Abstract
Background and objective
In low and middle-income countries where HIV infection is prevalent, identifying patients at high risk of dying from lower respiratory tract infections is challenging and validated prognostic models are lacking. Serum procalcitonin may be a useful prognostic tool in these settings. We sought to determine if elevated serum procalcitonin is associated with increased in-hospital mortality and to combine serum procalcitonin with available clinical characteristics to create a clinically useful prognostic model.
Methods
We conducted a prospective, nested case-control study of 241 HIV-infected adults admitted to Mulago Hospital in Kampala, Uganda with cough ≥2 weeks in duration. We collected demographic and clinical information, baseline serum for procalcitonin analysis, and followed patients to determine in-hospital mortality.
Results
Serum procalcitonin was a strong and independent predictor of inpatient mortality (aOR=7.69, p=0.01, sensitivity=93%, negative predictive value=97%). Best subset multivariate analysis identified 3 variables that were combined into a prognostic model to risk stratify patients; these variables included respiratory rate ≥30 breaths/minute (aOR=2.07, p=0.11), oxygen saturation <90% (aOR=3.07, p=0.02), and serum procalcitonin >0.5ng/ml (aOR=7.69, p=0.01). The predicted probability of inpatient mortality ranged from 1% when no variables were present, to 42% when all variables were present.
Conclusions
Elevated serum procalcitonin >0.5ng/ml is an independent predictor of in-hospital mortality. Elevated serum procalcitonin, tachypnea, and hypoxemia may be combined into a prognostic model to identify patients at high risk of dying in the hospital. This model may be used to estimate the probability of death and to guide triage and treatment decisions.
Keywords: HIV, Pneumonia, Procalcitonin, Prognosis, Tuberculosis
INTRODUCTION
Lower respiratory tract infections (LRTI), especially tuberculosis are a major cause of morbidity and mortality in HIV-infected people residing in low-income countries; however validated prognostic models to identify patients at high risk of dying are lacking. The diagnostic and prognostic utility of the serum biomarker procalcitonin (PCT) has been explored in several LRTI studies. Nyamande examined PCT in HIV-infected patients with bacterial pneumonia, Pneumocystis pneumonia (PCP), and tuberculosis1; he found that mean PCT levels were 19 times higher in patients with bacterial pneumonia than in those with PCP and nearly 5 times higher than in those with tuberculosis (19.479ng/mL vs. 1.138ng/mL vs. 4.164ng/mL, p<0.0004). Other studies have explored the prognostic value of PCT in patients with bacterial pneumonia and have shown results comparable to validated prognostic models such as CURB-652,3 and CRB-654,5. However, these prognostic studies excluded patients with HIV infection and those with tuberculosis and fungal pneumonia; thus the prognostic value of PCT in these patients remains unknown.
We performed a prospective, nested case-control study within the International HIV-associated Opportunistic Pneumonias (IHOP) Study and measured PCT using banked serum from a cohort of HIV-infected adults with suspected LRTI admitted to a large referral hospital in Uganda. We sought to determine if elevated serum PCT is associated with increased in-hospital mortality and combined PCT with available clinical characteristics to create a clinically useful prognostic model.
METHODS
Study Population
We enrolled 635 adults ≥18 years with known or suspected HIV, admitted to Mulago Hospital in Kampala, Uganda between September 2007 and July 2008 with cough ≥2 weeks but <6 months. These criteria were designed to select for patients with indolent LRTI such as tuberculosis and PCP, the primary pneumonias of interest for the IHOP Study. Patients were excluded if they were being treated for tuberculosis or had heart failure. The patients presented in this study have been included in other published studies, none of which measured PCT6-16.
Ethics Approval
The Institutional Review Board of Mulago Hospital, the Makerere University Faculty of Medicine Research Ethics Committee, the Ugandan National Council for Science and Technology, and the Committee on Human Research at the University of California, San Francisco all approved the study protocol. All participants signed written, informed consent.
Patient Evaluation
The study protocol has been described6-16. Briefly, clinical and demographic information was collected using standardized questionnaires. Vital signs were measured by study medical officers or nurses. HIV testing was performed in those without a documented positive HIV test and CD4 counts at the time of presentation were measured. Standardized evaluation for LRTI included chest radiography and two sputum specimens for acid-fast bacilli (AFB) smear (Ziehl-Neelsen) and mycobacterial culture (Lowenstein-Jensen media)8. If both sputum smears were negative for AFB, patients were referred for bronchoscopy with bronchoalveolar lavage (BAL) for further diagnosis. Bronchoscopic inspection was performed to assess for tracheobronchial Kaposi sarcoma and BAL fluid was examined for mycobacteria, Pneumocystis jirovecii, and other fungi. Patients were followed throughout their hospitalization to assess in-hospital mortality.
