Summary
Ulceration of primary melanomas is associated with poor prognosis yet is reported to predict benefit from adjuvant interferon.
To better understand the biological processes involved, clinico-pathological factors associated with ulceration were determined in 1804 patients. From this cohort, 348 primary tumor blocks were sampled to generate gene expression data using a 502-gene cancer panel and 195 blocks were used for immunohistochemistry to detect macrophage infiltration and vessel density. Gene expression results were validated using a whole genome array in two independent sample sets. Ulceration of primary melanomas was associated with more proliferative tumors, tumor vessel invasion and increased microvessel density. Infiltration of tumors with greater number of macrophages and gene expression pathways associated with wound healing and up-regulation of pro-inflammatory cytokines suggest that ulceration is associated with tumor related inflammation. The relative benefit from interferon reported in patients with ulcerated tumors may reflect modification of signalling pathways involved in inflammation.
Keywords: Melanoma, Primary, Ulceration, Gene expression, Clinico-pathological, Immunohistochemistry
Introduction
Ulceration of a primary melanoma is defined as absence of intact epidermis, including stratum corneum and basement membrane, with reactive changes in the skin (Spatz et al., 2003). It predicts poor survival, and is integral to the American Joint Committee on Cancer (AJCC) staging guidelines (Balch et al., 2009). Somewhat paradoxically, primary tumor ulceration has been reported to be associated with greater benefit in terms of longer relapse-free survival (RFS), distant metastasis-free survival (DMFS) and overall survival (OS) following treatment with adjuvant interferon-α (IFN) (Eggermont et al., 2012b ). There are currently no other confirmed predictive biomarkers for IFN therapy, therefore the association between ulceration and IFN benefit warrants further investigation (Eggermont et al., 2012a).
The biological explanation for ulceration and its impact on prognosis and IFN response is not yet known (Spatz et al., 2010b, Gogas et al., 2009) but there are broad hypotheses. Ulceration may simply result from increased proliferative activity and hypoxia during tumor expansion and the poor prognosis may purely be associated with the more proliferative phenotype. Mitotic rate, tumor thickness and ulceration are however independent histological prognostic markers in stage I/II melanoma, suggesting that a distinct biological process in addition to cell proliferation is occurring, which contributes to poorer prognosis (Balch et al., 2009, Gogas et al., 2009, Spatz et al., 2010b, Thompson et al., 2011, Balch et al., 1978).
The presence of ulceration may indicate differences in the way that tumor cells interact with the local tumor environment (Spatz et al., 2010b, Spatz et al., 2010a, Gogas et al., 2009). For example loss of adhesion to keratinocytes and associated control mechanisms (McGary et al., 2002, Haass et al., 2005, Spatz et al., 2010a) increases melanoma cell invasiveness, and loss of adhesion within the epidermis is clearly seen in ulcerated tumors.
Finally it has been hypothesised that the presence of ulceration may be associated with tumor related inflammation (Melnikova and Bar-Eli, 2009, Mantovani et al., 2008) and suppression of adaptive immunity (Spatz et al., 2010b, Gogas et al., 2009). Lower numbers of mature dendritic cell densities were reported in sentinel nodes containing micro-metastatic tumor deposits in association with an ulcerated primary tumor (Elliott et al., 2007). Gene expression studies have suggested that this reduction in adaptive immunity might result from tumor related inflammation as the gene encoding the pro-inflammatory cytokine interleukin-6 (IL6), reported to play a major role in tumor related inflammation (Mantovani et al., 2008), was differentially expressed in ulcerated tumors (Winnepenninckx et al., 2006).
We have previously reported that microvessel density and macrophage infiltration are increased in ulcerated primary melanomas (Storr et al., 2012). In the present study, we report the primary tumor and patient characteristics associated with tumor ulceration in participants in epidemiological studies. We generated tumor gene expression profiles for 502 cancer-related genes from a subset of the primary tumors using the Illumina, cDNA-mediated, annealing, selection, extension and ligation (DASL) cancer gene platform allowing identification of genes and biological pathways activated or suppressed in ulcerated tumors. We replicated our gene expression findings in two independent sample sets in which gene expression profiles were generated at whole genome level using the Illumina Whole Genome (WG) DASL platform. This integrated investigation of features associated with ulceration has identified factors independently associated with ulceration in multivariate analyses and revealed insight into biological processes related to the influence of ulceration on prognosis and putatively also to treatment response.
Results
Patient and tumor samples in the test data set
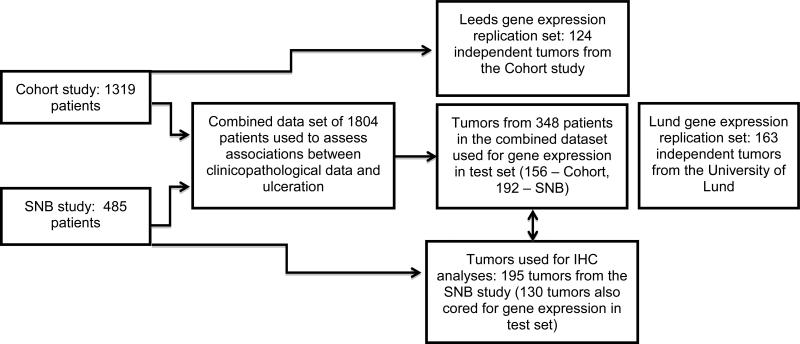
Figure 1 illustrates the patient and sample sets used in this study. Ulceration status data relating to primary melanoma were available for a total of 1804 patients recruited to the Cohort (n=1319) and SNB studies (n=485). Of these patients, 368 (20%) had centrally reviewed primary tumor histopathological data. Gene expression data were available for 348 individual primary tumors from this patient group (156 from the Cohort study and 192 from the SNB study), 339 (97%) of which had been centrally reviewed. This represents the gene expression test set.
Figure 1. Flow diagram illustrating the sample sets used for analyses described.
Ulceration status data relating to primary melanoma was available for a total of 1804 patients recruited to the Cohort and SNB studies. Gene expression data were available for 348 individual primary tumors from this patient group. This represents the gene expression test set. The first validation dataset (Leeds replication) consisted of an independent set of 124 primary tumors selected from within the Cohort Study. The second validation data set (Lund replication) consisted of 163 primary melanomas. One hundred and ninety-five tumors from the SNB study were used for IHC staining, 130 of these tumors were also cored for gene expression studies in the test set.
The first validation dataset (Leeds replication) consisted of an independent set of 209 primary tumors selected from within the Cohort Study. Ulceration status was available for 124 of these samples. The second validation data set (Lund replication) consisted of 223 primary melanomas (Harbst et al., 2012) of which ulceration status was available for 163 melanomas.
Out of the 218 tumor blocks received for the SNB study, 202 had sufficient tumor to allow IHC staining. Of these blocks, 130 (64%) had also been cored for gene expression. One hundred and ninety-five tumors had ulceration status recorded for use in the analyses described. Characteristics of the sample sets are described in Supplementary Table 1.
