Abstract
Purpose
To investigate systematically the retinal and optic disc changes in central retinal vein occlusion (CRVO) and their natural history.
Methods
The study comprised 562 consecutive CRVO patients [492 non-ischemic (NI-CRVO) and 89 ischemic CRVO (I-CRVO) eyes] seen within 3 months of onset, Ophthalmic evaluation at initial and follow-up visits included recording visual acuity, visual fields, and detailed anterior segment and fundus examinations and fluorescein fundus angiography.
Results
Retinal and sub-internal limiting membrane hemorrhages, and optic disc edema in I-CRVO were initially more marked (p<0.0001), and took longer to resolve (p<0.015) than in NI-CRVO. Initially, macular edema was more marked in I-CRVO than NI-CRVO (p<0.0001), but did not significantly differ in resolution time (p=0.238). Macular retinal epithelial pigment degeneration, serous macular detachment, and retinal perivenous sheathing developed at a higher rate in I-CRVO than in NI-CRVO (p<0.0001). I-CRVO had more retinal venous engorgement than NI-CRVO (p=0.003). Fluorescein fundus angiography showed significantly more fluorescein leakage, retinal capillary dilatation, capillary obliteration, and broken capillary foveal arcade (p<0.0001) in I-CRVO than NI-CRVO. Resolution time of CRVO was longer for I-CRVO than NI-CRVO (p<0.0001).
Conclusion
Characteristics and natural history of fundus findings in the two types of CRVO are different.
Keywords: Central retinal vein occlusion, Cotton-wool spots, Epiretinal membrane, Fluorescein fundus angiography, Macular edema, Macular pigmentary degeneration, Optic disc, Retina, Retinal hemorrhages, Retinal vein occlusion, Retinal veins
The clinical entity of central retinal vein occlusion (CRVO) has been known since 18781 and it is a common, visually disabling disorder. Yet, a Medline literature search revealed little comprehensive information on the various retinal and optic disc changes, from any study with large cohort of eyes with CRVO. We investigated this in 581 eyes of 562 patients with CRVO, as well as their natural history.
Patients and Methods
We have investigated fundus changes in CRVO systematically in the Ocular Vascular Clinic at the Tertiary Care University of Iowa Hospitals and Clinics since 1973. The current study was part of a prospective study on ocular vascular occlusive disorders funded by the National Institute of Health (RO1 grant), approved by the Institutional Review Board. The data for this study were collected prospectively. In the present study, we investigated the retinal and optic disc findings in CRVO and their evolution on follow-up. It included patients who were first seen in our clinic from 1973 to 2000. The data were compiled from 562 consecutive CRVO patients (543 patients with data for one eye and 19 patients with data from both– a total of 581 eyes) who strictly fulfilled our inclusion and exclusion criteria for this study. There was no built-in bias for the severity, type or laterality of CRVO seen in the Ocular Vascular Clinic because of extremely good co-operation from our referring community of ophthalmologists, who sent us all cases with CRVO.
Inclusion Criteria
Only those patients who were seen within 3 months of onset, and had a definite diagnosis of CRVO, were included. CRVO consists of two distinct clinical entities: non-ischemic CRVO (NI-CRVO) and ischemic (I-CRVO) types2–8. Differentiation between the two types of CRVO in this study was based on the following four functional tests investigated in our previous study8: relative afferent pupillary defect, electroretinography, visual fields plotted with a Goldmann perimeter and visual acuity. The various criteria for these tests to differentiate I-CRVO from NI-CRVO are discussed at length in our previous study8, which was specifically designed to establish the reliability of the various tests. Briefly, sensitivity and specificity for each of the these tests for I-CRVO were as follows: visual acuity 20/400 or less, sensitivity 91% and specificity 88%; as regards the visual fields, absent I-2e isopter, sensitivity 97% and specificity 73%, and defective or absent V-4e isopter, sensitivity 100% and specificity 100%; relative afferent pupillary defect of equal to or more than 0.9 log units, sensitivity 80% and specificity 97%; and in electroretinography, B-wave amplitude less than 60% of the normal amplitude, sensitivity 80% and specificity 80%. The distinction was based on the COMBINED data acquired from these 4 tests, and no one test was decisive per se. Our previous study8 showed that combined information from relative afferent pupillary defect and electroretinography differentiated 97% and that constituted the most reliable parameter.
Exclusion criteria
We excluded all other retinopathies mimicking CRVO, including diabetic retinopathy. All patients with inadequate information required for evaluation of retinal and optic disc changes, or doubtful diagnosis were excluded. Patients who had any other retinal or optic nerve lesion or any other factor (e.g., cataract), including any treatment for CRVO, which could have influenced the fundus findings, were excluded.
STUDIES PERFORMED
The intention was to document the retinal and optic disc changes serially during follow-up. The data were collected prospectively and systematically. At the initial visit, all patients were seen by one of us (SSH) in the Ocular Vascular Clinic and had a detailed ocular and medical history as well as a comprehensive bilateral ophthalmic evaluation. This included, (i) careful testing of the best corrected visual acuity using the Snellen visual acuity chart, (ii) visual field plotting with a Goldmann perimeter (using I-2e, I-4e and V-4e targets regularly), (iii) intraocular pressure recording with a Goldmann applanation tonometer, (iv) relative afferent pupillary defect, (v) a thorough anterior segment examination, including slit lamp examination of the anterior segment, lens and vitreous, (vi) a meticulous fundus evaluation by direct and indirect ophthalmoscopy, and, if required, by contact lens, (vii) stereoscopic color fundus photography, (viii) stereoscopic fluorescein fundus angiography (only in the involved eye), and (ix) electroretinography.
In addition, the patients had a full systemic evaluation performed either by an internist at the University of Iowa Hospitals & Clinics, or by their local internist/physician.
At each follow-up visit, the same ophthalmic evaluation and stereoscopic color fundus photography were done, except that fluorescein fundus angiography was performed only when considered essential; also many patients were reluctant to have repeated angiography because of nausea and other reasons. Electroretinography was usually performed only during the initial visit; however, if a change in status of CRVO from non-ischemic to ischemic was suspected, it was repeated. Both eyes were examined at each visit.
Follow-up protocol for all patients
All patients were followed (by SSH) according to a protocol practiced in this Clinic for CRVO patients - at about 3 monthly intervals for 3 visits, then 6 monthly intervals for 4 visits, then annually. Both eyes were examined at each visit. Since this was a natural history study of fundus changes, no intervention of any kind whatsoever was undertaken in this cohort of patients with CRVO.
Evaluation of fundus findings
In each eye the following retinal and optic disc changes were documented meticulously. Changes were evaluated in a masked fashion, by a single investigator (SSH), by comparing findings in 30° and 60° fundus photographs with the standard fundus photographs for various grades of retinal and optic disc changes.
1. Retinal hemorrhages
Final evaluation was always done from stereoscopic color fundus photographs (30° and 60° photographs), and also for peripheral hemorrhages based on the indirect and direct ophthalmoscopic evaluation because it is not possible to photograph that part of the fundus with the available fundus cameras. For each visit, the retinal hemorrhages at various locations in the retina were evaluated (comparing with the standard photos for various grades), using the following criteria:
Subjective grading of retinal hemorrhages
1= no hemorrhages; 2 = minimal/small/isolated hemorrhages; 3 = medium amount of hemorrhages; 4 = extensive hemorrhages; 5 = uncertain/questionable hemorrhages; 9 =no data.
Regions of the retina
For descriptive purposes, the entire fundus was divided into the following 5 regions: (1) foveal center, (2) perifoveal region, (3) rest of the macular region, (4) rest of posterior pole (in the region outside the vascular arcades), and (5) peripheral region. This is because different areas of the retina have different severity of hemorrhages and their effects on visual function. Retinal hemorrhages were evaluated separately in the 5 regions.
Ordinary retinal hemorrhages were intra-retinal; but when the hemorrhages were lying on the surface of the nerve fiber layer and under the internal limiting membrane, those were classified as “sub-internal limiting membrane hemorrhages”, while those lying in the subhyaloid space were labeled as “subhyaloid hemorrhages”.
2 Macular edema
Critical evaluation of the macular edema was done by using: (i) direct ophthalmoscopy, (ii) Hruby contact lens, (iii) stereoscopic fundus photographs of the macular region, and (iv) frequently stereoscopic fluorescein fundus angiography of the macular region. OCT or other modern technologies to evaluate macular edema were not available during the period of this study. The severity of macular edema was graded as minimal, medium, and marked (comparing with the standard photos for various grades).
3. Submacular serous retinal detachment
This was evaluated in our study from combined information provided by Hruby contact lens and stereoscopic color fundus photographs and fluorescein angiograms. We are aware that OCT most probably can show more subtle submacular serous retinal detachment than the methods we used.