Final diagnoses were assigned at 2-months by trained study personnel according to pre-specified criteria as follows: (1) Culture-positive tuberculosis– positive mycobacterial culture(s); (2) Fungal pneumonia – positive BAL fluid KOH smear or positive BAL fungal culture; (3) Pneumocystis pneumonia – positive BAL fluid Giemsa stain (Diff-Quik); (4) Pulmonary Kaposi sarcoma – presence of typical lesions in the tracheobronchial tree during bronchoscopic airways examination. Patients without a confirmed microbiologic etiology were categorized as presumed tuberculosis if they improved on empiric anti-tuberculosis therapy and as presumed bacterial pneumonia if they improved on empiric antibiotic therapy and were well at 2-month follow-up. Patients without a confirmed microbiologic etiology were categorized as having an unknown diagnosis if the diagnostic evaluation was incomplete and/or there was no 2-month follow-up (lost to follow-up). 13
Measurement of Procalcitonin and Cut-off Determination
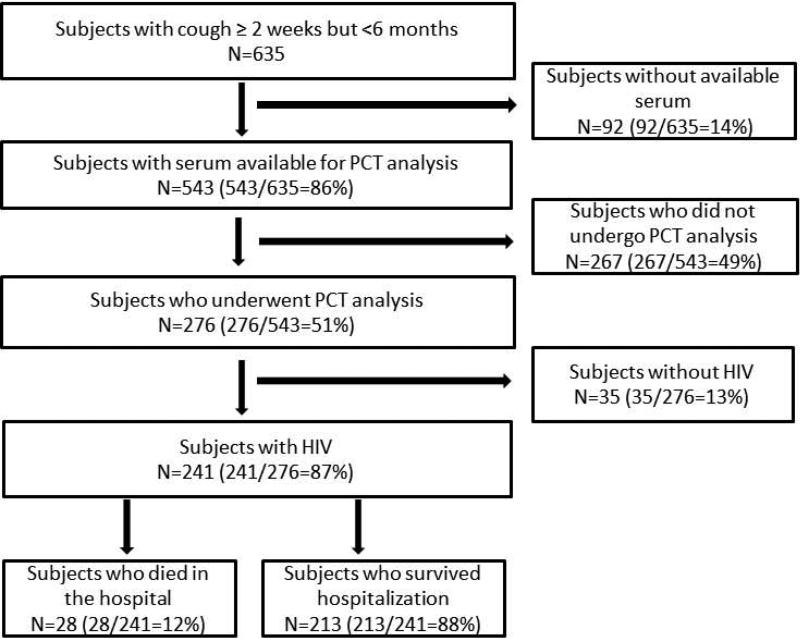
Serum was obtained at the time of study enrollment and frozen at −20°C until PCT analysis was performed. Serum was available from 543 subjects and PCT was measured from every other HIV-infected subject [Figure 1]. Procalcitonin cut-off values of 0.1ng/ml, 0.25ng/ml, and 0.5ng/ml have been used in previously published studies17. We chose a cut-off of >0.5ng/ml because too few patients had serum concentrations <0.25ng/ml. Procalcitonin levels were measured using the Kryptor immunoassay (B.R.A.H.M.S, Hennigsdorf, Germany), a functional assay with a sensitivity of 0.06ng/ml, inter-assay precision of 3.0–6.6%, and lower detection limit of 0.02ng/ml18. Biochemical testing was performed in a blinded fashion.
Figure 1.
Study Population
Statistical Analysis
The cut-off values for heart rate, respiratory rate, and oxygen saturation were borrowed from the Pneumonia Severity Index19. The temperature cut-off of ≥38.3°C is based upon the accepted definition of fever in non-neutropenic patients. Bivariate odds ratios were calculated using logistic regression with in-hospital mortality as the outcome and baseline clinical characteristics and serum PCT level as predictors. A stepwise sequence of 3-variable models was generated using a best subsets multivariate logistic regression to identify independent predictors of in-hospital mortality. This best subsets multivariate logistic regression was also performed on 200 bootstrapped data sets and the best 3 models in each set were examined20. In addition, PCT was assessed as a prognostic tool by calculating sensitivity, specificity, and predictive values of PCT level >0.5ng/ml in relation to survival to hospital discharge. Receiver Operator Characteristic (ROC) curves were constructed for the best clinical predictors of death, as well as for the best 3-variable model. Risk of mortality in groups with specific clinical characteristics was assessed using the Chi squared test. Survival analysis was performed and Kaplan Meier curves were constructed, stratified by PCT level >0.5ng/ml. All statistics were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Serum PCT was measured in 241 HIV-infected subjects [Figure 1]. The mean age was 34 years and 48.5% of subjects were male [Table 1]. The median CD4 count was 47cells/μl and 122 subjects (50.8%) had a CD4 count <50cells/μl. Among 168 subjects with known HIV infection, 35 (20.8%) were receiving antiretroviral therapy (ART) prior to their hospitalization.