Analyses of clinico-pathological factors and ulceration
Table 1 shows results of univariate and multivariate analyses from the Cohort and SNB studies combined. The multivariate model is based on 892 patients for whom data for all variables assessed were available. In univariate analysis, greater Breslow thickness and mitotic rate were associated with ulceration (p<0.001). Additional prognostic tumor factors associated with ulceration were presence of vascular invasion (p<0.001), perineural infiltration (p=0.003) and microsatellites (p<0.001). Forty-one percent of ulcerated tumors were nodular compared with 18% of non-ulcerated tumors (p<0.001). Furthermore, a higher proportion of ulcerated tumors were located at sun-protected sites compared with non-ulcerated tumors (defined as those arising in sites such as genital, subungual or acral skin) (p<0.001). Patients with ulcerated tumors were more likely to be older (p<0.0001) and male (p=0.001). In view of the prognostic influence of ulceration, it is unsurprising that patients with ulcerated tumors were more likely to have a positive sentinel node biopsy (p<0.001) and were more likely to relapse and die (p<0.001). The majority of these factors were also associated with ulceration when assessed singly in the smaller histopathologically centrally reviewed dataset (indicated by an asterisk in Table 1).
Table 1.
Associations between clinico-pathological features and ulceration in univariate and multivariate analyses.
| Univariate analysis | Multivariate logistic regression model (n=892) | ||||
|---|---|---|---|---|---|
| Factor | Non-ulcerated (n=1357) | Ulcerated (n=447) | Test statistic and P-value | OR (95% CI) | P-value |
| Study type, n (%): | |||||
| Cohort study | 1008 (74.3) | 311 (69.6) | |||
| SNB study | 349 (25.7) | 136 (30.4) | |||
| Breslow thickness, n (%): | χ2 (3) 365.5, p<0.001* | ||||
| ≤1mm | 425 (31.4) | 23 (5.2) | 1.0 | ||
| >1mm to ≤2mm | 561 (41.4) | 96 (21.7) | 3.22 (1.47-7.07) | 0.004 | |
| >2 to ≤4mm | 284 (21.0) | 174 (39.4) | 9.92 (4.46-22.06) | <0.001 | |
| >4mm | 84 (6.2) | 149 (33.7) | 21.02 (8.72-50.62) | <0.001 | |
| Mitotic rate per mm2, n (%): | χ2 (2) 100.4, p<0.001* | ||||
| <1 mitosis/mm2 | 181 (21.6) | 19 (6.4) | 1.0 | ||
| 1-5 mitoses/mm2 | 467 (55.8) | 122 (41.4) | 0.82 (0.44-1.53) | 0.54 | |
| >5 mitoses/mm2 | 189 (22.6) | 154 (52.2) | 1.45 (0.75-2.80) | 0.27 | |
| Tumor infiltrating lymphocytes, n (%): | χ2 (1) 0.002, p=0.97 | ||||
| None | 177 (15.7) | 59 (15.6) | |||
| Present | 951 (84.3) | 319 (84.4) | |||
| Vascular or lymphatic invasion, n (%): | χ2 (1) 50.2, p<0.001* | ||||
| None | 839 (95.5) | 250 (82.8) | 1.0 | ||
| Present | 40 (4.5) | 52 (17.2) | 1.57 (0.87-2.81) | 0.13 | |
| Perineural infiltration, n (%): | χ2 (1) 9.0, p=0.003 | Not included because of low numbers of observations | |||
| None | 345 (98.0) | 121 (92.4) | |||
| Present | 7 (2.0) | 10 (7.6) | |||
| Regression, n (%): | χ2 (1) 0.2, p=0.64 | ||||
| No | 980 (83.3) | 286 (82.2) | |||
| Yes | 197 (16.7) | 62 (17.8) | |||
| Microsatellites, n (%) | χ2 (1) 16.0, p<0.001 | ||||
| None | 791 (96.6) | 273 (90.7) | 1.0 | ||
| Present | 28 (3.4) | 28 (9.3) | 1.19 (0.57-2.52) | 0.64 | |
| Histological subtype, n (%): | χ2 (2) 111.8, p<0.001* | ||||
| SSM | 887 (68.3) | 177 (41.8) | 1.0 | ||
| NM | 234 (18.0) | 175 (41.3) | 1.17 (0.76-1.79) | 0.48 | |
| Other | 177 (13.6) | 72 (16.9) | 1.01 (0.57-1.79) | 0.97 | |
| Age at diagnosis, years, median (range) | 53.3 (14.4-87.0) | 58.8 (18.1-88.5) | Mann-Whitney z −6.7, p<0.0001* | 1.01 (1.00-1.02) | 0.13 |
| Age at diagnosis, n (%) | χ2 (3) 35.8, p<0.001 | ||||
| <40 years | 292 (21.5) | 50 (11.2) | |||
| 40-54 years | 442 (32.6) | 130 (29.1) | |||
| 55-64 years | 335 (24.7) | 129 (28.9) | |||
| ≥65 years | 288 (21.2) | 138 (30.9) | |||
| Patient gender, n (%) | χ2 (1) 10.5, p=0.001 | ||||
| Female | 778 (57.3) | 217 (48.6) | 1.0 | ||
| Male | 579 (42.7) | 230 (51.5) | 1.27 (0.87-1.84) | 0.22 | |
| Site of tumor, n (%): | χ2 (5) 74.9, p<0.001* | ||||
| Trunk | 484 (35.7) | 170 (38.0) | 1.0 | ||
| Leg | 440 (32.4) | 112 (25.1) | 1.08 (0.68-1.73) | 0.74 | |
| Arm | 268 (19.8) | 67 (15.0) | 0.98 (0.58-1.64) | 0.93 | |
| Head/neck | 133 (9.8) | 46 (10.3) | 0.90 (0.49-1.65) | 0.74 | |
| Sun protected | 31 (2.3) | 52 (11.6) | 4.55 (1.98-10.48) | <0.001 | |
| Unknown | 1 (0.1) | 0 (0.0) | |||
| SNB status, n (%) | χ2 (2) 27.1, p<0.001 | ||||
| No SNB | 725 (54.5) | 203 (47.1) | 1.0 | ||
| SNB performed – negative | 386 (29.0) | 108 (25.1) | 1.08 (0.68-1.72) | 0.74 | |
| SNB performed – positive | 219 (16.5) | 120 (27.8) | 0.74 (0.48-1.15) | 0.18 | |
| Relapse status, n (%) | χ2 (1) 125.4, p<0.001* | ||||
| Not relapse | 1138 (84.3) | 263 (59.0) | |||
| Relapsed | 212 (15.7) | 183 (41.0) | |||
| Overall survival, n (%) | χ2 (1) 106.6, p<0.001* | ||||
| Alive | 1147 (84.5) | 275 (61.5) | |||
| Died | 210 (15.5) | 172 (38.5) | |||
Results are presented for the Cohort and SNB studies combined. An asterisk indicates results that remain statistically significant in the smaller dataset with centrally reviewed histology data. Statistical tests (degrees of freedom) and p-values are presented for association between the factor and ulceration status in univariate analysis. Odds ratios, 95% confidence intervals and significance values are presented for the associations between each factor and ulceration in a multiple logistic regression model. The multivariate model is based on 892 patients for whom data for all variables assessed were available. A continuous variable for age at diagnosis was used in the multivariate model. Statistically significant results are highlighted in bold. Abbreviations used: OR, odds ratio; CI, confidence interval; SNB, sentinel node biopsy; n, number.
Factors found to be associated with an ulcerated tumor in univariate analysis were analysed using multiple logistic regression to identify features independently associated with ulceration. Perineural infiltration was omitted from the model because of insufficient numbers of observations. Greater Breslow thickness and tumors arising in sun-protected sites (OR 4.55 (95% CI 1.98-10.48) p<0.001, compared with tumors on the trunk) were independently associated with ulceration status. Mitotic rate was also independently associated with ulceration status when a test for trend was performed across the groups (OR 1.40 (95% CI 1.04-1.90), p=0.03 data not shown). In the smaller histopathologically centrally reviewed dataset, none of the factors were independently associated with ulceration status in multivariate analysis despite similar odds ratios. This is possibly indicative of the small sample size.
