Background: Chemotaxis is a fundamental process in many physiological and pathological events.
Results: An LPA gradient induces a spatiotemporally restricted decrease in the mobility of LPA2 indicative of its cytoplasmic anchorage to NHERF2, the cytoskeleton and PLCβ, which causes a gradient of localized Ca2+ puffs.
Conclusion: Asymmetrical macromolecular complex formation by LPA2 mediates gradient sensing.
Significance: Our finding provides a new mechanistic basis to help understand chemotactic gradient sensing.
Keywords: Calcium Intracellular Release, Cell Migration, Cell Surface Receptor, Fibroblast, G Protein-coupled Receptor (GPCR), PDZ Domain
Abstract
Chemotactic migration of fibroblasts toward growth factors relies on their capacity to sense minute extracellular gradients and respond to spatially confined receptor-mediated signals. Currently, mechanisms underlying the gradient sensing of fibroblasts remain poorly understood. Using single-particle tracking methodology, we determined that a lysophosphatidic acid (LPA) gradient induces a spatiotemporally restricted decrease in the mobility of LPA receptor 2 (LPA2) on chemotactic fibroblasts. The onset of decreased LPA2 mobility correlates to the spatial recruitment and coupling to LPA2-interacting proteins that anchor the complex to the cytoskeleton. These localized PDZ motif-mediated macromolecular complexes of LPA2 trigger a Ca2+ puff gradient that governs gradient sensing and directional migration in response to LPA. Disruption of the PDZ motif-mediated assembly of the macromolecular complex of LPA2 disorganizes the gradient of Ca2+ puffs, disrupts gradient sensing, and reduces the directional migration of fibroblasts toward LPA. Our findings illustrate that the asymmetric macromolecular complex formation of chemoattractant receptors mediates gradient sensing and provides a new mechanistic basis for models to describe gradient sensing of fibroblasts.
Introduction
Chemotaxis is a fundamental process in many physiological and pathological events, including embryonic development, angiogenesis, wound healing, inflammation, and metastasis. The capacity of cells to sense and respond to a chemoattractant gradient is central to chemotaxis. This response is achieved by integrating chemoattractant signals originating from a family of G-protein-coupled receptors (GPCRs),3 leading to dynamic organizational changes in signal transduction networks (1). In order to elucidate the mechanism of chemotaxis, two key questions must be answered. (i) What molecular events are initiated when a cell encounters a chemotactic gradient with as little as a 2% concentration difference across the cell perimeter? (ii) What is the dynamic process of signal amplification? The cells respond to external gradient signals by activation of chemoattractant receptors, which in turn initiates the intracellular signaling network, leading to directional motility (2–5). The crucial step in this response is how the low level and spatially distinct activation of GPCRs can provide the cell with an unambiguous cue for gradient sensing. Accumulating evidence suggests that the spatial organization of signaling molecules regulates chemotaxis (6–8). Previous studies of chemotaxis have shown that signaling events are restricted to the leading edge of the cell although chemoattractant receptors are uniformly distributed on the entire cell surface during migration (9). Recent studies utilizing fluorescence resonance energy transfer (FRET) methods have shown that the leading edge of Dictyostelium discoideum exhibits elevated cAMP receptor-mediated activation of the G proteins (10, 11). Single-particle tracking (SPT) imaging revealed that the dynamic properties of the cAMP GPCRs were involved in such gradient sensing (12, 13). These observations suggest that gradient sensing was initiated from a polarization in receptor activation in this organism. Until now, due to unresolved technical difficulties, the chemical gradient-induced activation pattern of chemoattractant receptors in fibroblasts has not yet been directly reported.