4. Optic disc edema
This was also graded as minimal, medium, and marked, comparing with the standard photos for various grades.
5. Retinal venous engorgement
This was graded as none, mild, moderate and marked, comparing with the standard photos for various grades.
6. Cotton wool spots
Their location and presence was documented, and when possible their number was recorded.
7. Other fundus parameters recorded
These included the presence of epiretinal membrane, macular retinal pigment epithelial degeneration, macular hole, retinal venous sheathing, retinal arterial abnormality, lipid deposits (“hard exudates”), optic disc hyperemia, optic disc collaterals, optic disc pallor, serous macular retinal detachment, and any other finding.
8. Fluorescein fundus angiography
In the fovea, perifovea, macula, the rest of the posterior pole (outside the vascular arcades) and peripheral retina, we evaluated the presence or absence of fluorescein staining, perivenous fluorescein staining, microaneurysms, retinal capillary obliteration, retinal capillary foveal arcade intact or broken, and arteriovenous filling time (in seconds). Severity of retinal capillary obliteration was graded as none, small patchy, moderate and extensive, according to a set of slides with different grades, and not in disc diameters. Since fluorescein angiography was mainly evaluated from angiograms covering the central 30° to 60° , findings in the extreme peripheral retina could not be evaluated; there were only some cases where the peripheral retinal was scanned. Also, when retinal hemorrhages were masking the retinal capillary bed, it was not possible to evaluate the extent of capillary non-perfusion correctly. These, and poor quality of angiogram, are the limitations with routine fluorescein fundus angiography.
RESOLUTION OF RETINOPATHY
Resolution of retinopathy was defined as when retinal hemorrhages, macular edema, optic disc edema, cotton wool spots and other retinal changes had all resolved.
Statistical Analysis
The CRVO was differentiated at the initial visit into NI-CRVO or I-CRVO, based on the criteria discussed above. Initial grades of hemorrhage severity, macular edema, optic edema, and severity of capillary obliteration were compared between NI-CRVO and I-CRVO as the initial diagnosis using exact Wilcoxon rank-sum test. Kaplan-Meier curves were constructed for resolution of hemorrhage, macular edema, optic disc edema, retinal vein engorgement, and CRVO, and were compared using log-rank test. For the NI-CRVO cases that later converted to I-CRVO, the analysis included only follow-up up to the time of conversion. To adjust for the effect of a covariate, Cox proportional hazard regression analysis was used. Similar analysis was performed to time to development of cotton wool spots, macular hole, epiretinal membrane, macular pigmentary degeneration, retinal vein sheathing, lipid deposits, retinal arterial attenuation, and serous detachment.
Results
Our study included 474 patients (492 eyes) with non-ischemic CRVO (NI-CRVO) and 88 patients (89 eyes) with ischemic CRVO (I-CRVO). There were 47 eyes that initially presented NI-CRVO that converted to ischemic CRVO. The demographic and clinical characteristics of these patients are presented in Table 1.
Table 1.
Demographic and Clinical characteristics
| Demographic/Clinical Variable | Non-ischemic CRVO n=474 patients 492 eyes | Ischemic CRVO
|
|
|---|---|---|---|
| As first diagnosis n=88 patients 89 eyes | Converted from non-ischemic CRVO n=46 patients* 47 eyes | ||
|
| |||
| Gender (Male) | 248 (53%) | 46 (52%) | 27 (59%) |
|
| |||
| Age at initial visit | |||
| Mean±SD | 60±16 | 69±14 | 70±13 |
| Range | 18–90 | 30–89 | 26–100 |
| Distribution by age (count, %) | |||
| <45 | 82 (17%) | 8 (9%) | 2 (4%) |
| 45-<65 | 173 (37%) | 15 (17%) | 11 (24%) |
| 65 and older | 219 (46%) | 65 (74%) | 33 (72%) |
|
| |||
| Eye involvement | |||
| Right eye | 229 (48%) | 37 (42%) | 18 (39%) |
| Left eye | 227 (48%) | 50 (57%) | 27 (59%) |
| Both eyes | 18 (4%) | 1 (1%) | 1 (2%) |
|
| |||
| Follow-up (of those with at least 1 month follow-up) | (n=422 eyes) | (n=80 eyes) | (n=46 eyes) |
| Median (25th-75th percentile) Minimum-Maximum |
2.1 (0.5–5.1) years (1 month-28 years) |
2.2 (0.7–6.0) years (1 month-15 years) |
4.2 (1.1–5.5) years (1 month-19 years) |
|
| |||
| Systemic Conditions | |||
| Arterial hypertension | 201 (42%) | 44 (50%) | 19 (41%) |
| Ischemic heart disease | 53 (11%) | 16 (18%) | 8 (17%) |
| Diabetes mellitus | 42 (9%) | 17 (19%) | 6 (13%) |
| TIA/CVA | 23 (5%) | 2 (2%) | 6 (13%) |
| (n=453) | (n=80) | (n=45) | |
|
| |||
| Smoked current/past | 202 (45%) | 33 (41%) | 21 (47%) |
CRVO=Central retinal Vein Occlusion
47 eyes (46 patients) with ischemic CRVO that converted from non-ischemic CRVO are also included among the eyes with non-ischemic CRVO
SD=Standard deviation
@ Follow-up of at least 3 months.
CVA = Cerebrovascular accident; TIA = Transient ischemic attack
RETINAL HEMORRHAGES
Fundus examination at the initial visit of NI-CRVO eyes for retinal hemorrhage at each location showed that more than 60% of eyes had mild to no retinal hemorrhage, and between 7% and 16% had severe hemorrhage (see Table 2). Overall, 3 (1%) did not have any retinal hemorrhage at any location, 42% had at most only a mild retinal hemorrhage, and 22% had at least 1 location with severe hemorrhage. In contrast, those with I-CRVO as the initial diagnosis presented with significantly worse hemorrhage in 6 locations, except for disc hemorrhage, with at least 40% of eyes having severe hemorrhage (all p<0.0001; p=0.105 for disc hemorrhage). Overall for I-CRVO, 12% had at most only a mild retinal hemorrhage, and 53% had at least 1 location with severe hemorrhage. The distribution of severity of hemorrhage at each location is shown in Table 2.
Table 2.
Retinal hemorrhage at initial visit and median time to resolution
| Location of Hemorrhage | Grade | Time to Resolution, months Median (25th-75th percentile) | |||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||
|
Non-ischemic CRVO (n=490, 2 missing; except peripheral 5 missing, disc 3 missing) |
|||||
| Fovea (center) | 255 (52%) | 140 (29%) | 61 (12%) | 34 (7%) | 8.5 (5.2–14.2) |
| Perifovea | 123 (25%) | 225 (46%) | 108 (22%) | 34 (7%) | 9.3 (5.2–17.3) |
| Macular | 71 (14%) | 228 (47%) | 151 (31%) | 40 (8%) | 9.4 (8.4–10.3) |
| Post. Pole | 49 (10%) | 240 (49%) | 149 (31%) | 52 (11%) | 9.5 (6.2–17.6) |
| Peripheral | 9 (2%) | 334 (69%) | 112 (23%) | 32 (7%) | 20.7 (8.5–40.9) |
| Disc | 299 (61%) | 122 (25%) | 58 (12%) | 10 (2%) | 4.6 (3.4–6.3) |
| Peripapillary | 143 (29%) | 158 (32%) | 109 (22%) | 80 (16%) | 6.8 (4.3–10.2) |
|
Ischemic CRVO as first diagnosis (n=88, 1 missing; except post. pole 2 missing, disc 4 missing) |
|||||
| Fovea (center) | 11 (13%) | 21 (24%) | 20 (23%) | 35 (40%) | 9.4 (6.2–17.7) |
| Perifovea | 5 (6%) | 15 (17%) | 31 (35%) | 37 (42%) | 12.2 (8.6–33.6) |
| Macular | 2 (2%) | 14 (16%) | 33 (38%) | 39 (44%) | 17.5 (10.5–40.0) |
| Post. Pole | 3 (3%) | 11 (13%) | 34 (39%) | 39 (45%) | 18.9 (14.5–48.0) |
| Peripheral | 1 (1%) | 15 (17%) | 36 (41%) | 36 (41%) | 34.4 (19.5–59.8) |
| Disc | 47 (55%) | 19 (22%) | 11 (13%) | 8 (9%) | 4.8 (3.7–7.7) |
| Peripapillary | 7 (8%) | 12 (14%) | 28 (32%) | 41 (47%) | 9.9 (7.2–14.4) |
|
Ischemic CRVO converted from non-ischemic CRVO
(n=46, 1 missing) |
|||||
| Fovea (center) | 6 (13%) | 7 (15%) | 9 (20%) | 24 (52%) | 8.5 (6.2–13.4) |
| Perifovea | 2 (4%) | 4 (9%) | 15 (33%) | 25 (54%) | 10.4 (7.9–14.7) |
| Macular | 2 (4%) | 1 (2%) | 15 (33%) | 28 (61%) | 13.4 (9.5–21.7) |
| Post. Pole | 1 (2%) | 4 (9%) | 15 (33%) | 26 (57%) | 14.7 (10.3–44.9) |
| Peripheral | 1 (2%) | 5 (11%) | 21 (46%) | 19 (41%) | 38.7 (16.9–69.1) |
| Disc | 24 (52%) | 15 (33%) | 3 (7%) | 4 (9%) | 3.3 (2.9–6.2) |
| Peripapillary | 4 (9%) | 6 (13%) | 10 (22%) | 26 (57%) | 8.2 (5.7–13.4) |
Kaplan-Meier curves for the resolution of retinal hemorrhage are shown in figure 1 for NI-CRVO and figure 2 for I-CRVO. Retinal hemorrhages in the disc had a shorter resolution time and peripheral retinal hemorrhages had the longest resolution time in both NI-CRVO and I-CRVO. The median times to resolution at each location are presented in Table 2. Comparing resolution time of retinal hemorrhage between NI-CRVO and I-CRVO as the initial diagnoses, except for hemorrhage in the fovea (p=0.23) and in the disc (p=0.56), hemorrhage in I-CRVO took significantly longer to resolve (all p<0.009).
