 to 1 in steps of 0.1. To account for the interaction between the inherent latency of complication onset and the variation in the follow up times of individual patients, we employed the method of Farewell.[9-11] Kaplan-Meier analysis was used to assess whether there was any significant difference in the risk of pulmonary toxicity between patients with tumors (GTV) within the NFZ and patients with tumors outside the NFZ but PTV approaching mediastinal structures.
For the purposes of future data synthesis,[12,13] dose-volume atlases of the incidence of ≥ grade 2 pulmonary complications, [14,15] based on physical dose, are provided in Excel files in electronic Appendix EA1 for each of the anatomic structures analyzed and for each fraction number. The format of these files is described in electronic Appendix EA2.
RESULTS
Patient and treatment characteristics
We identified 125 patients who received SBRT between 2006 and 2011 for single lung tumors within 2 cm of the proximal bronchial tree (n=81) or whose planning target volume (PTV) intersected mediastinal structures (n=44). Patient characteristics are detailed in Table 1. Ninety-one patients had primary NSCLC, 12 had locally recurrent NSCLC, and 22 had a metastatic tumor involving the lung. All primary NSCLC patients had early stage (I-II) disease, except one patient with multifocal T4N0 disease where one lesion was treated with SBRT and the other with surgery. Table 2 summarizes SBRT treatment characteristics and tumor volumes for the study population. A variety of fractionation schemes were used, with a median BED10 of 85.5 Gy (range 43.2-180 Gy). The most common doses were 45Gy in 5 fractions (n=56), 48Gy in 4 fractions (n=21), or 50Gy in 5 fractions (n=14). Forty-nine patients received a BED10 ≥ 100 Gy, while 76 received a BED10 < 100 Gy. Patients with BED10<80 (n=17) were excluded from the local failure analysis because we wished to report disease control results that are generalizable to contemporary practice, in which there is increasing consensus that higher-BED regimens are necessary for durable local control. All but two of the patients in the local failure analysis (98%) had a PTV D95 > 97% of the prescription dose.
Table 1.
Patient and Tumor Characteristics
| Characteristic | No. of Patients |
|---|---|
| Patients treated | 125 |
| Primary | 91 |
| Recurrent | 12 |
| Metastatic | 22 |
| Central definition | |
| Within 2cm of the PBT (“No-fly zone”) | 81 |
| PTV intersecting mediastinal structure | 44 |
| Heart/ pericardium | 12 |
| Aorta or great vessels | 22 |
| Vertebral Body | 7 |
| Trachea | 1 |
| Esophagus | 2 |
| Age at diagnosis, years | |
| Median (range) | 76 (32-95) |
| Sex | |
| Male | 62 |
| Female | 63 |
| Smoking History | |
| Never | 20 |
| Former | 94 |
| Current | 11 |
| Median Smoking Pack years | 45 |
| Baseline KPS | |
| ≥80 | 92 |
| <80 | 31 |
| Not available | 2 |
| COPD at diagnosis | |
| Yes | 55 |
| No | 70 |
| Histology | |
| Adenocarcinoma | 86 |
| Squamous Cell | 30 |
| Other | 9 |
| Stage | |
| IA (T1N0) | 61 |
| IB (T2aN0) | 26 |
| IIA (T2bN0) | 2 |
| IIB (T3N0) | 1 |
| III (T4N0) | 1 |
| IV (M1) | 22 |
| Recurrent | 12 |
Abbreviations: KPS, Karnofsky Perfomance Status; PTV, planning treatment volume; PBT, proximal bronchial tree
Table 2.