The sensitivity of gradient sensing is governed by signal amplification and adaption mechanisms. Dictyostelium and neutrophils perform strong internal signal amplification by means of feedback loops composed of phosphoinositide 3-kinase (PI3K) and its catalytic product, phosphatidylinositol 3,4,5-trisphosphate. It has been established that an early indicator of gradient sensing is the localized accumulation of phosphatidylinositol 3,4,5-trisphosphate that is accomplished through the regulation of PI3K and the PTEN (phosphatase and tensin homolog) activity at the leading edge (14). However, defective phosphatidylinositol 3,4,5-trisphosphate signaling was found only to affect the motility and not the chemotaxis of neutrophils (15) and Dictyostelium (16). Several types of cancer cells retain a highly invasive capacity despite loss of PTEN function, implying that chemoattractant sensing can be achieved independently of phosphatidylinositol 3,4,5-trisphosphate levels (17). Recently, a newly discovered pathway involving phospholipase A2 has been shown to mediate chemotaxis in cells lacking PI3K (18). These findings support the possibility that either there are multiple, redundant gradient-sensing mechanisms, or there is a common upstream compass coupled to redundant pathways of translating output into directional cell migration.
Calcium is known to play important roles in regulating cell migration (19, 20). Wei et al. (21) provided evidence that transient Ca2+ microdomains, known as Ca2+ flickers, are enriched near the leading edge of a motile fibroblast and that the asymmetry of Ca2+ flickers steers cells to turn. Highly localized Ca2+ elevations have been observed to trigger both attractive and repulsive growth cone turning (22). A study in zebrafish primordial germ cells found that transient Ca2+ elevations can locally induce protrusion formation (23). These studies support a model in which Ca2+ is a critical component in steering cells along a chemical gradient. Recent studies showed that depletion of extracellular Ca2+ disrupts the positive feedback loop that can be measured by either PI3K activity, actin polymerization, or protein kinase C (PKC) localization at the leading edge of polarized cells (24). Taken together, these observations suggest that Ca2+ gradients are indispensable to the positive feedback for gradient sensing of chemotactic cells.
The chemotactic migration of fibroblasts can be triggered by chemoattractants, including platelet-derived growth factor (PDGF) and LPA. LPA is one of the major motility-stimulating factors present in blood and other biological fluids. Although there has been great progress in understanding PDGF-induced chemotaxis in fibroblasts, the mechanisms of LPA-induced chemotactic migration remain poorly understood. LPA-initiated cellular responses are mediated through at least six GPCRs: LPA1/Edg2, LPA2/Edg4, LPA3/Edg7, LPA4/GPR23, LPA5/GPR92, and LPA6/P2Y5 (25). Recent studies have established that LPA2 mediates the migration of several types of cancer cells (26–28). In this paper, we report our findings that an asymmetric activation of LPA2 on the plasma membrane of fibroblasts elicits an asymmetric formation of macromolecular complexes of LPA2, NHERF2 (Na+/H+ exchange regulatory factor 2), and phospholipase C-β3 (PLC-β3) that, through an intracellular signal amplification mechanism, trigger the formation of spatially restricted, short lived, and high frequency Ca2+ microdomains (Ca2+ puffs), which govern the LPA gradient sensing and directional motility of fibroblasts.
EXPERIMENTAL PROCEDURES
Materials
Heat-inactivated fetal bovine serum (FBS) and CO2-independent medium were purchased from Invitrogen. Dulbecco's modified Eagle's medium (DMEM), 1-oleoyl-sn-glycero-3-phosphate (LPA, 18:1), 1-oleoyl-rac-glycero (oleoyl glycerol), fibronectin, and puromycin were purchased from Sigma-Aldrich. PLC-β inhibitor U73122 and its inactive analog U73343 and Polybrene were purchased from Santa Cruz Biotechnology, Inc. Anti-LPA2 antibody (rabbit 2143) and anti-NHERF2 antibody (rabbit 2346) were generated by Genemed Synthesis, Inc. (San Antonio, TX). Anti-PLC-β3 antibody was purchased from Abcam (Cambridge, MA).