Associations between IHC status and ulceration
Table 2 shows results of univariate and multivariate analyses. The multivariate model is based on 169 tumors where data for all variables assessed were available. In multivariate analysis the presence of lymphatic invasion (OR 3.59, 95% CI 1.64-7.87, p=0.001), greater microvessel density (OR 2.59, 95% CI 1.16-5.80, p=0.02) and macrophage count (OR 2.42, 95% CI 1.07-5.50, p=0.03) remained independently associated with ulceration status.
Table 2.
Associations between lymphovascular parameters and macrophage count (determined using immunohistochemistry) and ulceration status in primary melanoma tumors in univariate and multivariate analyses.
| Univariate analysis | Multivariate logistic regression model (n=169) | ||||
|---|---|---|---|---|---|
| Factor | Not ulcerated (n=145) | Ulcerated (n=50) | Test statistic and P-value | OR (95% CI) | P-value |
| Blood/lymphatic vessel invasion on H+E slide, n (%): | Fisher's exact p=0.36 | ||||
| No | 129 (90.9) | 48 (96.0) | |||
| Present/possible | 13 (9.1) | 2 (4.0) | |||
| Blood vessel invasion (CD34), n (%): | Fisher's exact p=0.39 | ||||
| Negative | 130 (97.0) | 46 (93.9) | |||
| Positive | 4 (3.0) | 3 (6.1) | |||
| Lymphatic invasion (D2-40), n (%): | χ2 (1) 22.6, p<0.001 | ||||
| Negative | 109 (82.6) | 22 (46.8) | 1.0 | ||
| Positive/possible | 23 (17.4) | 25 (53.2) | 3.59 (1.64-7.87) | 0.001 | |
| Any vessel invasion using IHC, n (%): | χ2 (1) 19.5, p<0.001 | ||||
| Both negative | 103 (79.8) | 22 (45.8) | |||
| Either positive/possible | 26 (20.2) | 26 (54.2) | |||
| Intratumoral lymphatic invasion, n (%): | χ2 (1) 14.7, p<0.001 | ||||
| Negative | 121 (89.0) | 31 (64.6) | |||
| Positive/possible | 15 (11.0) | 17 (35.4) | |||
| Peritumoral lymphatic invasion, n (%): | χ2 (1) 17.0, p<0.001 | ||||
| Negative | 119 (91.5) | 29 (65.9) | |||
| Positive/possible | 11 (8.5) | 15 (34.1) | |||
| Lymphatic density, n (%): | χ2(1) 0.1, p=0.74 | ||||
| Low | 69 (50.7) | 23 (47.9) | |||
| High | 67 (49.3) | 25 (52.1) | |||
| Intratumoral lymphatic density, n (%): | χ2(1) 0.7, p=0.41 | ||||
| Low | 69 (50.7) | 21 (43.8) | |||
| High | 67 (49.3) | 27 (56.2) | |||
| Peritumoral lymphatic density, n (%): | χ2(1) 0.1, p=0.73 | ||||
| Low | 66 (50.8) | 21 (47.7) | |||
| High | 64 (49.2) | 23 (52.3) | |||
| Microvessel density, n (%): | χ2 (1) 17.8, p<0.001 | ||||
| Low | 80 (59.7) | 12 (24.5) | 1.0 | ||
| High | 54 (40.3) | 37 (75.5) | 2.59 (1.16-5.80) | 0.02 | |
| Macrophage count, n (%): | χ2 (1) 12.2, p<0.001 | ||||
| Low | 79 (56.8) | 14 (28.0) | 1.0 | ||
| High | 60 (43.2) | 36 (72.0) | 2.42 (1.07-5.50) | 0.03 | |
Statistical tests (degrees of freedom) and p-values are presented for association between the factor and ulceration status in univariate analysis. Odds ratios, 95% confidence intervals and significance values are presented for the associations between each factor and ulceration in a multiple logistic regression model. The multivariate model is based on 169 tumors where data for all variables assessed were available. Vessel density and macrophage counts were categorised into high and low groups around the median value. The variable “any vessel invasion” was not included in the multivariate lymphovascular model as it was generated from the “blood vessel invasion” and lymphatic vessel invasion” variables. In addition, the variable “lymphatic invasion” was used in preference to “intratumoral lymphatic invasion” and “peritumoral lymphatic invasion” in the multivariate model as these variables were highly correlated with “lymphatic invasion” which was most associated with ulceration status in univariate analysis. Significance values quoted for Fisher's exact tests are 2-sided. Statistically significant results are highlighted in bold. Abbreviations used: H+E, haematoxylin and eosin; n, number.
Gene expression profiles associated with tumor ulceration
In the test data set analysis, 56 genes were differentially expressed in ulcerated tumors (34 downregulated and 22 upregulated) at a false discovery rate (FDR) <0.05. Table 3 shows the top 22 of this gene list, all with FDR<0.01. Approximately half of the genes in Table 3 were confirmed in the Leeds replication set with FDR<0.10. Of note, the replication dataset had a smaller size (124 samples compared to 348 in the test dataset) but the WG array generated data on many more genes, therefore imposing a higher multiple testing correction penalty. The Lund replication dataset reiterated 13 of the top 22 genes initially identified while data were not available for the other 9, as these probes did not pass quality control screens (Table 3). Overall, of the 22 top genes with FDR<0.01 in the test data set analysis, 7 were replicated in both Leeds and Lund replication datasets while another 9 genes were replicated in one of these datasets.
Table 3.
Genes differentially expressed in ulcerated tumors in the test dataset at FDR<0.01 and their significance in the two validation datasets. Genes replicated in both datasets are highlighted in bold. Underlined genes were replicated in either the Leeds or Lund replication datasets.