SBRT Characteristics
| Characteristic | No. of Patients | Median GTV size, cm3 (range) |
|---|---|---|
| BEDio ≥ 100 Gy | 49 | 11.1 (0.7-110.8) |
| 60 Gy in 3 fx (BED10= 180) | 4 | 10.7 (4.5-18.6) |
| 54 Gy in 3 fx (BED10= 151.2) | 9 | 10.1 (0.7-44.7) |
| 48 Gy in 4 fx (BED10= 105.6) | 21 | 8.4 (1.3-110.8) |
| 36 Gy in 2 fx (BED10= 100.8) | 1 | 10.6 (10.6) |
| 50 Gy in 5 fx (BED10= 100) | 14 | 15.2 (0.9-49.2) |
| BED10 < 100 Gy | 76 | 17.0 (0.7-195.4) |
| 44 Gy in 4 fx (BED10= 92.4) | 1 | 9.9 (9.9) |
| 45 Gy in 5 fx (BED10= 85.5) | 56 | 13.0 (0.6-25.9) |
| 40 Gy in 4 fx (BED10= 80) | 2 | 52.7 (32.6-72.8) |
| 36 Gy in 3 fx (BED10= 79.2) | 1* | 27.3 (27.3) |
| 40 Gy in 5 fx (BED10= 72) | 6* | 49.7 (6.1-71.9) |
| 30 Gy in 5 fx (BED10= 48) | 7* | 43.7 (19.4-186.6) |
| Other* | 3* | 38.2 (5.2-105.1) |
| GTV size | ||
| 0-10cm3 | 46 | |
| 10-20cm3 | 36 | |
| 20-50cm3 | 27 | |
| >50cm3 | 16 | |
| Median BED10 Gy (range) | 85.5 (43.2-180) | |
| Median PTV size, cm3 (range) | 63.0 (17.3-401.7) | |
| Median GTV size, cm3 (range) | 13.1 (0.6-195.4) | |
Excluded from local failure analysis.
Abbreviations: BED10, Biologically equivalent dose for α/β = 10; PTV, planning treatment volume; GTV, gross tumor volume; Gy, gray; fx, fraction.
Local control and survival
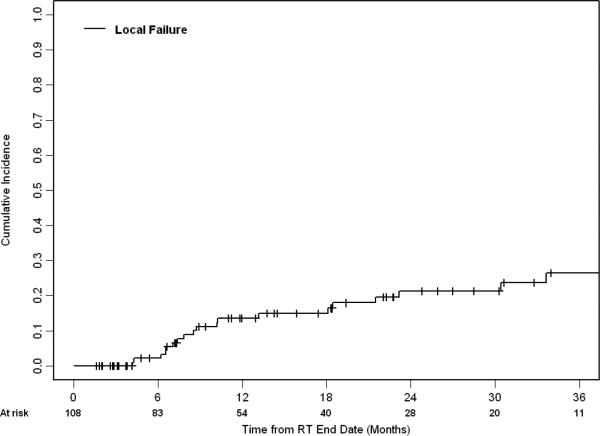
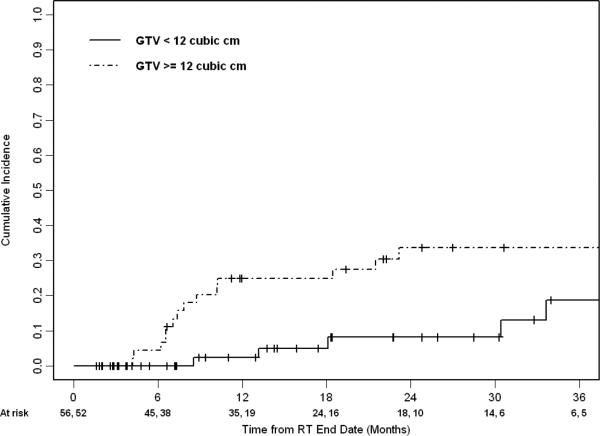
Median follow-up for living patients was 17.4 months (range 1.6-65.4 months). Of the 108 patients who were treated with a BED10 ≥ 80 Gy, the 1- and 2- year rate of local failure (LF) was 14% (95% CI 6-21%) and 21% (95% CI 12-31%), respectively. Figure 1 shows the cumulative incidence of LF. Nineteen patients experienced LF a median of 9 months after treatment. For patients with primary and recurrent NSCLC, the median survival was 29.1 months (95% CI 24.0 – 38.4 months). Their 1- and 2- year OS was 83% (95% CI 73-90%) and 64% (95% CI 52-74%), respectively. Table 3 describes variables associated with LF. Whether patients were treated with BED10≥100Gy or <100Gy (categorical variable) was not significantly associated with LF on univariate analysis. Of the variables analyzed, GTV size and BED10 (continuous variable) were candidates for stepwise multi-variable analysis (MVA). Only increasing GTV size remained associated with LF on the final MVA model (HR 1.52, 95% CI 1.05 - 2.20, p=0.03; see Figure 2). After adjusting for GTV size, increasing BED10 was not significantly associated with LF (HR 0.98, 95% CI 0.96 – 1.01, p=0.21).