Fig. 1.
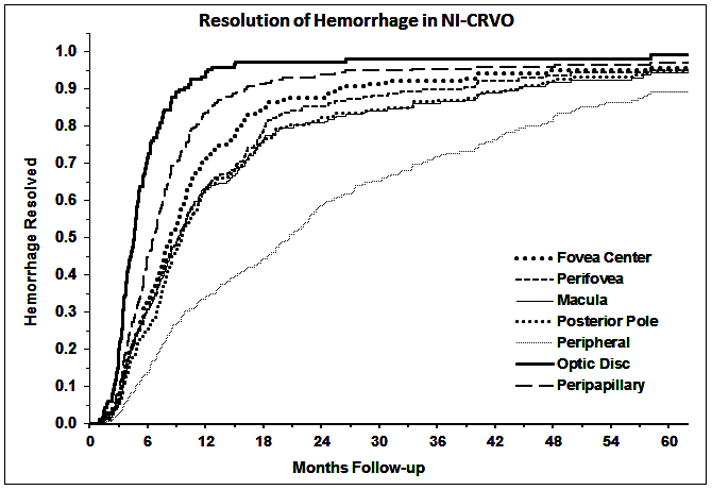
Kaplan-Meier curves for the resolution of retinal hemorrhage in different part of the retina in non-ischemic CRVO.
Fig. 2.
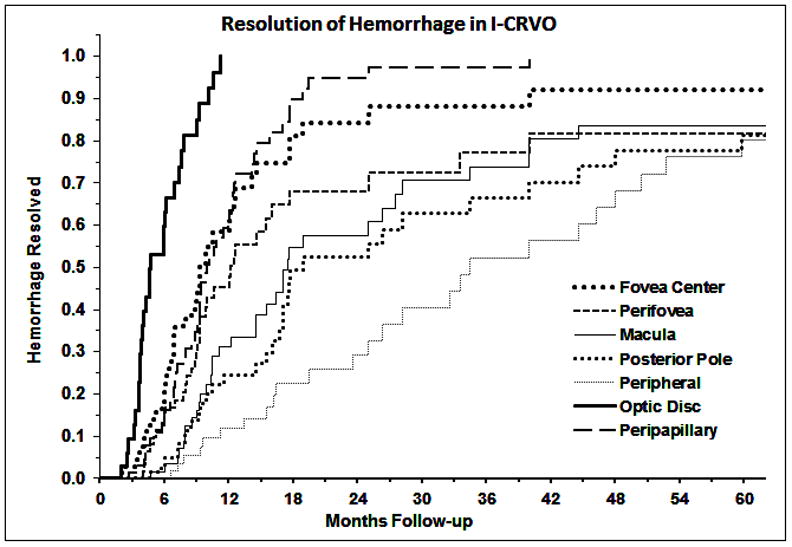
Kaplan-Meier curves for the resolution of retinal hemorrhage in different part of the retina in ischemic CRVO.
Sub-internal Limiting Membrane and Subhyaloid Hemorrhages
Sub-internal limiting membrane hemorrhages mostly developed within 5 months of onset for both NI-CRVO and I-CRVO as initial diagnoses. In NI-CRVO, the cumulative probability of sub-internal limiting membrane hemorrhage was 12.4%±1.6% within 3 months, and 16.9%±1.8% within 5 months from onset. In comparison, I-CRVO had a significantly higher rate of sub-internal limiting membrane hemorrhages, with 33.1%±5.1% within 3 months, and 38.3%±5.4% within 5 months from onset (p<0.0001). The sub-internal limiting membrane hemorrhages that developed in I-CRVO eyes also resolved at a significantly slower rate than those in NI-CRVO (p=0.015), with hemorrhage resolving in 52.7%±6.7% of NI-CRVO and 28.6%±9.3% of I-CRVO within 9 months.
There was also a significantly higher rate of development of subhyaloid hemorrhages in I-CRVO compared to NI-CRVO (p=0.047). Most appeared within 3 months from onset of I-CRVO, with cumulative probability of 6.1%±2.6%. In NI-CRVO, the cumulative probability of subhyaloid hemorrhages was 2.0%±0.7% within 3 months, and 2.5%±0.8% within 7.5 months from onset.
MACULAR CHANGES
At the initial visit, eyes with I-CRVO presented with more marked macular edema than NI-CRVO (p<0.0001). Macular edema grade in those with I-CRVO as the initial diagnosis was 12% with none-mild, 34% moderate, and 53% severe. In NI-CRVO, there were 68% none-mild, 24% moderate, and 9% severe.
Resolution time of macular edema did not significantly differ between I-CRVO and NI-CRVO (p=0.238). For eyes with I-CRVO at first diagnosis, 20.8%±6.0% had resolved within 12 months from onset, and 38.8%±8.6% had resolved within 24 months, with a median time to resolution of 28.8 (IQR: 17.6–50.4) months. For NI-CRVO, 31.5%±2.8% resolved within 12 months from onset, and 50.9%±3.2% resolved within 24 months, with a median time to resolution of 23.8 (IQR: 9.5–58.6) months. Limiting the comparison of resolution time among those who had severe macular edema at initial visit, also did not show any significant difference between I-CRVO ad NI-CRVO (p=0.362).
Kaplan-Meier product-limit estimates of the development of epiretinal membrane, macular retinal epithelial pigment degeneration, and serous macular detachment are shown in Table 3. Macular retinal epithelial pigment degeneration and serous macular detachment (including the bullous type) developed at a significantly higher rate in I-CRVO than in NI-CRVO (both p<0.0001). Within 12 months from onset, macular retinal epithelial pigment degeneration had developed in 39.1%±6.2% of eyes with I-CRVO and 21.5%±2.3% in NI-CRVO; within 24 months, it was 53.7%±6.5% in I-CRVO and 35.6%±2.9% in NI-CRVO. Serous macular detachment mostly developed within 3 months from onset of I-CRVO, with cumulative probability of 60.3%±5.4%. For NI-CRVO, serous macular detachment had developed in 39.1%±2.4% within 6 months from onset.
Table 3.