| Test data (92 ulcerated, 256 not ulcerated) | Leeds replication (68 ulcerated, 56 not ulcerated) | Lund replication (68 ulcerated, 95 not ulcerated) | ||||
|---|---|---|---|---|---|---|
| Gene | Fold change | BH - FDR | Fold change | BH - FDR | Fold change | q value |
| HLF | 0.67 | 8.7×10−6 | 0.60 | 0.07 | 0.68 | 0 |
| DSP | 0.68 | 8.8×10−6 | 0.34 | 0.03 | 0.68 | 0 |
| FGFR3 | 0.73 | 9.9×10−6 | 0.42 | 0.03 | 0.81 | 0 |
| GRB7 | 0.67 | 2.8×10−5 | 0.59 | 0.03 | 0.73 | 0 |
| FGFR2 | 0.59 | 4.2×10−5 | 0.65 | 0.05 | NA | NA |
| MAF | 0.92 | 3.1×10−4 | 0.87 | 0.40 | 0.78 | 0 |
| ITGB4 | 0.78 | 4.6×10−4 | 0.67 | 0.23 | 0.86 | 0.0027 |
| IL6 | 1.73 | 6.4×10−4 | 1.22 | 0.63 | 3.66 | 0 |
| RAD52 | 1.15 | 6.5×10−4 | 1.05 | 0.77 | NA | NA |
| EPHA1 | 0.74 | 6.5×10−4 | 0.56 | 0.04 | NA | NA |
| SPP1 | 1.34 | 8.6×10−4 | 1.54 | 0.32 | NA | NA |
| FGF7 | 1.35 | 9.1×10−4 | 1.08 | 0.66 | NA | NA |
| CDH1 | 0.83 | 9.3×10−4 | 0.85 | 0.76 | NA | NA |
| CEBPA | 0.77 | 9.4×10−4 | 0.60 | 0.06 | 0.76 | 0 |
| OSM | 1.80 | 0.001 | 0.97 | 0.91 | NA | NA |
| MMP1 | 1.52 | 0.002 | 1.22 | 0.75 | 1.55 | 0 |
| EGFR | 0.74 | 0.003 | 0.47 | 0.01 | 0.56 | 0 |
| PTGS1 | 0.80 | 0.003 | 0.66 | 0.04 | 0.60 | 0 |
| EPO | 0.81 | 0.003 | 0.97 | 0.92 | NA | NA |
| TIAM1 | 0.87 | 0.004 | 0.87 | 0.71 | 0.88 | 0.01 |
| PBX1 | 0.85 | 0.004 | 0.67 | 0.13 | 0.78 | 0 |
| IL8 | 1.46 | 0.009 | 1.81 | 0.03 | NA | NA |
Significance value for Leeds data is derived from linear regression adjusting for the study to which patients were recruited (test data) and batch effect (Leeds replication). Genes with FDR<0.10 in the Leeds replication set were considered replicated. The qvalues in Lund replication are empirically derived with permutation of ulceration status in SAM software. Abbreviation used: NA, not assessed.
When analyses in the test dataset were adjusted for Breslow thickness, a similar list of genes was generated to that presented in Table 3, however many of these genes were not significantly differentially expressed following adjustment for multiple testing.
A number of genes involved in cell adhesion (Desmoplakin, Integrin beta 4 and Cadherin 1) were down regulated in ulcerated tumors. Significantly, the changes in all three were replicated in all datasets. Fibroblast Growth Factor 7 (FGF7), otherwise known as Keratinocyte Growth Factor, was up-regulated in ulcerated tumors (although this was not replicated throughout the datasets), whilst in contrast Fibroblast Growth Factor Receptors 2 and 3 (FGFR2 and FGFR3) were down-regulated (replicated). The pro-inflammatory cytokine genes Interleukin-6 (IL6) and Interleukin 8 (IL8) were over-expressed in ulcerated tumors (both replicated). Other proinflammatory cytokine genes, such as Interleukin-1α and Interleukin-1β, were also over-expressed in ulcerated tumors in the test dataset (fold change ulcerated/non-ulcerated tumors 1.32 (FDR=0.17) and 1.74 (FDR=0.012), respectively), although only IL1β remained significant after multiple-testing correction.
Pathway analysis of the test dataset and Leeds replication dataset was performed using DAVID software to identify enriched pathways. Genes included in this analysis were those that demonstrated differential expression in ulcerated and non-ulcerated tumors at FDR level 0.05 in each dataset. The gene list generated without adjustment for Breslow thickness was used to maximise the number of significantly differentially expressed genes in ulcerated tumors for pathway analysis. Table 4 presents the GO terms and KEGG pathways significantly enriched in terms of genes up regulated or down regulated in ulcerated tumors in the test dataset analysis and in the Leeds replication data set. The key pathways associated with ulceration were regulation of cell proliferation and the cell cycle, the extracellular region and plasma membrane (including genes such as the metalloproteinases, fibroblast growth factors and pro-inflammatory cytokines), cell adhesion and response to wound healing, cytokine-cytokine receptor interaction, hematopoietic cell lineage and ECM-receptor interaction. Further details of the genes involved in each pathway are included in Supplementary Table 2.
Table 4.
Biological pathways enriched in differentially expressed genes in ulcerated tumors using test and Leeds replication datasets.
| Pathway | Test set (92 ulcerated, 256 not ulcerated) | Leeds replication (68 ulcerated, 56 not ulcerated) | |||
|---|---|---|---|---|---|
| Fold enrichment | BH - FDR | Fold enrichment | BH - FDR | ||
| Regulation of cell proliferation | GO - BP | 2.52 | 8.6×10−4 | 1.54 | 0.10 |
| Pathways in cancer | KEGG | 11.23 | 8.9×10−17 | 2.17 | 0.003 |
| Cell cycle process | GO - BP | 2.93 | 0.03 | 1.58 | 0.06 |
| Plasma membrane part | GO - CC | 2.29 | 0.03 | 2.26 | 2.15×10−12 |
| Response to wounding | GO - BP | 3.35 | 0.03 | 1.69 | 0.09 |
| Cell adhesion | GO - BP | 3.23 | 0.06 | 1.87 | 0.0008 |
| Cytokine-cytokine receptor interaction | KEGG | 12.30 | 6.3 × 10−6 | 2.17 | 0.01 |
| Hematopoietic cell lineage | KEGG | 17.09 | 0.001 | 3.07 | 0.04 |
| ECM-receptor interaction | KEGG | 34.18 | 0.001 | 2.90 | 0.08 |
The fold enrichment is calculated by DAVID as the proportion of genes that are part of a given pathway among those associated with ulceration divided by the equivalent proportion in the background list. Abbreviations used: GO-BP: Gene Ontology Biological Process; GO-CC: Gene Ontology Cellular Component; KEGG: Kyoto Encyclopedia of Genes and Genomes, BH FDR: Benjamini-Hochberg false discovery rate.
Hierarchical cluster analysis of the gene expression test dataset identified two clusters (Supplementary Figure 1). Cluster 1 (labelled as “ulcerated cluster”) contained a greater proportion of ulcerated tumors than cluster 2 (labelled as “non-ulcerated cluster”) with proportions of respectively 45% and 16%. The gene expression patterns of non-ulcerated tumors in cluster 1 had differential expression of genes involved in wound healing when compared with the non-ulcerated tumors of cluster 2 (Supplementary Table 3). In addition, non-ulcerated tumors in the ulcerated cluster had greater Breslow thickness (p=0.003), were older (p=4.2x10−5) and were more likely to have tumour-infiltrating lymphocytes (p=0.05). They also had higher mitotic rate, had greater levels of vascular invasion, more microsatellites and were more likely to be in males although these factors were not significant (Supplementary Table 4). Non-ulcerated tumors in the ulcerated cluster also had a poorer overall survival (Supplementary Figure 2). Furthermore, non-ulcerated tumors in the ulcerated cluster were more likely to have high macrophage counts compared with non-ulcerated tumors in the non-ulcerated cluster (78.3% versus 38.0% respectively, p=0.0005, Supplementary Table 4). These results indicate that there is a subgroup of non-ulcerated tumors with clinico-pathological and gene expression characteristics akin to ulcerated tumors.
Discussion
Tumor ulceration is a marker of poor prognosis for melanoma patients even after metastasis to regional nodes (Balch et al., 2009) and is reported to have a predictive influence on benefit from IFN therapy (Eggermont et al., 2012b). It is therefore important to understand the biology of this phenomenon.