Cell Culture, Transfection, and Lentivirus Infection
NIH 3T3 cells were purchased from ATCC (Manassas, VA). NIH 3T3 cells were transfected with an N-terminal FLAG-tagged WT-LPA2 construct (3T3-LPA2 cells) or the L351A PDZ motif mutant LPA2 (3T3-L351A cells). Double knock-out (DKO) mouse embryonic fibroblasts (MEFs), derived from Lpar1−/− and Lpar2−/− DKO mice and therefore deficient in LPA1 and LPA2, were reconstituted with either WT-LPA2 (DKO-LPA2 MEFs) or L351A-LPA2 (DKO-L351A MEFs) as described previously (29). Cells were cultured in DMEM supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FBS. The transfection was conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The FLAG-WT-LPA2 and FLAG-L351A-LPA2 lentiviral vectors were generated by the Viral Vector Core (University of Tennessee Health Science Center). Half-confluent cells were cultured in medium with 5 μg/ml Polybrene to which lentiviral vector was added and incubated overnight. After infection, cells were selected using 2 μg/ml puromycin for 5 days and used for experiments.
siRNA targeting NHERF2 was purchased from Santa Cruz Biotechnology. NIH 3T3 cells or DKO-LPA2 MEFs at 50% confluence were transfected with 200 pm NHERF2 siRNA using Lipofectamine 2000. As a control, a scrambled 21-nucleotide RNA duplex was used. Twenty-four hours after transfection, cells were used for experiments.
Chemotactic Stimulation of Cells Using an LPA Gradient Released from a Micropipette
Cells were seeded on fibronectin-coated glass-bottomed dishes, starved for 6 h, and then incubated in CO2-independent medium. Cells were stimulated by an LPA gradient generated by a micropipette at 37 °C. Micropipettes were prepared by pulling borosilicate capillaries to 1.0-mm outer diameter and 0.58-mm inner diameter on a dual stage glass micropipette puller (Narishige Co. Ltd., London). The micropipettes were filled with 200 nm LPA solution. The movement of cells was recorded using a Hamamatsu EM-CCD camera (C9100; Hamamatsu, Bridgewater, NJ) equipped on a phase-contrast microscope. The trajectories were analyzed using Slidebook version 5.0 software. The chemotactic index (CI) was calculated by taking the cosine of the angle between a line directly up the gradient and one that connects a cell's starting point to its end point. A value of 1 is directly up the gradient, whereas a CI of 0 indicates motion perpendicular to the direction of the gradient.
High Content Microscopy
Cells were grown for 24 h in fibronectin-coated 96-well Essen BioScience (Ann Arbor, MI) ImageLock microplates in a CO2 incubator. Wounds were made precisely using the 96-pin Wound-Maker provided with the IncuCyteTM system (Essen Bioscience) (30). After washing thoroughly with PBS to remove detached cells, the remaining cells were placed in the instrument in serum-free medium with or without LPA (1 μm), PLCβ inhibitor U73122 (2 μm), or its inactive analog U73343 (2 μm). The wound images were automatically acquired at 30-min intervals. The kinetics of the relative wound density (RWD) was analyzed using IncuCyteTM software.
Wound Healing Assay
Cells were grown on fibronectin-coated 35-mm diameter dishes and starved for 6 h prior to wounding. The wounds were made by scraping with a microtip. After being washed three times, cells were incubated in normal medium with or without 1 μm LPA for 6 h. A total of three different DKO, DKO-LPA2, and DKO-L351A MEF cultures were analyzed, and experiments were repeated four times. Cells were imaged using an Olympus (Center Valley, PA) IX51 inverted microscope.
Coimmunoprecipitation and Immunoblotting
For coimmunoprecipitation of LPA2, NHERF2, and PLC-β3, 3T3-LPA2 and DKO-LPA2 cells were starved in serum-free DMEM for 6 h and then stimulated with different concentrations of LPA for 5 min. After treatment, cells were harvested and resuspended in lysis buffer. The lysate was spun at 12,000 rpm for 10 min at 4 °C. The clear supernatant was subjected to immunoprecipitation using α-FLAG beads (Sigma-Aldrich) overnight at 4 °C. The immunoprecipitated beads were washed three times with lysis buffer, and proteins were eluted from the beads using 5× Laemmli sample buffer. The proteins were separated on 4–15% SDS-polyacrylamide gradient gels and immunoblotted for LPA2, NHERF2, and PLC-β3 using the respective antibodies.