Kaplan-Meier product-limit estimates for development of macular changes
| Macular change | Non-ischemic CRVO (n=492) | Ischemic CRVO at first diagnosis (n=89) |
|---|---|---|
|
| ||
| Premacular Fibrosis (Epiretinal membrane) | ||
| Total Developed | 74 | 20 |
| Cumulative % (SE): | ||
| 6 months | 2.2% (0.8%) | 2.8% (2.0%) |
| 9 months | 5.4% (1.2%) | 9.0% (3.5%) |
| 12 months | 11.6% (1.8%) | 12.5% (4.2%) |
| 24 months | 19.5% (2.4%) | 25.1% (6.0%) |
| 36 months | 22.7% (2.6%) | 38.0% (7.2%) |
|
| ||
| Macular pigmentary degeneration | ||
| Total Developed | 120 | 41 |
| Cumulative % (SE): | ||
| 6 months | 5.0% (1.1%) | 7.0% (3.0%) |
| 9 months | 14.5% (1.9%) | 22.6% (5.2%) |
| 12 months | 21.5% (2.3%) | 39.1% (6.2%) |
| 24 months | 35.6% (2.9%) | 53.7% (6.5%) |
| 36 months | 41.2% (3.1%) | 64.2% (6.5%) |
|
| ||
| Serous macular detachment | ||
| Total Developed | 185 | 57 |
| Cumulative % (SE): | ||
| 1 month | 11.4% (1.5%) | 22.6% (4.4%) |
| 2 months | 22.5% (1.9%) | 46.3% (5.4%) |
| 3 months | 29.9% (2.2%) | 60.3% (5.4%) |
| 6 months | 39.1% (2.4%) | 65.6% (5.3%) |
| 9 months | 41.5% (5.3%) | 68.9% (5.3%) |
Severity of macular edema at initial examination was significantly associated with macular retinal epithelial pigment degeneration and serous macular detachment in NI-CRVO. Those with moderate/severe macular edema were more likely to have epithelial pigment degeneration (hazard ratio: 3.78; 95% CI: 2.62, 5.45; p<0.0001) and serous macular detachment (hazard ratio: 7.99; 95% CI: 1.70, 37.65; p=0.002) relative to those with none/mild macular edema at initial visit. However, among eyes with I-CRVO, severity of macular edema at initial visit was not found to be significantly associated with epithelial pigment degeneration (p=0.646) and serous macular detachment (p=0.993).
There was no significant difference in the rate of development of epiretinal membrane between NI-CRVO and I-CRVO (p=0.126), where 11.6%±1.8% in NI-CRVO and 12.5%±4.2% had developed epiretinal membrane within 12 months from onset; by 24 months, it was 19.5%±2.4% in NI-CRVO and 25.1%±6.0% and I-CRVO. Severity of macular edema at initial examination was significantly associated with development of epiretinal membrane in NI-CRVO. Those with severe macular edema (hazard ratio: 10.09, 95% CI: 3.63, 28.09) and moderate macular edema (hazard ratio: 8.33, 95% CI: 3.22, 3.22) were more likely to develop epiretinal membrane relative to those with no macular edema at initial visit (both p<0.0001). This was also observed comparing severe relative to mild macular edema (hazard ratio: 2.43, 95% CI: 1.26, 4.65; p=0.008). However, among eyes with I-CRVO, severity of macular edema at initial visit was not found to be significantly associated with rate of development of epiretinal membrane (p=0.841).
Macular hole was seen in only 6 eyes with NI-CRVO and 1 eye with I-CRVO.
RETINAL VASCULAR CHANGES
Cotton Wool Spots
There was significantly more cotton wool spots in eyes with I-CRVO than NI-CRVO (p<0.0001). In I-CRVO, 25% had 8 or more cotton wool spots compared to 6% in NI-CRVO. Cotton wool spots also developed earlier in I-CRVO than in NI-CRVO (p=0.035). Within 3 months from onset, cotton wool spots had developed in 40.4%±5.3% in I-CRVO and 28.8%±2.1% in NI-CRVO; within 12 months it was 50.7%±5.6% in I-CRVO and 36.3%±2.3% in NI-CRVO.
Retinal Venous Engorgement and Sheathing
At the initial fundus examination, those with NI-CRVO had 39% moderate and 40% marked retinal vein engorgement, which was significantly less severe than with I-CRVO, which had 20% moderate and 61% marked (p=0.003). Comparing rate of resolution of retinal vein engorgement between NI-CRVO and I-CRVO, adjusted for severity of initial retinal vein engorgement, showed no significant difference between the CRVO types (p=0.918). In NI-CRVO, the cumulative proportion that resolved was 11.9%±1.8% within 12 months from onset, and 17.3%±2.2% within 24 months. For I-CRVO, 10.1%±3.9% had resolved within 12 months, and 12.2%±4.4% within 24 months.
I-CRVO had a significantly higher rate of retinal perivenous vein sheathing than NI-CRVO (p<0.0001). Retinal perivenous vein sheathing had appeared in 20.0%±5.0% within 12 months and 37.6%±6.5% within 24 months from onset of I-CRVO. For NI-CRVO, it was 8.9%±1.6% within 12 months, and 18.0%±2.4% within 24 months from onset.
Our study showed that cotton wool spots (and corresponding focal capillary obliteration on fluorescein angiography) were seen in both types of CRVO, although there were more cotton wool spots in eyes with I-CRVO compared to NI-CRVO (p<0.0001). Within 3 months from onset, cotton wool spots had developed in 40.4%±5.3% in I-CRVO and 28.8%±2.1% in NI-CRVO.
Retinal Arterial Attenuation and Sheathing
Ten eyes with I-CRVO developed retinal arterial attenuation, 7 of which were first observed within 8 months from onset. Only 1 eye with NI-CRVO had retinal arterial attenuation. Retinal arterial sheathing was found in only 2 eyes with I-CRVO and 1 eye with NI-CRVO.
Cilioretinal Artery
Normal cilioretinal artery was present in 3% of eyes with I-CRVO and 7% of NI-CRVO (p=0.23).
OPTIC DISC CHANGES
Optic Disc Edema
At the initial visit, eyes with I-CRVO presented with more marked optic disc edema than those with NI-CRVO (p<0.0001). The distribution of optic disc edema in those with I-CRVO as the initial diagnosis was none in 7%, mild in 25%, moderate in 35%, and severe in 33%. In NI-CRVO as the initial diagnosis, there were none in 19%, mild in 39%, moderate in 20%, and severe in 22%.
Resolution of optic disc edema significantly differed between I-CRVO and NI-CRVO (p<0.0001), with a slower rate of resolution in I-CRVO. For eyes with I-CRVO as the initial diagnosis, 33.9%±6.0% had resolved within 12 months from onset, and 53.6%±6.7% had resolved within 24 months, with a median time to resolution of 21.7 months. For NI-CRVO as the initial diagnosis, 57.5%±2.6% resolved within 12 months from onset, and 81.6%±2.2% resolved within 24 months, with a median time to resolution of 9.8 months. Comparing optic disc edema resolution time between I-CRVO and NI-CRVO stratified by initial optic disc edema grade, showed no significant difference between the CRVO types in resolution time of mild edema (p=0.619) and moderate edema (p=0.120). Resolution time of severe optic disc edema was significantly longer in I-CRVO compared to NI-CRVO (hazard ratio: 0.38; 95% CI: 0.19, 0.76; p=0.007).
Optic Disc Retinociliary Collaterals
The rate of development of these collaterals did not significantly differ between NI-CRVO and I-CRVO (p=0.53). For NI-CRVO, 19.0%±2.0% developed disc collaterals within 6 months, and 41.0%±2.7% within 12 months from onset. It was 21.4%±4.8% within 6 months, and 46.5%±6.3% within 12 months from onset for I-CRVO.
Optic Disc Pallor
Optic disc pallor appeared at a significantly higher rate in I-CRVO than in NI-CRVO (p<0.0001). Within 6 months from onset, 16.0%±4.2% in I-CRVO and 1.1%±0.5% in NI-CRVO had developed optic disc pallor. Within 12 months, it was 38.4%±6.0% in I-CRVO and 2.0%±0.8% in NI-CRVO
LIPID DEPOSITS
There were 15 eyes with I-CRVO that developed lipid deposits, of which 10 were first observed within 9.5 months from onset, corresponding to a cumulative probability of 15.0%±4.4%. For NI-CRVO, the cumulative probability at 12 months was 9.2%±1.5% and 10.4%±1.7% at 18 months. This suggested a higher rate of lipid deposit in I-CRVO than NI-CRVO (p=0.055), and a greater proportion of I-CRVO eyes develop lipid deposits earlier than do NI-CRVO.
FLUORESCEIN FUNDUS ANGIOGRAPHIC FINDINGS
The results of fluorescein angiography done within 4 months from onset are presented in Tables 4 and 5. Comparing fluorescein staining between NI-CRVO and I-CRVO as the initial diagnoses showed significantly higher proportion with posterior pole and perivenous staining in I-CRVO (both p<0.0001) (see Table 4). This may also apply for the foveal/perifoveal/macular region, if we accounted for the eyes where staining could not be determined due to overlying hemorrhages. Likewise for identifying microaneurysms in I-CRVO as the initial diagnosis, there were 11% with microaneurysm in foveal/perifoveal/macular region, with another 47% of those with angiography which could not be assessed because of overlying hemorrhages. For NI-CRVO as the initial diagnosis, it was 13% with microaneurysm in fovea/perifovea/macula, with additional 3% which could not be assessed because of overlying hemorrhages. Retinal capillary dilatation was more prevalent in I-CRVO (72%) compared to NI-CRVO (60%; p<0.0001).
Table 4.