FIGURE 1.
Cumulative incidence of local failure for patients receiving a BED10 ≥ 80 (n=108).
Table 3.
Analysis of local failure for patients receiving a BED10 ≥ 80 (n=108) using competing risks methods.
| Factor | Univariate Analysis p-value | Multivariate Analysis final model |
|
|---|---|---|---|
| HR (95% CI) | p-value | ||
| Age at diagnosis (year), mean ± SD | 0.22(HR=1.03) | ||
| Male (vs. female) | 0.41 | ||
| > 2cm from PBT (yes vs. no) | 0.27 | ||
| KPS < 80 at diagnosis (yes vs. no) | 0.75 | ||
| Tumor type | 0.27 | ||
| Primary NSCLC | |||
| Recurrent NSCLC | |||
| Metastatic NSCLC | |||
| GTV size (continuous) | 0.009(HR=1.62) | 1.52 (1.05 - 2.20) | 0.03 |
| BED10 (continuous) | 0.15 (HR=0.98) | ||
| BED10≥100 (yes vs. no) | 0.22 | ||
Abbreviations: SD, standard deviation; PBT, proximal bronchial tree; KPS, Karnofsky Perfomance Status; NSCLC, Non-small cell lung cancer; GTV, gross tumor volume; BED10, Biologically equivalent dose for α/β = 10; HR, hazard ratio.
FIGURE 2.
Cumulative incidence of local failure stratified by gross tumor volume for patients receiving a BED10 ≥ 80 (n=108), p=0.02 by Gray's test. The first and second number of patients at risk at each time point is for GTV < 12 cubic cm and GTV ≥ 12 cubic cm, respectively.
Toxicity
Table 4 describes the toxicity of the patients in this cohort. Ten patients experienced grade ≥3 toxicity, representing 8.0% of the cohort (9.3% if excluding patients with BED<80). Median time to toxicity was 4 months. Four patients experienced worsening dyspnea limiting self-care and activities of daily living. Two of these patients had chronic obstructive pulmonary disease prior to treatment. One patient developed “pneumonia” four months after treatment requiring hospitalization at an outside facility, suspicious for pneumonitis. There were 2 cases of grade 3 gastrointestinal complications: one patient with tumor abutting esophagus had esophagitis 4 months after treatment which then developed into a fistula, and the other patient (tumor 2.1 cm from esophagus) had upper gastrointestinal bleed requiring endoscopic intervention 2 weeks after radiation. The maximum esophageal dose for these patients was 46.0 Gy and 18.0 Gy, respectively, both treated in 5 fractions. Fourteen additional cases of grade 2 esophagitis occurred, for an overall 12.8% incidence of grade ≥2 esophageal toxicity. The median distance from PTV to esophagus for those with toxicity was 1.3 cm (range 0-5.6 cm). The range of maximum point doses to the esophagus for patients with toxicity was 16.5-47.0 Gy (median 29.5Gy). Among the 12 patients with PTV overlapping esophagus, 6 (50%) developed grade ≥2 esophageal toxicity, including one grade 3 event. For the 28 patients with PTV <2cm from esophagus, 4 (14%) had toxicity. Among the remaining 85 patients with PTV ≥2cm from esophagus, only 4 patients had toxicity (4.7%).