Table 1.
Bivariate analysis of in-hospital mortality in HIV-infected patients with pneumonia.
| Overall (N=241) N (%) | Dead (N=28) N (%) | Alive (N=213) N (%) | Bivariate OR (95%CI), p-value | |
|---|---|---|---|---|
| Mean age ±SD | 34.0±9.3 | 34.9±7.7 | 33.9±9.5 | 1.13 (0.75–1.71), 0.57 |
| Male sex | 117 (48.5) | 16 (57.1) | 101 (47.4) | 1.48 (0.67–3.28), 0.34 |
| CD4 cell count <50 cells/μl* | 122 (50.8) | 16 (57.1) | 106 (50.0) | 1.33 (0.60–2.95), 0.48 |
| Newly diagnosed HIV infection | 73 (30.3) | 11 (39.3) | 62 (29.1) | 1.58 (0.70–3.56), 0.27 |
| Antiretroviral therapy on admission | 35 (14.5) | 8 (28.6) | 27 (12.7) | 2.76 (1.10–6.87), 0.03 |
| Pneumocystis pneumonia prophylaxis* | 128 (53.3) | 14 (50.0) | 114 (53.8) | 0.86 (0.39–1.89), 0.71 |
| Antibiotic use prior to enrollment† | 125 (65.1) | 16 (66.7) | 109 (64.9) | 1.08 (0.44–2.68), 0.86 |
| Sputum production | 207 (85.9) | 25 (89.3) | 182 (85.5) | 1.42 (0.40–4.99), 0.58 |
| Hemoptysis | 47 (19.5) | 4 (14.3) | 43 (20.2) | 0.66 (0.22–2.00), 0.46 |
| Dyspnea at rest | 75 (31.1) | 16 (57.1) | 59 (27.7) | 3.48 (1.55–7.80), 0.002 |
| Chest pain | 150 (62.2) | 18 (64.3) | 132 (62.0) | 1.10 (0.49–2.51), 0.81 |
| Recent weight loss >5kg | 168 (69.7) | 21 (75.0) | 147 (69.0) | 1.35 (0.55–3.32), 0.52 |
| Temperature ≥38.3°C* | 58 (24.1) | 10 (35.7) | 48 (22.6) | 1.90 (0.82–4.38), 0.13 |
| Heart rate ≥125 beats/minute | 56 (23.2) | 5 (17.9) | 51 (23.9) | 0.69 (0.25–1.91), 0.48 |
| Respiratory rate ≥30breaths/minute‡ | 75 (31.6) | 15 (53.6) | 60 (28.7) | 2.87 (1.29–6.38), 0.01 |
| Oxygen saturation <90% | 38 (15.8) | 10 (35.7) | 28 (13.2) | 3.67 (1.54–8.75), 0.003 |
| Radiographic extent of disease§ Normal | 28 (15.0) | 3 (14.3) | 25 (15.1) | Referent |
| Minimal | 54 (28.9) | 6 (28.6) | 48 (28.9) | 1.04 (0.24–4.52), 0.96 |
| Moderately advanced | 60 (32.1) | 7 (33.3) | 53 (31.9) | 1.10 (0.26–4.62), 0.90 |
| Far advanced | 45 (24.1) | 5 (23.8) | 40 (24.1) | 1.04 (0.23–4.74), 0.96 |
| Procalcitonin level >0.5ng/ml | 162 (67.2) | 26 (92.9) | 136 (63.9) | 7.36 (1.70–31.9), 0.01 |
Total N = 240 (1 missing)
Total N = 192 (49 missing) and N dead = 24 (4 missing)
Total N = 237 (4 missing)
Definition of radiographic extent of disease found in the supplement. Total N = 187 (54 missing) and N dead = 21 (7 missing). The definition of radiographic extent of disease is found in the supplement.
Common vital sign abnormalities included fever (oral temperature ≥38.3°C, n=58, 24.2%), tachycardia (heart rate ≥125beats/minute, n=56, 23.2%), tachypnea (respiratory rate ≥30breaths/minute, n=75, 31.1%), and hypoxemia (oxygen saturation <90% on room air, n=38, 15.8%) [Table 1]. Admission chest radiograph was abnormal in the majority of patients (n=159, 85.0%).
A total of 203 diagnoses were assigned to 186 patients; a final diagnosis could not be made in 55 patients (22.8%) due to death or loss-to-follow-up prior to performance of diagnostic tests or 2-month assessment of treatment response. Tuberculosis was the most common diagnosis (n=146/203, 71.9%), followed by bacterial pneumonia (n=25, 12.3%). Other opportunistic pneumonias such as PCP (n=2, 1.0%), fungal pneumonia (n=11, 5.4%), and pulmonary Kaposi sarcoma (n=7, 3.4%) were uncommon. The inpatient mortality was 11.6% (n=28/241); 24% with an unknown diagnosis (n=13/55), 9% with tuberculosis (n=13/147) (p=0.01), and 14% (2/14) with other opportunistic pneumonias died (p=0.45).