Fluorescein Leak and Staining, Microaneurysms, and Capillary Dilatation on Angiography within 4 Months from Onset
| Location | NI-CRVO (n=447) | I-CRVO at (n=72) first diagnosis | I-CRVO converted from NI-CRVO (n=23) | |||
|---|---|---|---|---|---|---|
| Present | Not determined due to hemorrhage* | Present | Not determined due to hemorrhage* | Present | Not determined due to hemorrhage* | |
| Foveal/Perifoveal/Macular staining | 325 (73%) | 5 (1%) | 52 (72%) | 13 (18%) | 19 (83%) | 3 (13%) |
| Posterior pole staining | 86 (19%) | 1 (0.2%) | 34 (47%) | 17 (24%) | 11 (48%) | 2 (9%) |
| Optic disc staining | 392 (88%) | 1 (0.2%) | 67 (93%) | 1 (1%) | 20 (87%) | 1 (4%) |
| Perivenous staining | 252 (57%) | 6 (1%) | 53 (74%) | 7 (10%) | 17 (74%) | 0 (0%) |
| Foveal/Perifoveal/Macular microaneurysms | 60 (13%) | 14 (3%) | 8 (11%) | 34 (47%) | 6 (26%) | 7 (30%) |
| Posterior pole microaneurysm | 8 (2%) | 12 (3%) | 6 (8%) | 34 (47%) | 2 (9%) | 6 (26%) |
| Retinal capillary dilatation | 269 (60%) | 12 (3%) | 52 (72%) | 12 (17%) | 16 (70%) | 4 (17%) |
Not able to evaluate because of overlying extensive retinal hemorrhages
Table 5.
Retinal Capillary Obliteration on Angiography within 4 Months from Onset
| Location | Grade | ||||
|---|---|---|---|---|---|
| None | Patchy or Small | Moderate | Extensive | Not able to grade* | |
| Non-ischemic CRVO (n=447) | |||||
| Fovea (center) | 417 (93%) | 4 (0.9%) | 6 (1%) | 0 (0%) | 20 (4%) |
| Perifovea | 420 (94%) | 2 (0.5%) | 5 (1%) | 0 (0%) | 20 (4%) |
| Macular | 420 (94%) | 3 (0.7%) | 4 (0.9%) | 0 (0%) | 20 (4%) |
| Post. Pole | 417 (94%) | 5 (1%) | 4 (0.9%) | 0 (0%) | 20 (4%) |
| Peripheral | 443 (91%) | 0 (0%) | 6 (1%) | 1 (0.2%) | 33 (7%) |
| Ischemic CRVO at first diagnosis (n=72) | |||||
| Fovea (center) | 8 (11%) | 4 (6%) | 3 (4%) | 15 (21%) | 42 (58%) |
| Perifovea | 8 (11%) | 4 (6%) | 6 (8%) | 12 (17%) | 42 (58%) |
| Macular | 9 (12%) | 4 (6%) | 5 (7%) | 12 (17%) | 42 (58%) |
| Post. Pole | 5 (7%) | 3 (4%) | 6 (8%) | 16 (22%) | 42 (58%) |
| Peripheral | 6 (8%) | 0 (0%) | 2 (3%) | 18 (25%) | 46 (64%) |
| Ischemic CRVO converted from non-ischemic CRVO (n=23) | |||||
| Fovea (center) | 3 (13%) | 1 (4%) | 0 (0%) | 8 (35%) | 11 (48%) |
| Perifovea | 2 (9%) | 2 (9%) | 2 (9%) | 6 (26%) | 11 (48%) |
| Macular | 1 (4%) | 5 (22%) | 5 (22%) | 2 (9%) | 10 (43%) |
| Post. Pole | 2 (9%) | 4 (17%) | 4 (17%) | 4 (17%) | 9 (39%) |
| Peripheral | 2 (9%) | 1 (5%) | 3 (13%) | 6 (26%) | 11 (48%) |
Not able to grade severity of capillary obliteration because of overlying extensive retinal hemorrhages
Capillary obliteration was present and extensive in significantly more eyes with I-CRVO compared to NI-CRVO (all p<0.0001; see Table 5). Capillary obliteration was present in less than 10% of eyes with NI-CRVO. In contrast, at least 24% of eyes with I-CRVO at first diagnosis that had moderate to severe capillary obliteration in any of the five regions. Because of overlying retinal hemorrhages it was not possible to evaluate capillary obliteration in all eyes.
The capillary foveal arcade in eyes with NI-CRVO was intact in 90%, broken in 4%, and could not be determined in 6% due to overlying hemorrhage. In contrast, eyes with I-CRVO as the initial diagnosis had 12% intact, 36% broken, and could not be determined in 51% due to overlying hemorrhage (p<0.0001).
Time interval between the start of filling of the retinal arterioles and start of retinal venous filling within 4 months of onset of CRVO was measured in 318 eyes with NI-CRVO and in 40 eyes with I-CRVO at first diagnosis. The median time interval for NI-CRVO was 4 seconds (interquartile range: 3–5 seconds; range 3–35 seconds), and for I-CRVO, it was 5 seconds (interquartile range: 4–7 seconds; range 2–13 seconds) (p=0.0002).
RESOLUTION OF RETINOPATHY
The Kaplan-Meier curves for the resolution of NI-CRVO and I-CRVO are shown in figure 3. The time to resolution of CRVO was significantly longer for I-CRVO compared to NI-CRVO (p<0.0001). The hazard ratio of resolution for I-CRVO relative to NI-CRVO was 0.26 (95% CI: 0.16, 0.41).
Fig. 3.
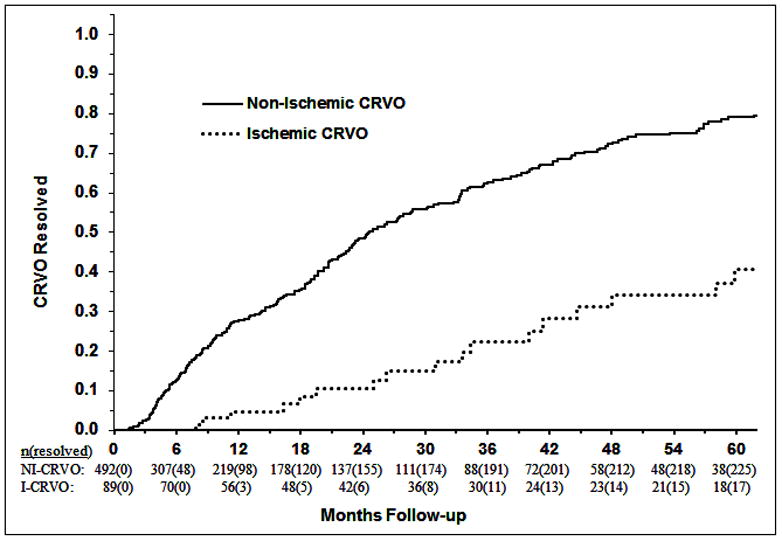
Kaplan-Meier curves for the resolution of non-ischemic and ischemic CRVOs.
There was a significant effect of diabetes on time to resolution of NI-CRVO (p=0.002). In those without diabetes, the cumulative proportion that resolved was 30.1%±2.6% within 12 months, and 50.9%±3.0% within 24 months from onset. In contrast, NI-CRVO resolution time was significantly longer in diabetics, with the proportion that resolved was 6.9%±4.7% within 12 months, and 25.2%±9.0% within 24 months.
Resolution time of I-CRVO did not significantly differ between those with and without diabetes (p=0.292). The cumulative proportion of I-CRVO that resolved in non-diabetics was 5.6%±3.26% within 12 months, and 10.2%±4.0% within 24 months from onset. For diabetics, none had resolved within 12 months, and 11.1%±10.5% within 24 months.
Discussion
As indicated earlier, there has not been previously a comprehensive study in a large cohort of eyes with CRVO eyes dealing in detail with retinal and optic disc changes and their natural history. We investigated that in 492 eyes with NI-CRVO and 89 eyes with I-CRVO as the initial diagnoses. Results showed that the retinal and optic disc findings in the two types of CRVO differ markedly. This information is important in the management of CRVO.
RETINAL HEMORRHAGES
At first diagnosis, I-CRVO eyes had significantly worse retinal (p<0.0001), sub-internal limiting membrane (i.e. lying on the nerve fiber layer) (p<0.0001) and subhyaloid hemorrhages (p=0.047) than in NI-CRVO. The retinal hemorrhages in both types of CRVO were more marked in the peripheral retina (particularly in the temporal periphery). In some eyes the retinal hemorrhages were located under the macular retina or in the macular cysts, as was also reported by Reyes et al.9 Retinal hemorrhages in I-CRVO took significantly longer to resolve than in NI-CRVO (p<0.009), and peripheral hemorrhages were the last to resolve in I-CRVO. Our study showed that peripheral retinal hemorrhages (particularly in the temporal periphery) usually were the first to appear and last to resolve. That information is important for the diagnosis and follow-up of CRVO.