Table 4.
Toxicity.
| Toxicity Grade | II | III | IV | V |
|---|---|---|---|---|
| Pulmonary | ||||
| Dyspnea | 2 | 4 | ||
| Cough | 4 | |||
| Radiation Pneumonitis | 18 | 1 | ||
| Pneumonia | 2 | 1 | 1 | |
| Esophagitis | 14 | 2 | ||
| Cardiac | 2 | 1 | ||
| Total (%) | 42 (34%) | 8 (6%) | 0 | 2 (2%) |
Two patients had deaths that were likely treatment-related. The first was a 75 year-old woman with a history of bronchiectasis, treated with 45Gy in 5 fractions for a 2.4 cm squamous cell carcinoma in the left hilum. She developed presumed pneumonia one month after treatment requiring intubation, recovered, then developed hemoptysis 7 months from treatment and expired. This patient had a mean bilateral lung dose (MLD) of 5.7 Gy, bilateral lung V20 of 9.8%, and ipsilateral lung V20 of 21.2%. Maximum point dose to the PBT and NFZ was 46.5 Gy and 48.6 Gy, respectively.
The second patient was a 67 year-old man with synchronous right upper lobe and left lower lobe NSCLC. The right-sided tumor was first treated with wedge resection, then the left-sided tumor with SBRT to 45 Gy in 5 fractions. This tumor measured 4cm and encased the left superior segmental bronchus. He developed hypoxemia 6 months after treatment and died two weeks later from presumed radiation-induced lung injury. This patient received a MLD of 7.5 Gy, bilateral lung V20 of 9.9%, and ipsilateral lung V20 of 25.6%, minimally exceeding our institutional guideline of V20 ≤ 25%. Maximum point dose to the PBT and NFZ was 47.7 Gy and 48.6 Gy, respectively
Though cardiac events were difficult to attribute to RT in this population with frequent comorbidities, we identified three cases of significant cardiac toxicity possibly attributable to SBRT: one case of pericardial effusion, one case of pericarditis, and one case of myocardial infarction.
In the DVH analysis, no significant models were found for gEUD values of the heart, ipsilateral and bilateral lung, esophagus, proximal bronchial tree and NFZ to grade ≥ 2 pulmonary toxicity. Results were not significantly different when excluding low-BED patients from the analysis. Figure EA3.1 in file EA3 in the electronic appendix shows the resulting t-statistic of the logistic correlation coefficient for each structure. Similarly, Cox proportional hazards analysis and logrank tests found no significant models of pulmonary toxicity based on the dose-volume metrics Dv or Vd for any of the investigated structures. No significant difference in the incidence of pulmonary toxicity was identified between central tumors located within the NFZ and those not in the NFZ but approaching mediastinal structures.
DISCUSSION
This is the largest series of SBRT for centrally located lung tumors reported to date. SBRT achieved local control in most patients, but at lower rates than those reported in RTOG 0236 and other series of peripheral lung SBRT utilizing high-BED regimens such as 54 Gy in 3 fractions.[2] Other prospective and retrospective series have reported 2-year rates of local control for centrally-located tumors ranging from as low as 60%[16] to as high as 94%.[17] No significant correlation between local control and BED was found on our analysis, and a multivariate model incorporating both BED and tumor size indicated that across the dose regimens we used, only tumor size was independently correlated with local control. However, it is likely that our modest rates of local control are due to our use of relatively low-BED regimens. A clearer impact of BED on local control may have been seen if we had treated more patients with higher-BED regimens, given that BED ≥100 has been established as a significant predictor of local control in other larger series.[18] Prior tumor control probability models have suggested a correlation between BED and tumor size with respect to local control[19]. It is likely that fractionation schedules such as 45 Gy in 5 fractions are inadequate to control larger tumors, and our current practice and recommendation is to prescribe 50 Gy in 5 fractions for lesions in the central lung zone. However, larger lung tumors have also been associated with increased risk of severe toxicity [20], and thus optimizing the therapeutic ratio remains a major challenge when treating large lesions in the central lung zone.