SPT and Data Analysis
3T3-LPA2 and 3T3-L351A cells were grown on 35-mm fibronectin-coated glass-bottomed dishes (MatTeK, Ashland, MA). Cells were washed twice with serum-free medium and then incubated with biotin α-FLAG antibody (1 μg/ml; Sigma-Aldrich) for 30 min after an initial block (DMEM containing 4% BSA, 10 min) and washed five times, followed by a second incubation with streptavidin-conjugated quantum dots (QDs; Qdot-655, 0.1 nm; Invitrogen) for 5 min. Cells were washed with DMEM without phenol red for 6–10 min and immediately mounted on an Olympus inverted microscope (IX51) equipped with a ×100 oil immersion objective (numerical aperture = 1.4) and xenon (300-watt lamp) light source. Images were captured with a Hamamatsu EM-CCD camera at 10 frames/s for ∼2 min. QD excitation and emission was controlled by a Qdot 655-A BrightLine® high brightness and contrast single-band filter set (Semrock, Rochester, NY). Acquisition of images and trajectory analysis were performed using SlideBook version 5.0 software. Single QDs were identified by characteristic blinking, and periods where the fluorescence signal disappeared due to the blinking of single QDs were omitted from the analysis. The trajectory was reconstructed by joining trajectory fragments immediately before and after the dark period of the blink. Trajectories were considered for 10 or more consecutive frames. The mean square displacement (MSD) of a trajectory was calculated using SlideBook version 5.0 software. The diffusion coefficients (D) were calculated with the equation, MSD = 4Dt using an analysis software and fitting the first 4–50 time points of the MSD curves versus the lag time.
Quantitation of LPA2 Internalization
Cells were plated on glass-bottomed dishes for internalization assays. The cells were serum-starved for 6 h and then incubated with biotin-α-FLAG primary antibody for 30 min after initial block with 4% BSA, followed by a second incubation with streptavidin-conjugated QDs for 5 min. Cells were then rinsed and processed for time lapse microscopy to measure LPA2 internalization.
Ca2+ Imaging with Non-fluorescent Acetoxymethyl Ester (Fluo-4 AM)
Cultured DKO, DKO-LPA2, or DKO-L351 MEFs were loaded with 5 μm Fluo-4 AM in the presence of 1.5 μm pluronic acid F-127 and incubated for 30–45 min at 37 °C in the dark in KREB buffer, washed, and equilibrated for de-esterification for 15 min. Fluorescence images of Fluo-4 were acquired with 1 frame/s using the ×10 objective at 37 °C. The fluorescence intensity of Fluo-4 for each region of interest was divided by the averaged resting fluorescence intensity of 10 frames before stimulation of the same region, after which the background fluorescence was subtracted. The resultant relative fluorescence intensity (F/F0) was used as an indicator of intracellular Ca2+ levels ([Ca2+]i). Image processing and analysis were performed using NIH Image software.
Total internal reflection fluorescence (TIRF) microscopy imaging of changes in [Ca2+]i was performed in an in-house built TIRF microscopy system based on an Olympus microscope with a ×100 oil objective. Fluo-4 AM was excited at 488 nm, and images of emitted fluorescence were captured at a resolution of 128 × 128 pixels (1 pixel = 0.33 μm) at 60 frames/s with an EM-CCD camera. After subtraction of background, fluorescence intensity within a defined circular region of interest was expressed as F/F0, where F is the fluorescence intensity at time t, and F0 is the average fluorescence intensity from the same region of interest collected from the first ∼10 frames.
Statistical Analyses
The Kolmogorov-Smirnov test (KS-test) was used to evaluate the differences between the different populations of the determined D. Statistical analyses also were performed using a two-tailed, unpaired Student's t test. Results are presented as mean ± S.E., and differences were considered statistically significant at p < 0.05.