This study has shown that a number of poor prognostic tumor characteristics were associated with the presence of ulceration. Ulcerated tumors were more likely to arise in sun-protected sites (such as acral or subungual melanomas) and in older and male patients. The increased proportion of ulcerated tumors in males is not explained but it is of note that Naugler et al. suggested that pro-inflammatory cytokine IL6 production by macrophages is down regulated in response to oestrogens (Naugler et al., 2007). Factors remaining independently associated with ulceration in multivariate analysis were Breslow thickness, mitotic rate and tumor site. Significantly, Breslow thickness and mitotic rate are two primary tumor features that contribute to current AJCC staging in stage I and II disease (Balch et al., 2009). Therefore, it is unsurprising that these prognostic tumor features, representing an aggressive, proliferative phenotype are associated with ulceration and contribute to the effect of ulceration on prognosis. These associations have been previously identified in large sample sets (Balch et al., 2001, Thompson et al., 2011). The gene expression data generated in the study correspondingly showed that the Gene Ontology (GO) pathways associated with ulceration included those associated with cell proliferation and the cell cycle.
Tumors of sun-protected sites, such as acral, subungual and mucosal tumors were independently more likely to be ulcerated which is consistent with previous reports (Phan et al., 2007, Tan et al., 2007). This work demonstrates that the association with site is independent of Breslow thickness and mitotic rate, suggesting that aggressiveness of the tumor or delay in diagnosis, which have been suggested as contributing factors to the poorer prognosis of acral tumors, does not fully explain the association with ulceration (Durbec et al., 2012, Stalkup et al., 2002). The histopathological description of ulceration defines changes that are active (Spatz et al., 2003). The definition requires more than simply loss of the epidermis, which could occur as a result of trauma. The site association does not exclude the possibility that trauma plays some part in the evolution of ulceration, but the data suggest that ulceration may be associated with the inherent biology of melanomas arising in sun-protected sites.
Analysis of IHC data identified independent associations between ulceration and the presence of lymphatic invasion, greater microvessel density and macrophage infiltration in these primary melanomas. The presence of lymphovascular invasion and greater microvessel density in primary tumors has been associated with poorer prognosis (Rose et al., 2011, Xu et al., 2012, Straume et al., 1999, Depasquale and Thompson, 2005, Kashani-Sabet et al., 2002). These associations are therefore likely to contribute to increased propensity to metastasis associated with ulcerated tumors. It has been suggested that there is a common biological process causing lymphovascular invasion and ulceration in melanomas, which may be related to hypoxia (Rose et al., 2011). Tumor cell death may result from anoxia, but the hypoxia in surrounding zones is thought to drive angiogenesis promoting tumor progression (Pouyssegur et al., 2006, Bedogni and Powell, 2009). Our data support the theory that ulceration is associated with angiogenesis since greater microvessel density was also independently associated with tumor ulceration in this study as has been previously described (Straume et al., 1999, Depasquale and Thompson, 2005).
We report here gene expression patterns associated with primary tumor ulceration. A strength of our study is that we were able to validate the biological pathways associated with ulceration in two independent data sets using a whole genome array. Recently Rakosy et al. have reported a smaller study of 36 cryopreserved tumors utilising the Affymetrix U133 Plus 2.0 array (Rakosy et al., 2013). Ten genes associated with ulceration and identified by Rakosy et al. were replicated in both the test and validation datasets of the current study (including: FGFR2, FGFR3, HLF, DSP, EGFR, CEBPA, PTGS1, KCNK6) and 137 replicated in at least one. As Rakosy et al. used a relatively small sample set of tumors, their data are more variable and their estimates of fold change are more extreme than ours but the findings remain well correlated (correlation between fold changes in test data and Rakosy et al. study 0.76, correlation for Leeds replication data 0.67).Although many small studies of gene expression in melanoma have reported different findings, this work suggests that profiling larger tumor samples using platforms developed for formalin-fixed paraffin wax-embedded tumors generates reproducible data.
Our data suggest that ulceration may be driven by tumor related inflammation. Macrophage count was independently associated with ulceration status. Although macrophage subtype could not be confirmed using the limited immunohistochemistry performed, we postulate that the increased numbers of cells described here are M2 macrophages usually found in association with tissue remodelling and hypoxic environments, such as in tumors (Biswas and Mantovani, 2010, Mantovani et al., 2002, Movahedi et al., 2010 ). M2 macrophages release proangiogenic growth factors, support tumor growth, improve attachment of melanoma cells to extracellular matrix, suppress anti-tumor T-cell dependent adaptive immune responses and modify inflammatory responses, leading to chronic inflammation (Schmidt and Carmeliet, 2010, Fantin et al., 2010, Biswas and Mantovani, 2010, Mantovani et al., 2008). The association between inflammatory processes and cancer has been well documented (Mantovani et al., 2008). Evidence exists that both higher microvessel density and macrophage count in melanoma tumors are associated with poorer prognosis (Biswas and Mantovani, 2010, Erdag et al., 2012, Kashani-Sabet et al., 2002); therefore the presence of these factors may contribute to prognostic significance of ulceration. Suppression of adaptive immune responses by M2 macrophages may also contribute to why ulcerated tumors are more amenable to the effects of exogenous IFN therapy. Our gene expression data support this hypothesis. Genes corresponding to the “response to wounding” pathway were over represented in ulcerated tumors, which included the pro-inflammatory cytokines IL 1B, IL6 and IL8. Interrogation of the IHC characteristics of non-ulcerated tumors which clustered with ulcerated tumors on gene expression analysis did show that a proportion of primary tumors have similar characteristics and in particular more macrophages. We would argue with this supports the suggestion that inflammation is driving the presence of ulceration rather than the reverse.
We considered if there was evidence of a particular “Interferon signature” in ulcerated tumors. Over-expression of STAT5B was seen in ulcerated tumors in our data set (test set fold change 1.08, FDR 0.04). This gene encodes one of the two highly related STAT5 proteins and is a component of a number of pathways identified as having altered gene expression in ulcerated tumors (Supplementary Table 2). Evidence exists in melanoma cell lines that STAT5 activation antagonizes the effects of STAT1 (Wellbrock et al., 2005) which is part of the Jak-STAT signalling pathway by which IFN predominantly exerts it's anti-proliferative, immune, cytotoxic and anti-angiogenic effects (Pestka et al., 2004, Bracarda et al., 2010). STAT5 protein expression has been shown to be greater in IFN resistant melanoma cell lines and ectopic expression of STAT5 can reduce in vitro IFN sensitivity (Wellbrock et al., 2005). This contrasts with our demonstration of increased STAT5B expression in ulcerated tumors, which are reported to be relatively IFN-sensitive. Furthermore, STAT1 was not differentially expressed, but there was down regulation of factors downstream of STAT1, such as PIM1 (test set, fold change 0.88, FDR=0.10), MYC (test set, fold change 0.95, FDR=0.06) and CCND3 (or cyclin D3, test set, fold change 0.92, FDR=0.08). These findings indicate that Jak-STAT signalling may be especially suppressed in ulcerated melanoma and it may therefore be that interferon is more effective in patients with these tumors because of this, but a potential mechanism is not clear.
Cluster analysis has identified non-ulcerated tumors with similar clinico-pathological features to ulcerated tumors and similar expression of genes in the wound-healing pathway. Specifically, these non-ulcerated tumors have over-expression of the pro-inflammatory genes IL1B, IL6 and over-expression of STAT5B. This raises the possibility that these tumors may have similar biological properties to ulcerated tumors, particularly being associated with worse prognosis and perhaps improved responses to IFN therapy. Use of gene expression profiles as a predictive tool to identify IFN sensitive tumors that are not ulcerated would clearly be useful clinically. We have been unable to investigate this further as we have limited data regarding response to IFN therapy, however analysis of tumors from patients recruited to large trials of IFN therapy (Eggermont et al., 2005, Eggermont et al., 2011) would provide this opportunity and should be pursued. An inflammatory gene expression signature and presence of macrophage infiltration may have prognostic significance and be involved in the aetiology of ulceration.