The incidence of severe toxicity was acceptably low in this cohort at 8% overall, indicating that with the use of conservative fractionation, rates of SBRT-related toxicity in central lung tumors are comparable to those for peripheral lung lesions. Our findings are similar to the 8.6% incidence of grade ≥ 3 toxicity reported by Senthi et al. in their systematic review on outcomes after SBRT for central lung tumors.[21] Other major series on this topic have shown comparable results. Haasbeck et al. found no significant differences in toxicities when comparing 63 patients with central lung tumors treated with 60Gy in 8 fractions to peripheral tumors.[22] A report from MD Anderson Cancer Center similarly found that SBRT can be safely delivered to these patients with excellent disease control.[23] Lastly, Rowe et al. found a 94% 2-year actuarial lobar control rate in their cohort of 47 patients with central lung tumors, the majority of which were treated with 62.5 Gy in 5 fractions.[20] Our larger study contributes to the body of evidence that SBRT can be safely delivered to centrally located lung tumors, and ongoing studies such as RTOG 0813 should clarify the optimal dose fractionation in a prospective fashion. It is also worth noting that updated results from the seminal trial from Timmerman et al. showed that longer-term rates of severe toxicity and survival were equivalent between central and peripheral tumors [1].
Two patients in our cohort died of pulmonary events likely attributable to SBRT. This incidence of treatment-related mortality (2%) is acceptable compared to that with surgical resection in this subset of tumors, especially considering that most patients receiving SBRT are medically or technically inoperable. [24] However, over 10% of patients developed clinically significant esophageal toxicity, including 50% of patients with PTV overlapping esophagus. These findings contrast with those recently reported by the MD Anderson Cancer Center, where the rate of significant esophagitis was much lower, likely because tumors approaching esophagus would have been treated with a more highly fractionated regimen (7Gy x 10).[25] Given that the maximum esophageal dose in our series was 47Gy in 5 fractions, and that a minimum of 50Gy in 5 fractions would be required to achieve BED of 100 to an adjacent tumor, such patients should be offered SBRT with particular caution, perhaps using more than five fractions. Three patients also had significant cardiac events possibly attributable to SBRT. The risk of esophageal and cardiac toxicity with lung SBRT warrants further study.
The precise mechanisms and risk factors for severe pulmonary toxicity in patients with central lung tumors remain unclear, a fact underlined by the presence of two different definitions of “central” in recent RTOG trials. Our analysis did not demonstrate a signficant difference in pulmonary toxicity according to the definition of “central” used. Previous analyses have demontrated V20 and mean lung dose correlated with rates of radiation pneumonitis after SBRT.[26] However, we did not identify any DVH characteristics with respect to the NFZ, heart, or lungs that were predictive of significant pulmonary toxicity. It is possible that studying an larger cohort of patients may reveal clearer dosimetric predictors of toxicity. However, as this report adds to the increasing experience indicating that moderate-dose SBRT is safe and feasible in central lung tumors, it appears likely that the high rates of severe toxicity observed in the early report from Timmerman et al. was related to the high-BED regimens used, and that central tumor location is not a predictor of enhanced pulmonary toxicity when using moderate-dose SBRT.