RESULTS
LPA Gradient Induces a Spatially Restricted Reduction in the Mobility of LPA2 in the Plasma Membrane
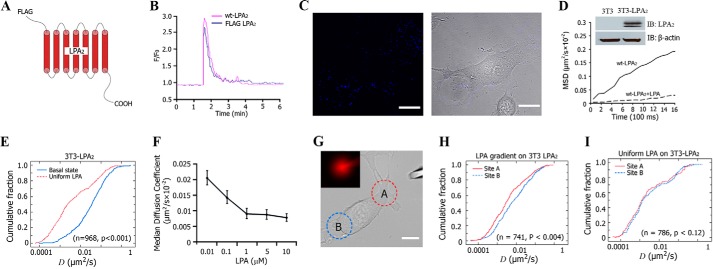
The dynamics of GPCRs in the plasma membrane and its relationship with receptor function are important to the actively developing field of research. In order to analyze the mobility of receptors in detail, it is necessary to monitor an individual receptor. We engineered an LPA2 construct containing a FLAG tag at the N terminus (Fig. 1A) and tested its function using an intracellular calcium mobilization assay in rat hepatoma cells (RH777, an LPA-receptor null cell line). We found that cells transiently expressing FLAG-tagged LPA2 exhibited the same function as non-tagged WT-LPA2-expressing cells (Fig. 1B). We then used the SPT method to monitor the dynamic properties of LPA2 in living fibroblasts. The membrane localization of LPA2 labeled with streptavidin-conjugated QDs in 3T3-LPA2 cells was observed (Fig. 1C). The expression of FLAG-tagged LPA2 was confirmed by Western blotting (Fig. 1D, inset). Time lapse imaging showed the diffusion of LPA2 confined at the plasma membrane during the 2-min observation period with a median diffusion coefficient (Dmed) of ∼0.0301 ± 0.0610 μm2/s, which is typical for many membrane proteins, including GPCRs. Exposure to 1 μm LPA within seconds reduced the Dmed of LPA2 13-fold to 0.0023 ± 0.0013 μm2/s (Fig. 1D and supplemental Video 1). The KS-test revealed a significant difference in the lateral mobility of LPA2 between the basal and LPA-activated states (Fig. 1E). We next examined whether the LPA effect was dose-dependent and found that LPA decreased the lateral mobility of LPA2 in a dose-dependent manner within the range of 10 nm to 1 μm (Fig. 1F). At ∼100 nm of LPA, the Dmed of LPA2 reached half of that without LPA stimulation. At 1 μm LPA, the stimulation rose to a plateau; further increase in LPA concentration did not elicit a significant decrease in LPA2 mobility.
FIGURE 1.
Lateral mobility of LPA2 on the plasma membrane of cells. A, schematic representation of FLAG-tagged LPA2. B, FLAG-LPA2 functioned the same as WT-LPA2 in LPA-induced intracellular Ca2+ mobilization. C, left, fluorescence image of 3T3-LPA2-stable cells labeled with QDs. Right, phase-contrast image. Scale bar, 10 μm. D, MSD versus time plots for FLAG-LPA2 in 3T3-LPA2 cells at basal (without LPA) or LPA (1 μm)-activated state. Plots are averaged from 16–36 individual trajectories. The inset blots show the expression of LPA2 in 3T3-LPA2 cells. E, comparison of cumulative fraction plots from a KS-test of the difference in the diffusion coefficient (D) of LPA2 at the basal state or after exposure to uniform LPA. The respective number of trajectories (n) and p value are listed. F, lateral mobility of LPA2 in response to different concentration of LPA treatment. G, a representative image of a cell exposed to an LPA gradient generated by placing a micropipette releasing LPA (200 nm) in close proximity to a cell. Sites A and B (A and B) are designated relative to the site of micropipette. The inset shows the gradient generated by including fluorescent dextran in the pipette. Scale bar, 10 μm. H, comparison of the cumulative fraction plot from the KS-test of the difference in the diffusion coefficient of LPA2 in site A and site B after exposure to an LPA gradient. The respective number of trajectories (n) and p value are listed. I, comparison of cumulative fraction plot from the KS-test of the difference in the diffusion coefficient of LPA2 in site A and site B after exposure to uniformly distributed LPA. The respective number of trajectories (n) and p value are listed. IB, immunoblot; Error bars, S.E.
We next examined the effect of an LPA gradient on the lateral mobility of LPA2 by placing a micropipette releasing LPA in close proximity to a cell. To verify the establishment of an LPA gradient, we included fluorescent dextran in the micropipette with LPA and used the fluorescence intensity to calculate the gradient (Fig. 1G). Under our experimental conditions, we created >10% gradient steepness across the cell. After exposure to this LPA gradient, SPT data indicated that the lateral mobility of LPA2 in the portion facing the LPA source (site “A”) was more restricted, indicated by a significantly lower D, than that of LPA2 in the portion farthest away from the LPA source (site “B”) (Fig. 1H). In contrast, when the cells were exposed to LPA uniformly, the lateral mobility of LPA2 in site A versus site B was indistinguishable (Fig. 1I). This suggests that the LPA gradient induced a spatially polarized reduction in the mobility of LPA2, which reflects the spatial differences in ligand exposure of LPA2 at different sites of the plasma membrane.