MACULAR CHANGES
At the initial visit, microcystic macular edema was more marked in I-CRVO than in NI-CRVO (p<0.0001); however, its resolution time did not differ significantly between the two types (p=0.238). One can only speculate as to the reason for this disparity. In I-CRVO ischemic damage of the thick ganglion cell layer may result in their loss, resulting in reduced thickness of the macular retina, which may give a mistaken impression of resolution of macular edema. McIntosh et al.10 on a review of literature, found that in nonischemic CRVO cases, macular edema resolved in approximately 30% of eyes over time. In our study, macular edema resolved within 2 years in 50.9% in NI-CRVO and in 38.8% in I-CRVO.
Associated with macular edema, serous macular retinal detachment (including the bullous type) developed at a significantly higher rate in I-CRVO than in NI-CRVO (p<0.0001) (Table 3). This association is well known and is well documented currently by OCT. During the period of our study from 1973 onwards, OCT or other modern technologies to evaluate macular edemas were not yet available. Macular edema and serous macular detachment evaluation was based on clinical evaluation only (see above). However, for subretinal fluid, the baseline results of the SCORE Study report 111 using OCT were similar to our results based on examination using the Hruby contact lens and stereoscopic color fundus photographs and fluorescein angiograms. This suggests that our results were comparable to those on OCT. The relationship between macular edema and visual outcome was investigated by us in a previous study12 and is discussed in detail there. Briefly, that study showed that on resolution of macular edema, in eyes with initial visual acuity 20/70 or worse, visual acuity improved in 59% of the non-ischemic CRVO eyes, with no significant (p=0.55) improvement in ischemic CRVO. Similarly, on resolution of macular edema, in eyes with moderate to severe initial visual field defect, improvement was seen in 86% of non-ischemic CRVO eyes but no significant (p=0.83) improvement in ischemic CRVO.
One of us (SSH), in his clinical studies of CRVO, has found that patients with NA-CRVO usually complain that their visual acuity is worse when they wake up in the morning and improves later on in the day. Since poor visual acuity is related to macular edema, that suggests a circadian fluctuation of macular edema. This has also been reported by others13,14. Paques et al.13 suggested that it is due to nocturnal arterial fall of blood pressure. This circadian change may also be due to supine position during sleep, which may increase the venous pressure in the retinal veins and causing an increase in macular edema.
Rarely, CRVO eyes may develop macular ischemia (Fig 4) due to impairment of macular blood flow at onset of occlusion. Blood flow in the retinal circulation depends upon the difference between its arterial and venous pressures. A sudden occlusion of the central retinal vein may result in abrupt, marked rise of retinal venous pressure, and if that reaches the retinal arterial pressure, it results in hemodynamic block, causing this macular ischemia. In such a situation nocturnal arterial hypotension during the acute phase can also result in the same. There are several reports of simultaneous development of CRVO and central retinal artery, which is a variant of the above, as discussed elsewhere.14
Fig. 4.
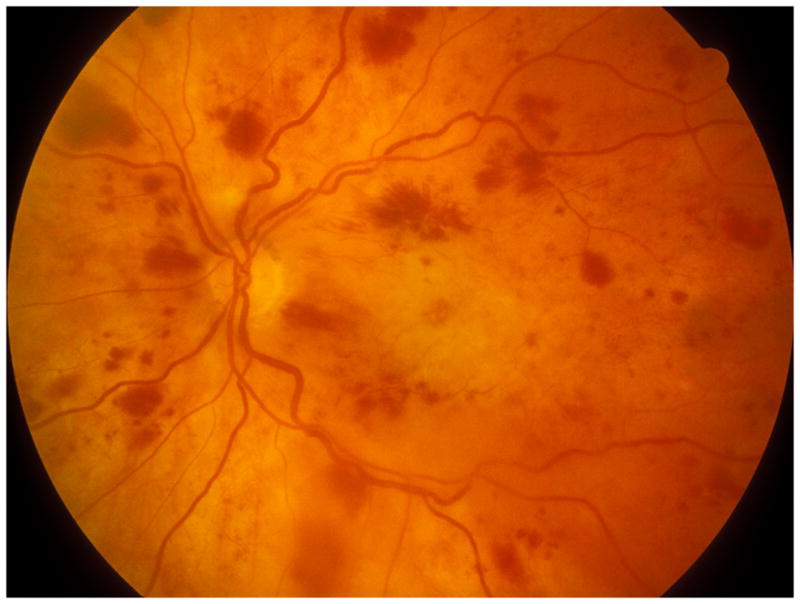
Fundus photograph of left with ischemic CRVO. It shows macular retinal ischemic opacity, scattered retinal hemorrhages and engorged retinal veins.
Our study showed that CRVO eyes with macular edema can develop epiretinal membrane and macular retinal epithelial pigment degeneration. The latter developed at a significantly higher rate in I-CRVO than NI-CRVO (p<0.0001) (Table 3), but not so in epiretinal membrane (p=0.126). Severity of macular edema at initial examination was significantly associated with development of both epiretinal membrane and retinal epithelial pigment degeneration in NI-CRVO, but not in I-CRVO. Foveal pigmentation and epiretinal membrane have detrimental effects on visual outcome in CRVO, as shown by our previous study.12
RETINAL VASCULAR CHANGES
Cotton wool spots were more common in I-CRVO than in NI-CRVO (p<0.0001) – within 3 months from onset in 40.4% versus 28.8%. This shows that contrary to almost a universal impression, cotton wool spots are seen in some eyes with classical NI-CRVO (this was also shown by our previous studies8). Since in the region of the cotton wool fluorescein angiography shows retinal capillary obliteration, presence of some patches of retinal capillary obliterations does occur in some NI-CRVO eyes.
Retinal venous engorgement was more marked in I-CRVO than in NI-CRVO (p=0.003). Resolution was seen within 24 months in 17.3% of NI-CRVO and 12.2% of I-CRVO. This shows that engorgement of the retinal veins resolved in only a small number of eyes, even up to 2 years. This is an important piece of information which contradicts a common impression that as retinopathy resolves in CRVO, the retinal veins return to their normal caliber. This persistence of some degree of venous engorgement being permanent is due to the fact that recanalization of the thrombosed central retinal vein (as shown by histopathological studies16) does not return the lumen of the vein to normal, resulting in a certain amount of persistent obstruction. This finding is also supported by the fact that in our fluorescein fundus angiographic study, the arteriovenous filling time interval did not return to normal in these eyes on resolution of CRVO.
Retinal perivenous sheathing developed within 2 years in 37.6% of I-CRVO and 18.0% of NI-CRVO (p<0.0001). In some eyes there was complete sheathing of small venules (mostly in the temporal peripheral retina) and those corresponded to retinal capillary obliteration. Some eyes with CRVO may show retinal perivenous sheathing at onset (Fig. 5). There may be two reasons for that. (1) It may be due to retinal phlebitis in these eyes, where the vitreous usually shows many cells. (2) Experimental studies by one of us (SSH) found similar perivenous sheathing develop soon after experimental occlusion of the branch retinal vein. This is due to breakdown of the blood retinal barrier caused by sudden distension of the lumen of the vein, as shown by fluorescein fundus angiography (Fig. 5B); this may be misinterpreted as retinal phlebitis. For example, Foss et al.17 showed similar perivenous sheathing in 3 eyes with CRVO (which cleared within 3 weeks), and Paques and Gaudric18 showed perivenous sheathing in 2 eyes during the acute phase of CRVO.
Fig. 5.