In addition to the constraints of any retrospective study, our study had several limitations. Because we included patients treated over a time period where institutional standards for SBRT dose were rapidly evolving, our cohort was heterogeneous with respect to fractionation and dose. This heterogeneity was potentially valuable in trying to identify underlying dosimetric factors associated with toxicity and local control, but makes it difficult to extrapolate a single recommended dose for clinical practice. Also, most patients in our cohort were treated with conservative fractionation schemes with BED<100Gy. RTOG 0813 may eventually demonstrate that higher-BED regimens are preferable for central tumors, which could limit the clinical generalizability of our results. We also note that our SBRT technique involved prescribing to the 100% isodose line, which results in significantly lower hotspots compared to the RTOG technique of prescribing to the 60-90% isodose line and as such may not be a fully comparable population. Finally, because Grade 3+ toxicity events were rare, it was necessary to include less clinically significant Grade 2 events in order to facilitate statistically meaningful analysis. Even so, no significant correlations were found.
Despite these limitations, this analysis demonstrates the feasibility and effectiveness of SBRT for central lung tumors in a large number of patients. It also represents a comprehensive attempt to identify dosimetric factors predictive of severe pulmonary toxicity, and the first attempt to determine whether a significant difference in toxicity profile exists between two different definitions of “central” lung tumors.
CONCLUSION
SBRT using attenuated fractionation schemes for central lung tumors achieves acceptable local control with low rates of severe toxicity. However, death from pulmonary complications remains a possible though rare event even with conservative fractionation such as 45 Gy in 5 fractions. Although the enhanced risk of severe pulmonary toxicity in central tumors was evident from the prior prospective experience of Timmerman et al., comprehensive DVH analysis in this cohort failed to demonstrate any dosimetric factors predictive of pulmonary toxicity. Because dose to the NFZ as defined in RTOG 0236 did not predict for pulmonary toxicity, and there was no difference in toxicity profile between the two definitions of central tumor, the underlying risk factors and mechanisms of severe pulmonary toxicity in these patients remain unclear. Other significant toxicities, particularly esophageal and cardiac complications, are also possible and patients with tumors approaching the esophagus may require modified treatment approaches.
Supplementary Material
Summary.
We reviewed local control and toxicity in 125 patients receiving SBRT for central lung tumors, and attempted to identify dosimetric predictors of pulmonary toxicity. With moderate-dose regimens, SBRT achieved acceptable local control with low rates of severe toxicity. Dosimetric analysis showed no correlations between lung, heart or central airway dose and pulmonary toxicity. Esophagitis occurred in 13% of patients, and was particularly common when the PTV overlapped with the esophagus.
Acknowledgments
Sources of support: Supported in part by NCI R01 CA129182. The research of Weiji Shi and Zhigang Zhang were partly supported by the Core Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: Andreas Rimner has served as a consultant for Varian Medical Systems and GE Healthcare. Andreas Rimner has received financial research support from Varian Medical Systems. Abraham Wu has served as a consultant for Pfizer, Inc.
This abstract was presented as an oral presentation at the 55th Annual Meeting of the American Society for Radiation Oncology (ASTRO) in Atlanta, GA, September 22-25, 2013.
References
- 1.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase ii study. International journal of radiation oncology, biology, physics. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA : the journal of the American Medical Association. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase ii study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Bezjak A. Seamless phase i/ii study of stereotactic lung radiotherapy (sbrt) for early stage, centrally located, non-small cell lung cancer (nsclc) in medically inoperable patients. Editor, editor^editors. Book Seamless phase i/ii study of stereotactic lung radiotherapy (sbrt) for early stage, centrally located, non-small cell lung cancer (nsclc) in medically inoperable patients. 2008 http://clinicaltrials.gov/show/NCT00750269.