In conclusion, we report clinico-pathological evidence that ulceration of melanoma primaries is associated with more proliferative, mitotically active tumors with corresponding perturbation of genes involved in these pathways. In ulcerated tumors there is histological evidence of loss of cohesion between keratinocytes and we confirmed that the expression of genes involved in cell adhesion was correspondingly perturbed. The infiltration of ulcerated tumors with greater number of macrophages and gene expression pathways associated with wound healing with up regulation of pro-inflammatory cytokines suggest that tumor related inflammation is associated with ulceration and we postulate that the relative benefit from interferon reported in patients with ulcerated tumors might reflect modification of signalling pathways involved in inflammation. These data support the view expressed by others (Melnikova and Bar-Eli, 2009, Dunn et al., 2012) that inflammation may play a significant role in melanoma progression.
Methods
Description of the data sets used for the clinico-pathological correlation with ulceration study, the immunohistochemistry study, and the gene expression data sets (test and Leeds replication set)
Samples and data from patients recruited to two previously described studies (the Leeds Melanoma Cohort Study (Cohort study) (Newton-Bishop et al., 2009) and the Retrospective Sentinel Node Biopsy Study (SNB study) (Mitra et al., 2010)) were combined and used for this work in Leeds. UK national ethics committees (MREC and PIAG) approved these studies. Tumor specimens from these studies were analysed immunohistochemically and used to generate gene expression data in the test set. The Leeds replication data were generated from an independent sample set of tumors from the Cohort study. Figure 1 illustrates the patient and sample sets used in the analyses described.
Histology data were derived from clinical histopathology reports. For a subset of tumors, diagnostic H+E slides were reviewed centrally by dermatopathologists (Dr Andy Boon (Cohort study) and Professor Martin Cook (SNB study)) to standardize reporting across the specimens.
Gene expression assays
For gene expression analysis, tumor blocks from both studies were sampled horizontally through the vertical growth phase using a TMA needle as previously described (Conway et al., 2009). RNA was extracted and gene expression data were generated for 502 cancer-related genes using the Illumina Human Cancer panel DASL assay as previously described (Conway et al., 2009) and the data from the two groups were then merged to make the test dataset (Jewell et al., 2010).
In the first validation dataset (Leeds replication), we generated whole genome gene expression profiles using Illumina human HT12 v4 (~30,000 probes) from an independent set of tumors from within the Cohort Study using the sampling technique previously described (Conway et al., 2009).
Independent validation (Lund replication) was provided in a separate sample set of primary melanoma tumors using the whole-genome DASL assay at Lund University (Harbst et al., 2012).
Immunohistochemistry
Immunohistochemistry was performed upon consecutive tissue sections of the sampled blocks originating from the SNB study as previously reported (Storr et al., 2012). Expression of D2-40, CD34 and CD68 were assessed at the University of Nottingham to measure microvessel density, macrophage count, lymphatic density and vascular invasion as previously described (Storr et al., 2012). We were unable to assess subtypes of tumor-associated macrophages because of limited tissue availability.
Statistical analysis of clinico-pathological features and lymphovascular parameters
Pearson's Chi-squared (χ2) tests or Fisher's exact tests were used for categorical variables and Mann-Whitney U tests for continuous variables to identify associations between ulceration status and clinico-pathological features in the Cohort and SNB studies combined, and between ulceration and lymphovascular parameters in the subset of tumors with IHC staining. Breslow thickness and mitotic rate were analysed as categorical variables based on AJCC staging criteria (Balch et al., 2009) due to skewed frequency distributions. For IHC data, vessel density and macrophage counts were categorised into high and low groups around the median value.
To identify factors independently associated with ulceration status, multiple logistic regression was performed using factors significantly associated with ulceration in univariate analysis. A continuous variable for age at diagnosis was used in the multivariate model. To maximise the number of observations assessed in each analysis, multiple logistic regression was performed separately for clinico-pathological data and lymphovascular parameters.
For the subset of samples with haematoxylin and eosin (H+E) sections centrally reviewed by pathologists, analyses to identify clinico-pathological associations with ulceration were repeated to confirm results found in the pooled Cohort and SNB dataset. For all analyses a significance level of 0.05 was used.
Statistical analysis of gene expression data
In the test dataset, gene profiles (502 genes) were normalised as described previously (Conway et al., 2009, Jewell et al., 2010). In the Leeds replication dataset (whole genome), the gene expression dataset was normalised using the robust spline method in the Lumi R package. All normalised gene profiles were log2-transformed prior to statistical analysis.
Associations between gene expression and ulceration status were identified using linear regression adjusting for the study type to which the patient was recruited in the test data set and for a batch effect in the Leeds validation set. Analyses in the test set were further adjusted by Breslow thickness.
The Lund replication data set was pre-processed as described previously (Harbst et al., 2012) with each probe being median-centered. For each GeneSymbol the probe with the highest variation was then selected. To detect significant differentially expressed genes, Significance of Microarray (SAM) analysis was used (Tusher et al., 2001).
In all 3 gene expression datasets (test set, Leeds replication and Lund replication) the false discovery rate was applied to correct for multiple-testing (permutation-based within SAM software in Lund dataset and Benjamini-Hochberg method in Leeds datasets) (Benjamini and Hochberg, 1995) and fold changes in mean expression between ulcerated and non-ulcerated tumors were calculated using data that had not been log-transformed.
Pathway analysis of gene expression data
In the test dataset, pathway analysis was performed in DAVID (the database for annotation, visualisation and integrated discovery) to test the enrichment of specific pathways among the differentially expressed genes using the list of 502 cancer genes as background (Dennis et al., 2003). Significantly over- and under-expressed genes (FDR <0.10) were inputted into DAVID and functional annotation analysis was performed to identify Gene Ontology (GO) terms from three ontologies (Biological processes, Molecular Functions and Cellular components) (Ashburner et al., 2000) and KEGG (Kyoto Encylopedia of Genes and Genomes) (Kanehisa and Goto, 2000) pathways enriched in those genes.
This analysis was repeated in the Leeds validation dataset using the whole genome as background. A slight modification of the Fisher exact test was used in DAVID to calculate the significance of enrichment scores and Benjamini-Hochberg false discovery was used to adjust for multiple testing.
Cluster analysis of gene expression data and statistical analysis of clinico-pathological features of tumors in clusters
Hierarchical clustering of genes and tumours was conducted using the average linkage method with uncentred correlation distance metric in CLUSTER 3.0 (de Hoon et al., 2004) and graphical representation in Java Treeview (Saldanha, 2004). Clustering was based on 73 genes associated with ulceration status at FDR<0.10 and log2 transformed centre-normalised expression values were used. After cluster analysis, non-ulcerated tumours from different clusters identifiable on the dendrogram were compared using Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
Supplementary Material
Significance.
Primary melanoma tumor ulceration is associated with poor prognosis yet predicts benefit from adjuvant interferon therapy. There are currently no other confirmed predictive biomarkers for interferon therapy. This integrated study of clinico-pathological factors and gene expression profiles reveals that ulcerated tumors are infiltrated with greater numbers of macrophages and differentially express genes involved in wound healing pathways associated with up-regulation of pro-inflammatory cytokines. This suggests that tumor related inflammation is associated with ulceration. The relative benefit from interferon reported in patients with ulcerated tumors may reflect modification of signalling pathways involved in inflammation.