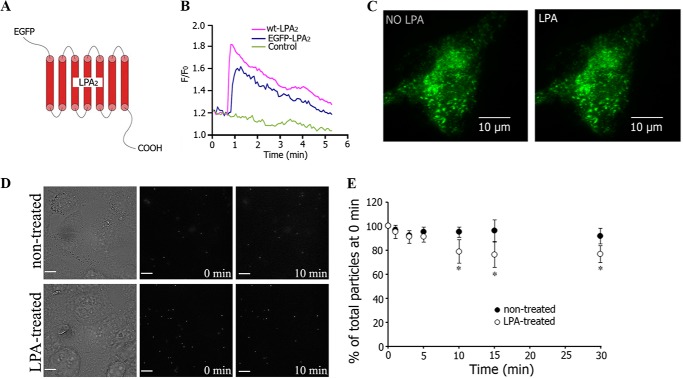
Chemoattractant receptors are uniformly distributed along the cell surface during cheomotaxis. However, some proteins can relocalize during cell migration, such as PTEN dissociating along the leading edge and accumulating at the cell rear during chemotaxis (14, 31). We studied the cellular distribution of LPA2 during chemotaxis by transiently overexpressing an EGFP-tagged LPA2 (Fig. 2A). A calcium mobilization assay in RH777 cells showed that EGFP-LPA2 functioned similarly to WT-LPA2 (Fig. 2B). TIRF microscopy imaging was used to examine the distribution of EGFP-LPA2 with or without an LPA gradient stimulation. We found that EGFP-LPA2 was distributed evenly throughout the plasma membrane regardless of whether an LPA gradient was present (5-min treatment) or not (Fig. 2C), suggesting that the LPA gradient-induced polarized decrease in LPA2 mobility was not due to redistribution of LPA2.
FIGURE 2.
LPA did not induce LPA2 internalization within 5 min of exposure. A, schematic representation of EGFP-tagged LPA2. B, EGFP-LPA2 functioned similar as WT-LPA2 in LPA-induced intracellular Ca2+ mobilization. C, TIRF imaging showing the distribution of EGFP-LPA2 in 3T3-LPA2 cells before and after treatment with an LPA gradient for 5 min. Scale bar, 10 μm. D, SPT images of FLAG-LPA2 expressed in HEK293 cells in the absence or presence of LPA. Scale bar, 10 μm. E, quantitative analysis of the percentage of LPA2 internalization for 30 min with or without LPA treatment (mean ± S.E. (error bars), n = 4). *, significant differences compared with 0 min in each group (p < 0.05).
Internalization of several types of GPCRs occurs in response to agonist activation and can cause a redistribution of receptors away from the plasma membrane. LPA1 has been reported to undergo ligand-induced internalization (32). In contrast, the ligand-induced internalization of LPA2 has not been reported. We investigated whether internalization of LPA2 would occur upon exposure to LPA stimulus by using time lapse microscopy. HEK293 cells overexpressing FLAG-LPA2 were labeled with biotin-α-FLAG primary antibody and then streptavidin-conjugated QDs. The cells were subjected to fluorescence time lapse microscopy with (5 μm LPA for maximal stimulation) or without LPA (Fig. 2D). The maximal stimulation of LPA2 induced no detectable LPA2 internalization within the first 5 min of exposure (Fig. 2E), although changes in receptor dynamics and receptor-activated Ca2+ responses were robustly triggered in the cells. Internalization of LPA2 became detectable only after 10 min of stimulation and affecting only 25% of the receptors expressed uniformly on the cell surface (Fig. 2E). These observations emphasize the discordant time course of receptor dynamics that was apparent as early as 2 min of LPA application, and LPA2 internalization was absent during the first 10 min of stimulant exposure. Taken together, our data suggest that the spatial regulation of the membrane dynamics of ligand-activated LPA2 preceded the partial internalization of this receptor.