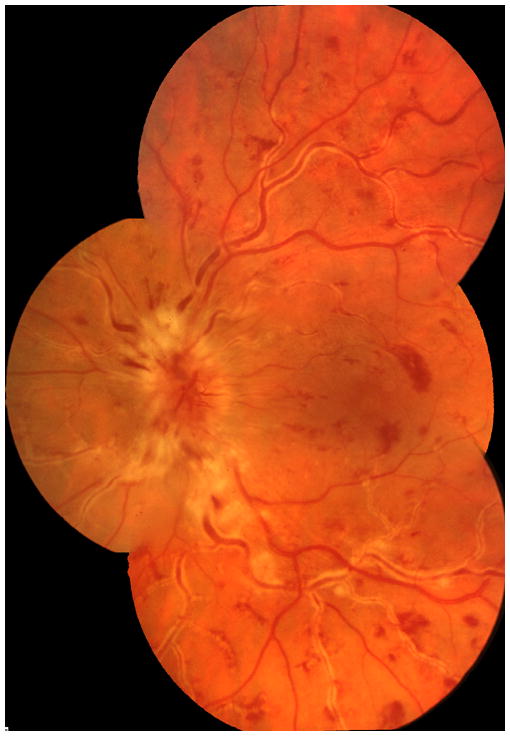
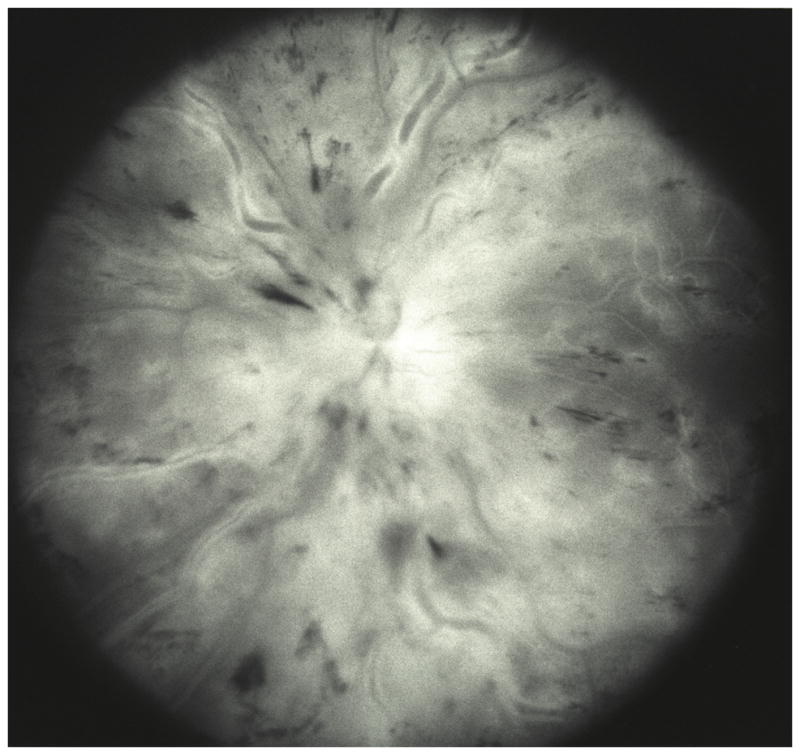
Fundus photograph (A) and fluorescein angiogram (B) of left with non-ischemic CRVO 4 days after onset of visual complaint.
A. This compost fundus photograph shows extensive perivenous sheathing, optic disc edema, macular edema and scattered retinal hemorrhages. .
B. Late phase of fluorescein angiography showing marked perivenous staining due to fluorescein leakage.
Retinal arterial attenuation was seen in 10 eyes with I-CRVO and in only one eye with NI-CRVO. Retinal arterial sheathing was found in only 2 eyes with I-CRVO and 1 eye with NI-CRVO.
OPTIC DISC CHANGES
Optic disc hemorrhages were not significantly different between the two types of CRVO at onset (p=0.099) and in their resolution (p=0.56). Optic disc edema was more marked in I-CRVO than in NI-CRVO at the initial visit (p<0.0001). Resolution of optic disc edema was significantly longer for I-CRVO compared to NI-CRVO (p<0.0001). Optic disc retinociliary collaterals developed in 41.0% within 12 months in NI-CRVO and in 46.5% in I-CRVO. Giuffre et al.19, in a study of 94 CRVO eyes, found these collaterals in 30.4% after a follow up of more than 1 year and no difference between NI-CRVO and I-CRVO. Takahashi et al.20, on indocyanine green angiography in 10 CRVO eyes with these collaterals, found that blood ultimately drained into the vortex veins. The effect of retinociliary collaterals on visual outcome in CRVO was investigated by us in a previous study.12
Optic disc pallor developed within 12 months in 38.4% of I-CRVO and 2.0% of NI-CRVO (p<0.0001). We investigated cup-to-disc ratio in CRVO in a previous study and a detailed account of that is given there.21 This study contradicted the concept that the “compartment syndrome” plays any role in the prevalence of various types of retinal vein occlusion or in their severity, the resolution of retinopathy or the visual outcome.
DEVELOPMENT OF RETINAL AND OPTIC DISC NEOVASCULARIZATION
We discussed this in detail in a previous study.22 That study, of 673 NI-CRVO and 239 I-CRVO eyes, showed that retinal and optic disc neovascularization attributable to CRVO occurred only in I-CRVO. In ischemic CRVO, within 6 months from time of onset, the cumulative probability of development of iris neovascularization was 49%, angle neovascularization 37%, neovascular glaucoma 29%, retinal neovascularization 9% and disc neovascularization 6%.
PROGRESSION OF NI-CRVO TO I-CRVO
This was investigated by us in detail in a previous study and reported in detail there.23 In that study, based on 550 episodes of NI-CRVO, the cumulative proportion of NI-CRVO cases that converted to the ischemic type was 9.4%±1.3% within six months and 12.6%±1.6% within 18 months from onset of nonischemic central retinal vein occlusion.
FLUORESCEIN FUNDUS ANGIOGRAPHIC FINDINGS
Fluorescein fundus angiography findings at the initial visit are given in Tables 4 and 5. Because of the presence of overlying retinal hemorrhages, it was not possible to evaluate various parameters in all eyes – a universal limitation. Available findings showed significantly (p<0.0001) more fluorescein leakage, retinal capillary dilatation, capillary obliteration and broken capillary foveal arcade in I-CRVO than in NI-CRVO. Similarly there were more microaneurysms in I-CRVO than in NI-CRVO. Time interval between the start of filling of the retinal arterioles and of retinal venous was significantly (p=0.0002) longer in I-CRVO than in NI-CRVO. Some eyes may be left with permanent microaneurysms or intraretinal microvascular abnormalities.
In most of the previous CRVO studies, differentiation between I-CRVO and NI-CRVO has been based on “10 disc diameters capillary non-perfusion” on fluorescein fundus angiography. Our previous study, specifically designed to find sensitivity and specificity of various clinical tests to differentiate two types of CRVO8, showed “10 disc diameters capillary non-perfusion” not a reliable parameter compared to the various functional tests. Hayreh has discussed this subject in detail elsewhere.15
RESOLUTION OF RETINOPATHY
The time to resolution of CRVO was significantly longer for I-CRVO compared to NI-CRVO (p<0.0001) (Fig. 3). Compared to non-diabetics, in diabetics it took significantly longer time for resolution in NI-CRVO (p=0.002) but it did not differ in I-CRVO (p=0.292). During this study, one of us (SSH) found that in diabetics, NI-CRVO retinopathy often gradually merges into the development of diabetic retinopathy in the involved eye only, as if CRVO acts as an additive effect to stimulate development of diabetic retinopathy earlier than would have occurred otherwise.
MORPHOLOGIC SEQUELAE OF A RESOLVED CRVO
Resolution of CRVO may leave several permanent footprints in the fundus of some eyes; these include: (1) retinociliary collaterals on the optic disc (Fig. 6), (2) retinal perivenous sheathing of major retinal veins (Fig. 7), (3) retinal pigment epithelial degeneration in the macular region (Figs. 6,7), (4) epiretinal membrane and (5) microaneurysms in the macular region (Fig. 8). One of us (SSH) has found from time to time that discovery on routine ophthalmic examination of these footprints, when there is no active CRVO, has baffled ophthalmologists about their etiology. For example, macular retinal pigment epithelial degeneration has been diagnosed as unilateral age-related macular degeneration; discovery of retinociliary collaterals on the optic disc has resulted in (a) extensive neuro-ophthalmologic investigations, because those are assumed to be due to optic nerve meningioma, and (b) in the glaucoma literature those have been called “abnormal optic disc vessels” caused by glaucoma.
Fig. 6.
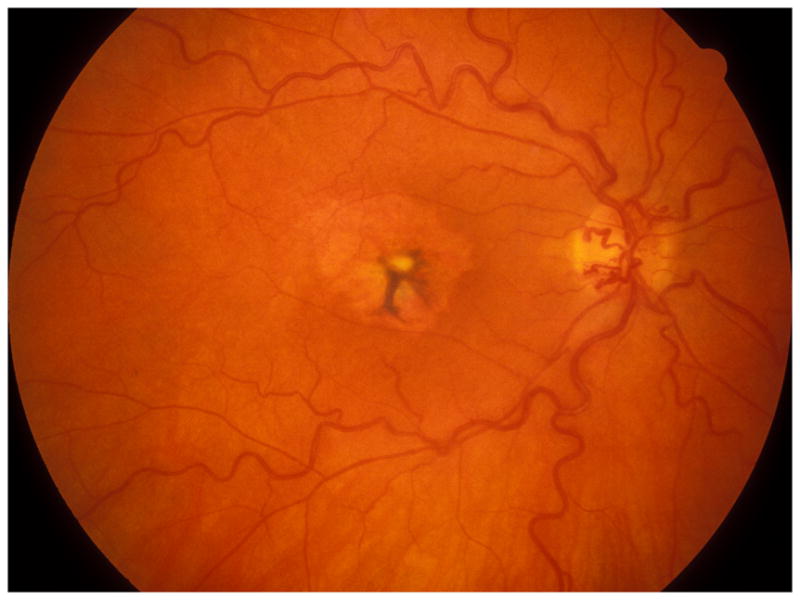
Fundus photograph of a resolved non-ischemic CRVO right eye. It shows retinociliary collaterals on the optic disc, macular retinal pigmentary degeneration and engorged tortous retinal veins.