Acknowledgments
Funding summary:
This work was supported by Cancer Research UK (project grants C8216/A6129 and C8216/A8168), and program grants C588/A4994 and C588/A10589), and by the NIH (R01 CA83115). RJ was in receipt of a Bramall Fellowship and a Medical Research Council Clinical Research Training Fellowship (G0802123). AM was funded by a grant from the Leeds Teaching Hospitals Trust Charitable Fund. ML, KJ and GJ receive funding from the Swedish Cancer Society and the Swedish Research Council.
References
- ASHBURNER M, BALL CA, BLAKE JA, BOTSTEIN D, BUTLER H, CHERRY JM, DAVIS AP, DOLINSKI K, DWIGHT SS, EPPIG JT, HARRIS MA, HILL DP, ISSEL-TARVER L, KASARSKIS A, LEWIS S, MATESE JC, RICHARDSON JE, RINGWALD M, RUBIN GM, SHERLOCK G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALCH CM, GERSHENWALD JE, SOONG SJ, THOMPSON JF, ATKINS MB, BYRD DR, BUZAID AC, COCHRAN AJ, COIT DG, DING S, EGGERMONT AM, FLAHERTY KT, GIMOTTY PA, KIRKWOOD JM, MCMASTERS KM, MIHM MC, JR., MORTON DL, ROSS MI, SOBER AJ, SONDAK VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALCH CM, MURAD TM, SOONG SJ, INGALLS AL, HALPERN NB, MADDOX WA. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978;188:732–42. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALCH CM, SOONG SJ, GERSHENWALD JE, THOMPSON JF, REINTGEN DS, CASCINELLI N, URIST M, MCMASTERS KM, ROSS MI, KIRKWOOD JM, ATKINS MB, THOMPSON JA, COIT DG, BYRD D, DESMOND R, ZHANG Y, LIU PY, LYMAN GH, MORABITO A. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- BEDOGNI B, POWELL MB. Hypoxia, melanocytes and melanoma - survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 2009;22:166–74. doi: 10.1111/j.1755-148X.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENJAMINI Y, HOCHBERG Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- BISWAS SK, MANTOVANI A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- BRACARDA S, EGGERMONT AM, SAMUELSSON J. Redefining the role of interferon in the treatment of malignant diseases. Eur J Cancer. 2010;46:284–97. doi: 10.1016/j.ejca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- CONWAY C, MITRA A, JEWELL R, RANDERSON-MOOR J, LOBO S, NSENGIMANA J, EDWARD S, SANDERS DS, COOK M, POWELL B, BOON A, ELLIOTT F, DE KORT F, KNOWLES MA, BISHOP DT, NEWTON-BISHOP J. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009;15:6939–46. doi: 10.1158/1078-0432.CCR-09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE HOON MJ, IMOTO S, NOLAN J, MIYANO S. Open source clustering software. Bioinformatics. 2004;20:1453–4. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- DENNIS G, JR., SHERMAN BT, HOSACK DA, YANG J, GAO W, LANE HC, LEMPICKI RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome biology. 2003;4:P3. [PubMed] [Google Scholar]
- DEPASQUALE I, THOMPSON WD. Microvessel density for melanoma prognosis. Histopathology. 2005;47:186–94. doi: 10.1111/j.1365-2559.2005.02193.x. [DOI] [PubMed] [Google Scholar]
- DUNN JH, ELLIS LZ, FUJITA M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33. doi: 10.1016/j.canlet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- DURBEC F, MARTIN L, DERANCOURT C, GRANGE F. Melanoma of the hand and foot: epidemiological, prognostic and genetic features. A systematic review. Br J Dermatol. 2012;166:727–39. doi: 10.1111/j.1365-2133.2011.10772.x. [DOI] [PubMed] [Google Scholar]
- EGGERMONT A, SUCIU S, SANTINAMI M, KRUIT W, TESTORI A, MARSDEN J, PUNT CJ, GORE M, MACKIE R, DUMMER R, SCHADENDORF D, PATEL P, SPATZ A, KEILHOLZ U, GROUP EM. EORTC 18991 phase III trial: Long-term adjuvant pegylated interferon-α2b (PEG-IFN) versus observation in resected stage III melanoma: Long-term results at 7.6-years follow-up.. J Clin Oncol; ASCO Annual Meeting; Chicago, Illinois. 2011; abstr 8506b. [DOI] [PubMed] [Google Scholar]
- EGGERMONT AM, SPATZ A, LAZAR V, ROBERT C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol. 2012a;24:137–40. doi: 10.1097/CCO.0b013e32834fcb0d. [DOI] [PubMed] [Google Scholar]
- EGGERMONT AM, SUCIU S, MACKIE R, RUKA W, TESTORI A, KRUIT W, PUNT CJ, DELAUNEY M, SALES F, GROENEWEGEN G, RUITER DJ, JAGIELLO I, STOITCHKOV K, KEILHOLZ U, LIENARD D. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–96. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- EGGERMONT AM, SUCIU S, TESTORI A, KRUIT WH, MARSDEN J, PUNT CJ, SANTINAMI M, SALES F, SCHADENDORF D, PATEL P, DUMMER R, ROBERT C, KEILHOLZ U, YVER A, SPATZ A. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012b;48:218–25. doi: 10.1016/j.ejca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- ELLIOTT B, SCOLYER RA, SUCIU S, LEBECQUE S, RIMOLDI D, GUGERLI O, MUSAT E, SHARMA RN, LIENARD D, KEILHOLZ U, TESTORI A, EGGERMONT A, MACKIE R, ROBERT C, COOK M, THOMPSON JF, ANGEVIN E, SPATZ A. Long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. Clin Cancer Res. 2007;13:3825–30. doi: 10.1158/1078-0432.CCR-07-0358. [DOI] [PubMed] [Google Scholar]
- ERDAG G, SCHAEFER JT, SMOLKIN ME, DEACON DH, SHEA SM, DENGEL LT, PATTERSON JW, SLINGLUFF CL., JR. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTIN A, VIEIRA JM, GESTRI G, DENTI L, SCHWARZ Q, PRYKHOZHIJ S, PERI F, WILSON SW, RUHRBERG C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOGAS H, EGGERMONT AM, HAUSCHILD A, HERSEY P, MOHR P, SCHADENDORF D, SPATZ A, DUMMER R. Biomarkers in melanoma. Ann Oncol. 2009;20(Suppl 6):vi8–13. doi: 10.1093/annonc/mdp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAASS NK, SMALLEY KS, LI L, HERLYN M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–9. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- HARBST K, STAAF J, LAUSS M, KARLSSON A, MASBACK A, JOHANSSON I, BENDAHL PO, VALLON-CHRISTERSSON J, TORNGREN T, EKEDAHL H, GEISLER J, HOGLUND M, RINGNER M, LUNDGREN L, JIRSTROM K, OLSSON H, INGVAR C, BORG A, TSAO H, JONSSON G. Molecular profiling reveals low- and high-grade forms of primary melanoma. Clin Cancer Res. 2012;18:4026–36. doi: 10.1158/1078-0432.CCR-12-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL R, CONWAY C, MITRA A, RANDERSON-MOOR J, LOBO S, NSENGIMANA J, HARLAND M, MARPLES M, EDWARD S, COOK M, POWELL B, BOON A, DE KORT F, PARKER KA, CREE IA, BARRETT JH, KNOWLES MA, BISHOP DT, NEWTON-BISHOP J. Patterns of expression of DNA repair genes and relapse from melanoma. Clin Cancer Res. 2010;16:5211–21. doi: 10.1158/1078-0432.CCR-10-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEHISA M, GOTO S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHANI-SABET M, SAGEBIEL RW, FERREIRA CM, NOSRATI M, MILLER JR., 3RD Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–31. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A, ALLAVENA P, SICA A, BALKWILL F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A, SOZZANI S, LOCATI M, ALLAVENA P, SICA A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- MCGARY EC, LEV DC, BAR-ELI M. Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther. 2002;1:459–65. doi: 10.4161/cbt.1.5.158. [DOI] [PubMed] [Google Scholar]