- 5.Mohan R, Barest G, Brewster LJ, Chui CS, Kutcher GJ, Laughlin JS, Fuks Z. A comprehensive three-dimensional radiation treatment planning system. International journal of radiation oncology, biology, physics. 1988;15:481–495. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 6.Chui CS, LoSasso T, Spirou S. Dose calculation for photon beams with intensity modulation generated by dynamic jaw or multileaf collimations. Medical physics. 1994;21:1237–1244. doi: 10.1118/1.597206. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R, Grannis FW, Jr., Grant SC, Horn L, Jahan TM, Komaki R, Kong FM, Kris MG, Krug LM, Lackner RP, Lennes IT, Loo BW, Jr., Martins R, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Shapiro TA, Swanson SJ, Tauer K, Wood DE, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 2.2013. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. quiz 653. [DOI] [PubMed] [Google Scholar]
- 8.Wheldon TE, Deehan C, Wheldon EG, Barrett A. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1998;46:285–295. doi: 10.1016/s0167-8140(97)00162-x. [DOI] [PubMed] [Google Scholar]
- 9.Farewell VT. The use of mixture models for the analysis of survival data with long-term survivors. Biometrics. 1982;38:1041–1046. [PubMed] [Google Scholar]
- 10.Schultheiss TE, Thames HD, Peters LJ, Dixon DO. Effect of latency on calculated complication rates. International journal of radiation oncology, biology, physics. 1986;12:1861–1865. doi: 10.1016/0360-3016(86)90331-7. [DOI] [PubMed] [Google Scholar]
- 11.Tucker SL, Dong L, Bosch WR, Michalski J, Winter K, Mohan R, Purdy JA, Kuban D, Lee AK, Cheung MR, Thames HD, Cox JD. Late rectal toxicity on rtog 94-06: Analysis using a mixture lyman model. International journal of radiation oncology, biology, physics. 2010;78:1253–1260. doi: 10.1016/j.ijrobp.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deasy JO, Bentzen SM, Jackson A, Ten Haken RK, Yorke ED, Constine LS, Sharma A, Marks LB. Improving normal tissue complication probability models: The need to adopt a “data-pooling” culture. International journal of radiation oncology, biology, physics. 2010;76:S151–154. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, Constine LS, Deasy JO. The lessons of quantec: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. International journal of radiation oncology, biology, physics. 2010;76:S155–160. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: A proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–268. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. International journal of radiation oncology, biology, physics. 2012;83:e661–667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshiro Y, Aruga T, Tsuboi K, Marino K, Hara R, Sanayama Y, Itami J. Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2010;186:274–279. doi: 10.1007/s00066-010-2072-y. [DOI] [PubMed] [Google Scholar]
- 17.Bral S, Gevaert T, Linthout N, Versmessen H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De Ridder M, Storme G. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: Results of a phase ii trial. International journal of radiation oncology, biology, physics. 2011;80:1343–1349. doi: 10.1016/j.ijrobp.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Hirokawa Y, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K. Stereotactic hypofractionated high-dose irradiation for stage i nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 19.Ohri N, Werner-Wasik M, Grills IS, Belderbos J, Hope A, Yan D, Kestin LL, Guckenberger M, Sonke JJ, Bissonnette JP, Xiao Y. Modeling local control after hypofractionated stereotactic body radiation therapy for stage i non-small cell lung cancer: A report from the elekta collaborative lung research group. International journal of radiation oncology, biology, physics. 2012;84:e379–384. doi: 10.1016/j.ijrobp.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 21.Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: A systematic review. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;106:276–282. doi: 10.1016/j.radonc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:2036–2043. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- 23.Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, Bucci MK, McAleer MF, Mehran RJ, Roth JA, Komaki R. Stereotactic body radiation therapy in centrally and superiorly located stage i or isolated recurrent non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Bao F, Zheng H, Zhou YM, Bao MW, Xie HK, Jiang GN, Ding JA, Gao W. Local extension at the hilum region is associated with worse long-term survival in stage i non-small cell lung cancers. The Annals of thoracic surgery. 2012;93:389–396. doi: 10.1016/j.athoracsur.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 25.Chang JY, Li QQ, Xu QY, Allen PK, Rebueno N, Gomez DR, Balter P, Komaki R, Mehran R, Swisher SG, Roth JA. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone”. International journal of radiation oncology, biology, physics. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, Johnstone PA, Fakiris AJ. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. International journal of radiation oncology, biology, physics. 2012;82:457–462. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.