Procalcitonin Level
Procalcitonin levels varied widely (0.02-515.70ng/ml). The median PCT level was 1.45ng/ml and 162 patients (67.2%) had PCT levels >0.5ng/ml. Of the 162 patients with PCT >0.5ng/ml, 26 (16.1%) died; of the 79 patients with PCT <0.5ng/ml, two (2.5%) died (aOR=7.69, 95% confidence interval (CI)=1.74–34.0, p=0.01). There was no correlation between CD4 count and PCT level. Patients with tuberculosis and unknown final diagnosis had higher PCT levels than patients with LRTI due to other pathogens (both p= 0.04). Among patients with tuberculosis, there was no significant difference between PCT levels in those with pulmonary tuberculosis compared to those with extrapulmonary disease (p=0.24).
Predictors of In-hospital Mortality
Dyspnea at rest, respiratory rate ≥30breaths/minute, oxygen saturation <90%, treatment with ART prior to hospitalization, and serum PCT >0.5ng/ml were associated with increased inhospital mortality in bivariate analysis [Table 1]. Fever, tachycardia, CD4 count <50cells/μl, and advanced extent of radiographic disease were not associated with mortality.
A 3-variable clinical prediction rule for in-hospital mortality consisting of respiratory rate ≥30breaths/minute, oxygen saturation <90%, and PCT >0.5ng/ml was identified via best subsets regression analysis. This rule was validated via logistic regression of 200 bootstrapped data sets, and these 3 predictors were consistently chosen as the best predictors of death. Of the three variables identified within the best subsets model only two, oxygen saturation <90% and PCT >0.5ng/ml, showed a statistically significant and independent association with inpatient mortality [Table 2]. Of the three variables included in the model, PCT >0.5ng/ml showed the strongest association with inpatient mortality with an adjusted odds ratio (aOR) of 7.69 (95% CI=1.74-34.0, p=0.01).
Table 2.
Best subsets multivariate analysis of in-hospital mortality of HIV-infected patients with pneumonia.
| aOR (95%CI), p value | |
|---|---|
| Respiratory rate ≥30breaths/minute | 2.07 (0.86–4.99), 0.11 |
| Oxygen saturation <90% | 3.07 (1.16–8.10), 0.02 |
| Procalcitonin >0.5ng/ml | 7.69 (1.74–34.0), 0.01 |
Total N=237 (4 missing)
Virtually all patients who died in the hospital had a PCT >0.5ng/ml (n=26/28, 93% sensitivity for in-hospital death) and similarly virtually all who survived to hospital discharge had a PCT ≤0.5ng/ml (n=211/213, 99% specificity). Thus, PCT ≤0.5ng/ml has a high negative predictive value (97%).