- MELNIKOVA VO, BAR-ELI M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009;22:257–67. doi: 10.1111/j.1755-148X.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- MITRA A, CONWAY C, WALKER C, COOK M, POWELL B, LOBO S, CHAN M, KISSIN M, LAYER G, SMALLWOOD J, OTTENSMEIER C, STANLEY P, PEACH H, CHONG H, ELLIOTT F, ILES MM, NSENGIMANA J, BARRETT JH, BISHOP DT, NEWTON-BISHOP JA. Melanoma sentinel node biopsy and prediction models for relapse and overall survival. Br J Cancer. 2010;103:1229–36. doi: 10.1038/sj.bjc.6605849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAHEDI K, LAOUI D, GYSEMANS C, BAETEN M, STANGE G, VAN DEN BOSSCHE J, MACK M, PIPELEERS D, IN'T VELD P, DE BAETSELIER P, VAN GINDERACHTER JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- NAUGLER WE, SAKURAI T, KIM S, MAEDA S, KIM K, ELSHARKAWY AM, KARIN M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- NEWTON-BISHOP JA, BESWICK S, RANDERSON-MOOR J, CHANG YM, AFFLECK P, ELLIOTT F, CHAN M, LEAKE S, KARPAVICIUS B, HAYNES S, KUKALIZCH K, WHITAKER L, JACKSON S, GERRY E, NOLAN C, BERTRAM C, MARSDEN J, ELDER DE, BARRETT JH, BISHOP DT. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–44. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESTKA S, KRAUSE CD, WALTER MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- PHAN A, TOUZET S, DALLE S, RONGER-SAVLE S, BALME B, THOMAS L. Acral lentiginous melanoma: histopathological prognostic features of 121 cases. Br J Dermatol. 2007;157:311–8. doi: 10.1111/j.1365-2133.2007.08031.x. [DOI] [PubMed] [Google Scholar]
- POUYSSEGUR J, DAYAN F, MAZURE NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- RAKOSY Z, ECSEDI S, TOTH R, VIZKELETI L, HERNANDEZ-VARGAS H, LAZAR V, EMRI G, SZATMARI I, HERCEG Z, ADANY R, BALAZS M. Integrative genomics identifies gene signature associated with melanoma ulceration. PLoS One. 2013;8:e54958. doi: 10.1371/journal.pone.0054958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE AE, CHRISTOS PJ, LACKAYE D, SHAPIRO RL, BERMAN R, MAZUMDAR M, KAMINO H, OSMAN I, DARVISHIAN F. Clinical relevance of detection of lymphovascular invasion in primary melanoma using endothelial markers D2-40 and CD34. Am J Surg Pathol. 2011;35:1441–9. doi: 10.1097/PAS.0b013e31822573f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALDANHA AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–8. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- SCHMIDT T, CARMELIET P. Blood-vessel formation: Bridges that guide and unite. Nature. 2010;465:697–9. doi: 10.1038/465697a. [DOI] [PubMed] [Google Scholar]
- SPATZ A, BATIST G, EGGERMONT AM. The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol. 2010a;22:163–8. doi: 10.1097/CCO.0b013e328337fe8f. [DOI] [PubMed] [Google Scholar]
- SPATZ A, COOK MG, ELDER DE, PIEPKORN M, RUITER DJ, BARNHILL RL. Interobserver reproducibility of ulceration assessment in primary cutaneous melanomas. Eur J Cancer. 2003;39:1861–5. doi: 10.1016/s0959-8049(03)00325-3. [DOI] [PubMed] [Google Scholar]
- SPATZ A, STOCK N, BATIST G, VAN KEMPEN LC. The biology of melanoma prognostic factors. Discov Med. 2010b;10:87–93. [PubMed] [Google Scholar]
- STALKUP JR, ORENGO IF, KATTA R. Controversies in acral lentiginous melanoma. Dermatol Surg. 2002;28:1051–9. doi: 10.1046/j.1524-4725.2002.02082.x. discussion 1059. [DOI] [PubMed] [Google Scholar]
- STORR SJ, SAFUAN S, MITRA A, ELLIOTT F, WALKER C, VASKO MJ, HO B, COOK M, MOHAMMED RA, PATEL PM, ELLIS IO, NEWTON-BISHOP JA, MARTIN SG. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod Pathol. 2012;25:493–504. doi: 10.1038/modpathol.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUME O, SALVESEN HB, AKSLEN LA. Angiogenesis is prognostically important in vertical growth phase melanomas. Int J Oncol. 1999;15:595–9. doi: 10.3892/ijo.15.3.595. [DOI] [PubMed] [Google Scholar]
- TAN KB, MONCRIEFF M, THOMPSON JF, MCCARTHY SW, SHAW HM, QUINN MJ, LI LX, CROTTY KA, STRETCH JR, SCOLYER RA. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902–12. doi: 10.1097/PAS.0b013e318073c600. [DOI] [PubMed] [Google Scholar]
- THOMPSON JF, SOONG SJ, BALCH CM, GERSHENWALD JE, DING S, COIT DG, FLAHERTY KT, GIMOTTY PA, JOHNSON T, JOHNSON MM, LEONG SP, ROSS MI, BYRD DR, CASCINELLI N, COCHRAN AJ, EGGERMONT AM, MCMASTERS KM, MIHM MC, JR., MORTON DL, SONDAK VK. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29:2199–205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUSHER VG, TIBSHIRANI R, CHU G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLBROCK C, WEISSER C, HASSEL JC, FISCHER P, BECKER J, VETTER CS, BEHRMANN I, KORTYLEWSKI M, HEINRICH PC, SCHARTL M. STAT5 contributes to interferon resistance of melanoma cells. Curr Biol. 2005;15:1629–39. doi: 10.1016/j.cub.2005.08.036. [DOI] [PubMed] [Google Scholar]
- WINNEPENNINCKX V, LAZAR V, MICHIELS S, DESSEN P, STAS M, ALONSO SR, AVRIL MF, ORTIZ ROMERO PL, ROBERT T, BALACESCU O, EGGERMONT AM, LENOIR G, SARASIN A, TURSZ T, VAN DEN OORD JJ, SPATZ A. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–82. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- XU X, CHEN L, GUERRY D, DAWSON PR, HWANG WT, VANBELLE P, ELDER DE, ZHANG PJ, MING ME, SCHUCHTER L, GIMOTTY PA. Lymphatic invasion is independently prognostic of metastasis in primary cutaneous melanoma. Clin Cancer Res. 2012;18:229–37. doi: 10.1158/1078-0432.CCR-11-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.