Fig. 7.
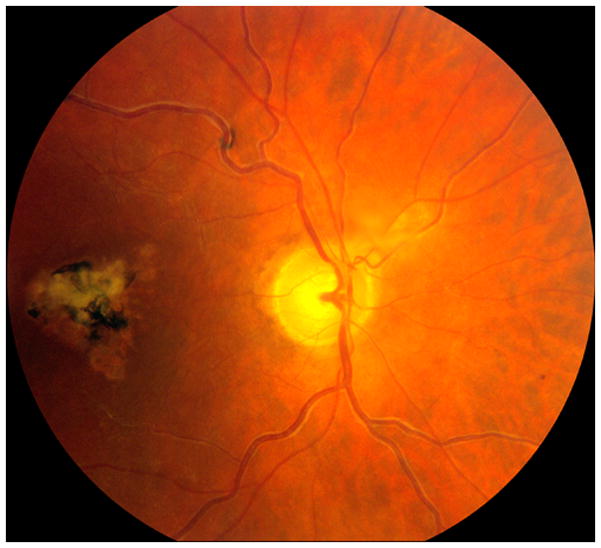
Fundus photograph of a resolved non-ischemic CRVO right eye. It shows engorged tortuous retinal veins, perivenous sheathing and macular pigmentary degeneration.
Fig. 8.
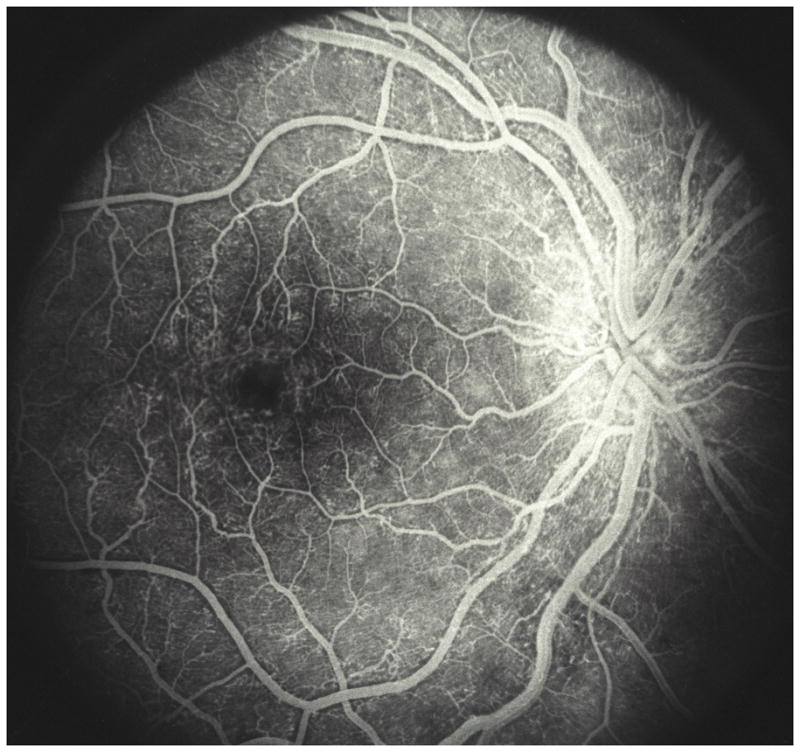
Fluorescein fundus angiogram of a right eye with resolved non-ischemic CRVO. It shows macular aneurysms.
The natural history of a disease always acts as the gold standard against which the effect of various therapies can be judged. We hope that our natural history findings on fundus changes in CRVO will serve as a reference for future therapeutic trials.
Acknowledgments
Supported by grant EY-1151 from the National Institutes of Health, Bethesda, Maryland
Footnotes
Authors have no financial interest or conflict.
References
- 1.Michel J. Ueber die anatomischen Ursachen von Veranderungen des Augenhintergrundes bei einigen Allgemeinerkrankungen. Deutsch Arch Klin Med. 1878;22:339–45. [Google Scholar]
- 2.Hayreh SS. Occlusion of the central retinal vessels. Br J Ophthalmol. 1965;49:626–45. doi: 10.1136/bjo.49.12.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayreh SS. So called "central retinal vein occlusion": 1. Pathogenesis, terminology, clinical features. Ophthalmologica. 1976;172:1–13. doi: 10.1159/000307579. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS. Pathogenesis of occlusion of the central retinal vessels. Am J Ophthalmol. 1971;72:998–1011. doi: 10.1016/0002-9394(71)91706-5. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, van Heuven WAJ, Hayreh MS. Experimental retinal vascular occlusion I. Pathogenesis of central retinal vein occlusion. Arch Ophthalmol. 1978;96:311–23. doi: 10.1001/archopht.1978.03910050179015. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983;90:458–74. doi: 10.1016/s0161-6420(83)34530-9. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- 8.Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Klin Exp Ophthalmol. 1990;228:201–17. doi: 10.1007/BF00920022. [DOI] [PubMed] [Google Scholar]
- 9.Reyes ME, Barr CC, Gamel JW. Blood levels in macular cystoid spaces and their relationship to retinal vein obstruction. Retina. 1994;14:14–18. doi: 10.1097/00006982-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, Kowalski JW, Nguyen HP, Wong TY. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113–23. doi: 10.1016/j.ophtha.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 11.Scott IU1, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, Jumper JM, Figueroa M SCORE Study Investigator Group. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116:504–12. doi: 10.1016/j.ophtha.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119–33. doi: 10.1016/j.ophtha.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paques M, Massin P, Sahel JA, Gaudric A, Bergmann JF, Azancot S, Levy BI, Vicaut E. Circadian fluctuations of macular edema in patients with morning vision blurring: correlation with arterial pressure and effect of light deprivation. Invest Ophthalmol Vis Sc. 2005;46:4707–11. doi: 10.1167/iovs.05-0638. [DOI] [PubMed] [Google Scholar]
- 14.Gupta B, Grewal J, Adewoyin T, Pelosini L, Williamson TH. Diurnal variation of macular oedema in CRVO: prospective study. Graefe's Arch Clin Experiment Ophthalmol. 2009;247:593–6. doi: 10.1007/s00417-008-1011-4. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases 1981. Retina. 2005;25(5 Suppl):27–55. doi: 10.1097/00006982-200507001-00008. [DOI] [PubMed] [Google Scholar]
- 17.Foss AJ, Headon MP, Hamilton AM, Lightman S. Transient vessel wall sheathing in acute retinal vein occlusions. Eye. 1992;6:313–6. doi: 10.1038/eye.1992.62. [DOI] [PubMed] [Google Scholar]
- 18.Paques M, Gaudric A. Perivenular macular whitening during acute central retinal vein occlusion. Arch Ophthalmol. 2003;121:1488–91. doi: 10.1001/archopht.121.10.1488. [DOI] [PubMed] [Google Scholar]
- 19.Giuffre G, Palumbo C, Randazzo-Papa G. Optociliary veins and central retinal vein occlusion. Br J Ophthalmol. 1993;77:774–7. doi: 10.1136/bjo.77.12.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Muraoka K, Kishi S, Shimizu K. Formation of retinochoroidal collaterals in central retinal vein occlusion. Am J Ophthalmol. 1998;126:91–9. doi: 10.1016/s0002-9394(98)00069-5. [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS, Zimmerman MB, Podhajsky PA. Retinal vein occlusion and the optic disk. Retina. 2012;32:2108–18. doi: 10.1097/IAE.0b013e31825620f2. [DOI] [PubMed] [Google Scholar]
- 22.Hayreh SS, Zimmerman MB. Ocular neovascularization associated with central and hemicentral retinal vein occlusion. Retina. 2012;32(8):1553–65. doi: 10.1097/IAE.0b013e318246912c. [DOI] [PubMed] [Google Scholar]
- 23.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 24.Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–94. doi: 10.1097/IAE.0b013e31815ec29b. [DOI] [PubMed] [Google Scholar]