Procalcitonin as a Predictor of In-hospital Mortality
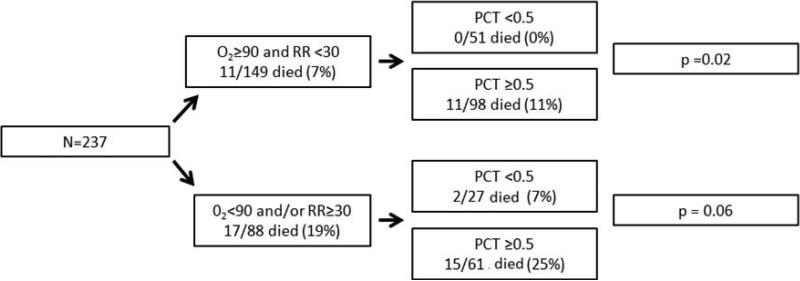
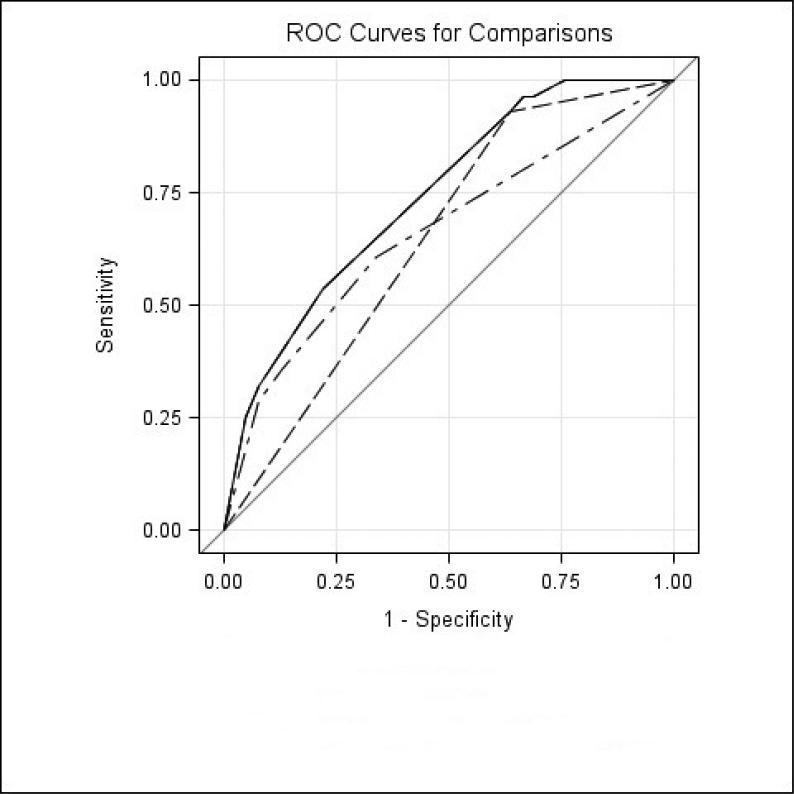
The presence of an elevated PCT, even in patients without tachypnea or hypoxemia, was associated with an increased predicted probability of mortality (1% mortality in those with PCT ≤0.5ng/ml vs. 10% mortality in those with PCT >0.5ng/ml p=0.004 [Table 3]). The predicted probability of mortality as determined by best subsets logistic analysis [Table 3] was confirmed with the actual mortality in our cohort [Figure 2]. Patients who were not tachypneic or hypoxemic with a low PCT had a mortality of 0%, while those with an elevated PCT had a mortality of 11% (p=0.02) [Figure 2]. Patients who were not tachypneic or hypoxemic with an elevated PCT had a similar mortality as those who were tachypneic and hypoxemic with a low PCT (11% vs. 7% p=0.73). Finally, patients who were tachypneic and/or hypoxemic with a low PCT had a mortality of 7%, while those with an elevated PCT had a mortality of 25% (p=0.06) [Figure 2]. A model that includes PCT, tachypnea, and hypoxemia has better prognostic accuracy than a model comprised of tachypnea and hypoxemia or a model with PCT alone, as illustrated via ROC curves (AUC=0.741, 0.659, and 0.645, accordingly p=0.05 and p=0.01, respectively) [Figure 3].
Table 3.
Predicted probability of in-hospital mortality stratified by procalcitonin level.
| Oxygen saturation <90% | Respiratory rate ≥30 breaths/minute | Procalcitonin ≤0.5ng/ml | Procalcitonin >0.5ng/ml | p-value | ||
|---|---|---|---|---|---|---|
| Mean PP* (95%CI) | n | Mean PP* (95%CI) | n | |||
| No | No | 1% (0.3–6) | 51 | 10% (6–17) | 98 | 0.004 |
| Yes | No | 4% (1–19) | 5 | 26% (11–49) | 8 | 0.32 |
| No | Yes | 3% (1–13) | 1 5 | 19% (10–33) | 36 | 0.25 |
| Yes | Yes | 9% (2–30) | 7 | 42% (24–62) | 17 | 0.17 |
Total N=237 (missing 4)
PP=predicted probability
Figure 2.
In-hospital mortality of HIV-infected patients stratified via the 3-variable prognostic model.
PCT: procalcitonin
O2<90: oxygen saturation <90%
RR≥30: respiratory rate ≥30 breaths/minute
Figure 3.
ROC curves for components of the three-variable prognostic model. (---) PCT > 0.5 (AUC = 0.645, 95% CI = 0.59-0.70, p = 0.01), (-‧-) O2<90 + RR ≥ 30 (AUC = 0.659, 95% CI = 0.55-0.77, p = 0.05), (—) PCT > 0.5 + O2<90 + RR ≥ 30 (AUC = 0.741, 95% CI = 0.65-0.83, Referent). PCT, procalcitonin; O2 <90: oxygen saturation <90%; RR ≥ 30: respiratory rate ≥30 breaths/minute.
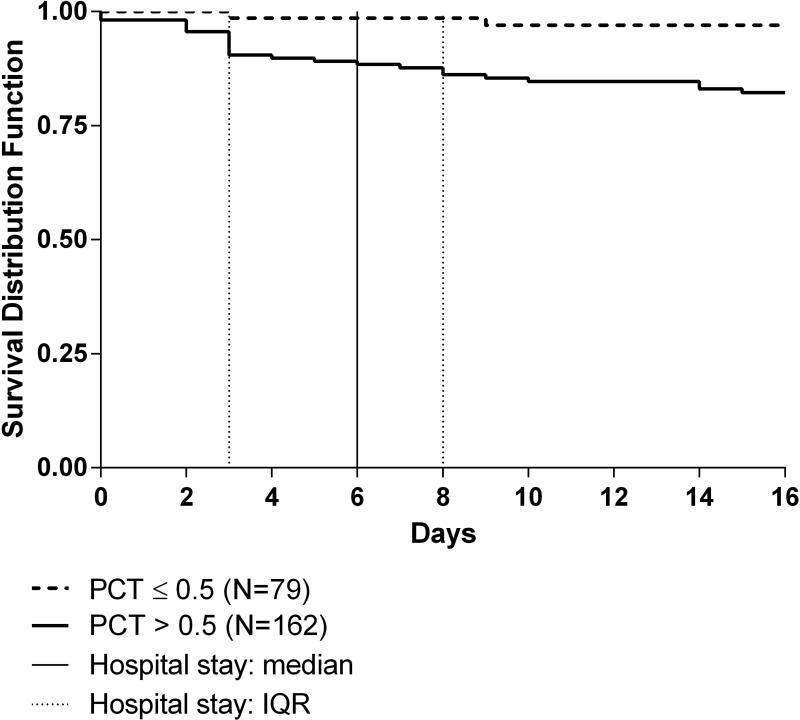
A Kaplan Meier curve illustrates survival to hospital discharge stratified by PCT level [Figure 4]. Subjects with PCT >0.5ng/ml had significantly lower survival rates than those with PCT ≤0.5ng/ml. A significant survival difference occurred at 3 days and persisted for the duration of the hospitalization; at 3 days, 90.4% of patients with PCT >0.5ng/ml survived as compared to 98.6% of patients with PCT ≤0.5ng/ml, p=0.02.
Figure 4.
Kaplan Meier curve of survival to hospital discharge stratified by procalcitonin level
PCT: procalcitonin
DISCUSSION
We examined the prognostic utility of serum PCT level in a Ugandan cohort of HIV-infected subjects admitted with cough ≥2 weeks in duration, in a setting with a high prevalence of tuberculosis, and identified several important findings. First, very few demographic and clinical characteristics were predictive of in-hospital death. Second, elevated PCT was the strongest independent predictor of in-hospital mortality. Third, the combination of tachypnea (respiratory rate ≥30breaths/minute), hypoxemia (oxygen saturation <90%), and PCT >0.5ng/ml was highly predictive of in-hospital mortality in our cohort, and may be a useful clinical prediction tool for clinicians.
In this study, only five of the 18 demographic and clinical variables evaluated showed an association with mortality. In the best subset multivariate analysis, only oxygen saturation <90% and PCT >0.5ng/ml remained statistically significant. Factors that often impact physicians’ triage decisions such as CD4 cell count or extent of radiographic disease were not associated with increased mortality in our study. The paucity of demographic and clinical characteristics associated with increased mortality further emphasizes the potential utility of a sensitive tool such as PCT to aid in decisions at the time of admission.
Serum PCT was a strong and independent predictor of in-hospital mortality in this cohort. Although earlier studies have shown an association between elevated PCT and mortality, these studies only included HIV-uninfected patients with typical bacterial pneumonia3,5,21. The findings of our study contrast with those of a study by Schleicher and colleagues22 who found that patients with bacterial pneumonia had higher PCT levels than those with tuberculosis; furthermore PCT level was not predictive of mortality in their cohort. The difference in PCT level may be due to under-representation of bacterial pneumonia in our cohort; the difference in prognostic utility of PCT may be due to the low mortality in Schleicher's cohort (4.5% vs. 11.6%).
In a busy, resource-limited setting, prognostic models must be practical and easy to use. The model presented, composed of three variables, respiratory rate, oxygen saturation, and PCT, has the potential to quickly identify the sickest patients thereby aiding in appropriate patient triage and treatment. Measurement of PCT is especially important because it improves the prognostic accuracy of the model [Figure 3] and has the strongest association with in-hospital mortality [Table 2].
The assay used to detect PCT has an assay time of less than 20 minutes making it ideal for busy settings. Elevated PCT can be used to identify severely ill patients and may alter their management by guiding triage, speed of diagnostic work up, and breadth of antibiotic therapy. The cost of PCT testing limits its use in resource-constrained settings; however, our study provides initial evidence to support its prognostic utility. Studies to assess the cost-effectiveness of adopting PCT testing as a clinical tool are needed.
At least two hypotheses have been proposed to explain the ubiquitous synthesis of PCT during infection. The first postulates that infection activates a transcription factor that upregulates transcription of PCT. The second proposes that infection leads to suppression of an inhibitor and subsequent transcription of PCT. It is unclear whether these modifications occur due to the action of bacterial components, pathogen induced cytokines such as such as TNFa, IL-1b, IL-6, and IL-8, or a combination of both23.
In addition to being a marker of infection, PCT may also be an immune-modulatory molecule24,25. Several studies have shown that elevated PCT is associated with higher death rates in animal models of sepsis26-30. For example, Nylen reported that administration of anti-PCT antibodies decreased mortality in septic hamsters and that the administration of exogenous PCT significantly increased the rate and magnitude of the mortality27. If these findings are generalizable to humans, PCT may be a future therapeutic target in patients at high risk of death.
This study is limited by its inclusion criteria and small cohort size. The inclusion criterion of cough ≥2 weeks in duration selects for patients with indolent LRTIs such as tuberculosis, fungal infections, and PCP; thus, bacterial pneumonia, which produces symptoms more acutely, is under-represented in this cohort. However, given the known association of elevated PCT and increased mortality in persons with bacterial pneumonia, the significance of elevated PCT as an independent predictor of in-hospital mortality in our cohort composed predominantly of persons with tuberculosis (71.9%) is notable. Also, our study includes only hospitalized patients and therefore may not be generalizable to the outpatient setting where mortality is generally lower.
In conclusion, there are few clinical characteristics independently associated with mortality among HIV-infected Ugandans with LRTI. In our study, PCT level >0.5ng/ml is a strong and independent predictor of in-hospital mortality. Serum PCT >0.5ng/ml, respiratory rate ≥30 breaths/minute, and oxygen saturation <90% can be combined into a prognostic model to calculate the predicted probability of death and to identify patients at high risk of dying in the hospital. Future prospective studies must be performed to validate these findings in larger cohorts and ultimately to determine if implementation of this model leads to improved resource allocation and patient outcomes.
Summary at a Glance.
Lower respiratory tract infections carry a high mortality in HIV-infected Ugandans. We sought to determine whether serum procalcitonin can be used to predict in-hospital mortality. Serum procalcitonin level >0.5ng/ml was highly predictive of mortality and could be incorporated into a simple prognostic model along with respiratory rate and oxygen saturation.
Acknowledgments
The authors thank Drs. Musinguzi, Nankya, and Kyeyune, and Patrick Byanyima and John Kiidha who enrolled the patients. We thank the Mulago Hospital Administration for facilitating this research; Joy, Hannah, Justine, and Olivia for assisting with bronchoscopy; Elisha Hatanga, Peter Awongo, John Matovu, and the NTRL staff for performing smear microscopy and mycobacterial culture. We thank Steven Carter at University of California in San Diego for performing the PCT analysis. Source of funding: NIH K24 HL087713 (LH), NIH R01 HL090335 (LH).
Abbreviations
- PCT
Procalcitonin
- HIV
Human immunodeficiency virus
- PCP
Pneumocystis jirovecii pneumonia
- IHOP study
International HIV-associated Opportunistic Pneumonias Study
- AFB
Acid-fast bacilli
- ROC
Receiver operator characteristic
REFERENCES
- 1.Nyamande K, Lalloo UG. Serum procalcitonin distinguishes CAP due to bacteria, Mycobacterium tuberculosis and PJP. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2006;10:510–5. [PubMed] [Google Scholar]
- 2.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang DT, Weissfeld LA, Kellum JA, Yealy DM, Kong L, Martino M, Angus DC. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–58. e2. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260:93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 5.Kruger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H, Suttorp N, Welte T. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31:349–55. doi: 10.1183/09031936.00054507. [DOI] [PubMed] [Google Scholar]
- 6.Cattamanchi A, Davis JL, Worodria W, Yoo S, Matovu J, Kiidha J, Nankya F, Kyeyune R, Andama A, Joloba M, Osmond D, Hopewell P, Huang L. Poor performance of universal sample processing method for diagnosis of pulmonary tuberculosis by smear microscopy and culture in Uganda. J Clin Microbiol. 2008;46:3325–9. doi: 10.1128/JCM.01175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, Matovu J, Hopewell PC, Huang L. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis. 2009;9:53. doi: 10.1186/1471-2334-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JL, Worodria W, Kisembo H, Metcalfe JZ, Cattamanchi A, Kawooya M, Kyeyune R, den Boon S, Powell K, Okello R, Yoo S, Huang L. Clinical and radiographic factors do not accurately diagnose smear-negative tuberculosis in HIV-infected inpatients in Uganda: a cross-sectional study. PLoS One. 2010;5:e9859. doi: 10.1371/journal.pone.0009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattamanchi A, Ssewenyana I, Davis JL, Huang L, Worodria W, den Boon S, Yoo S, Andama A, Hopewell PC, Cao H. Role of interferon-gamma release assays in the diagnosis of pulmonary tuberculosis in patients with advanced HIV infection. BMC Infect Dis. 2010;10:75. doi: 10.1186/1471-2334-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deok-jong Yoo S, Worodria W, Davis JL, Cattamanchi A, den Boon S, Kyeyune R, Kisembo H, Huang L. The prevalence and clinical course of HIV-associated pulmonary cryptococcosis in Uganda. J Acquir Immune Defic Syndr. 2010;54:269–74. doi: 10.1097/QAI.0b013e3181ce6b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worodria W, Davis JL, Cattamanchi A, Andama A, den Boon S, Yoo SD, Hopewell PC, Huang L. Bronchoscopy is useful for diagnosing smear-negative tuberculosis in HIV-infected patients. Eur Respir J. 2010;36:446–8. doi: 10.1183/09031936.00010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyeyune R, den Boon S, Cattamanchi A, Davis JL, Worodria W, Yoo SD, Huang L. Causes of early mortality in HIV-infected TB suspects in an East African referral hospital. J Acquir Immune Defic Syndr. 2010;55:446–50. doi: 10.1097/qai.0b013e3181eb611a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JL, Huang L, Worodria W, Masur H, Cattamanchi A, Huber C, Miller C, Conville PS, Murray P, Kovacs JA. Nucleic acid amplification tests for diagnosis of smear-negative TB in a high HIV-prevalence setting: a prospective cohort study. PLoS One. 2011;6:e16321. doi: 10.1371/journal.pone.0016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo SD, Cattamanchi A, Den Boon S, Worodria W, Kisembo H, Huang L, Davis JL. Clinical significance of normal chest radiographs among HIV-seropositive patients with suspected tuberculosis in Uganda. Respirology. 2011;16:836–41. doi: 10.1111/j.1440-1843.2011.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SM, Meshnick SR, Worodria W, Andama A, Davis JL, Cattamanchi A, den Boon S, Yoo SD, Goodman CD, Huang L. Low prevalence of Pneumocystis jirovecii lung colonization in Ugandan HIV-infected patients hospitalized with non-Pneumocystis pneumonia. Diagn Microbiol Infect Dis. 2012;72:139–43. doi: 10.1016/j.diagmicrobio.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattamanchi A, Davis JL, Worodria W, den Boon S, Yoo S, Matovu J, Kiidha J, Nankya F, Kyeyune R, Byanyima P, Andama A, Joloba M, Osmond DH, Hopewell PC, Huang L. Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2009;13:1130–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B, Pro HSG. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;302:1059–66. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JS, Chan MC, Hsu JY, Kuo BI, Wu CL. Procalcitonin is a valuable prognostic marker in ARDS caused by community-acquired pneumonia. Respirology. 2008;13:505–9. doi: 10.1111/j.1440-1843.2008.01293.x. [DOI] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. The New England journal of medicine. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Yeo D MH, Liu TP. American Statistical Association 1999 Proceedings of the Section on Survey Research Methods American Statistical Association Proceedings of the Section on Survey Research Methods. 1999 [Google Scholar]
- 21.Masia M, Gutierrez F, Shum C, Padilla S, Navarro JC, Flores E, Hernandez I. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128:2223–9. doi: 10.1378/chest.128.4.2223. [DOI] [PubMed] [Google Scholar]
- 22.Schleicher GK, Herbert V, Brink A, Martin S, Maraj R, Galpin JS, Feldman C. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. The European respiratory journal. 2005;25:688–92. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 23.Matwiyoff GN, Prahl JD, Miller RJ, Carmichael JJ, Amundson DE, Seda G, Daheshia M. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res. 2012 doi: 10.1007/s00011-012-0439-5. [DOI] [PubMed] [Google Scholar]
- 24.Dahaba AA, Hagara B, Fall A, Rehak PH, List WF, Metzler H. Procalcitonin for early prediction of survival outcome in postoperative critically ill patients with severe sepsis. Br J Anaesth. 2006;97:503–8. doi: 10.1093/bja/ael181. [DOI] [PubMed] [Google Scholar]
- 25.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, Vadas L, Pugin J. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. American journal of respiratory and critical care medicine. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 26.Steinwald PM, Whang KT, Becker KL, Snider RH, Nylen ES, White JC. Elevated calcitonin precursor levels are related to mortality in an animal model of sepsis. Crit Care. 1999;3:11–6. doi: 10.1186/cc300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nylen ES, Whang KT, Snider RH, Jr., Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001–6. doi: 10.1097/00003246-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Wagner KE, Martinez JM, Vath SD, Snider RH, Nylen ES, Becker KL, Muller B, White JC. Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med. 2002;30:2313–21. doi: 10.1097/00003246-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Tavares E, Minano FJ. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemia: neuroendocrine and systemic studies. Clin Sci (Lond) 2010;119:519–34. doi: 10.1042/CS20100007. [DOI] [PubMed] [Google Scholar]
- 30.Martinez JM, Wagner KE, Snider RH, Nylen ES, Muller B, Sarani B, Becker KL, White JC. Late immunoneutralization of procalcitonin arrests the progression of lethal porcine sepsis. Surg Infect (Larchmt) 2001;2:193–202. doi: 10.1089/109629601317202678. discussion -3. [DOI] [PubMed] [Google Scholar]