LPA Gradient Induces Congruent Spatiotemporal Patterns of Localized Ca2+ Microdomains in Fibroblasts
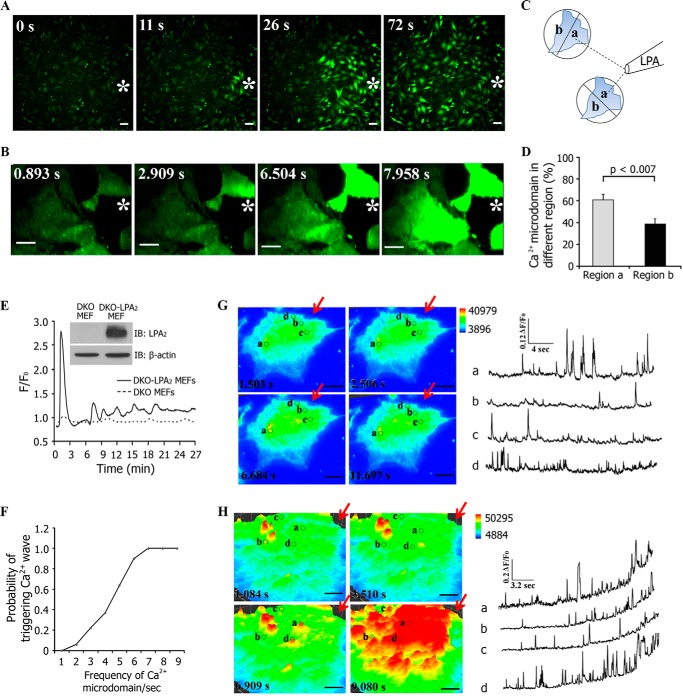
Our results thus far had indicated that an LPA gradient induced a mirroring decrease in the lateral mobility of LPA2 along the length of the cells without a detectable change in the number of receptors present in the plasma membrane. We next examined how this spatiotemporal change of LPA2 mobility is translated into localized intracellular signaling. LPA can activate changes in intracellular Ca2+ levels ([Ca2+]i) through receptor-mediated PLC activation (33). We used time lapse imaging to investigate the spatiotemporal patterns of [Ca2+]i in Fluo-4 AM-labeled DKO MEFs that were reconstituted with WT-LPA2 (DKO-LPA2 MEFs) or L351A-LPA2 (DKO-L351A MEFs). Note that DKO MEFs do not express LPA1, LPA2, or LPA3 receptors and do not develop Ca2+ transients when exposed to LPA unless reconstituted with WT-LPA2 or L351A-LPA2 (29, 34). The expression of FLAG-tagged LPA2 was confirmed by Western blot (Fig. 3E, inset). Exposure of Fluo-4 AM-labeled DKO-LPA2 MEFs to an LPA gradient revealed that the transient fluorescence intensity of Fluo-4 increased in every cell (Fig. 3A and supplemental Video 2). In most cells, this [Ca2+]i signaling initiated from the region nearest to the micropipette source of LPA. Using TIRF imaging, we observed that LPA elicited localized and short-lived high frequency Ca2+ microdomains analogous to Ca2+ puffs (35). These Ca2+ puffs rapidly triggered Ca2+ waves along the LPA gradient throughout the DKO-LPA2 MEFs (Fig. 3B and supplemental video 3). Thus, DKO-LPA2 MEFs can spatiotemporally sense and respond to an LPA gradient by directionally organized changes in [Ca2+]i signals.
FIGURE 3.
Spatiotemporal patterns of intracellular Ca2+ puffs stimulated by an LPA gradient. A, Fluo-4 AM fluorescence images of [Ca2+]i responses in DKO-LPA2 MEFs exposed to an LPA gradient emitted by a micropipette containing 200 nm LPA. Scale bar, 50 μm. B, TIRF imaging of Fluo-4 AM-stained DKO-LPA2 MEFs exposed to an LPA gradient produced by a micropipette containing 200 nm LPA. Scale bar, 10 μm. White stars, sites of the micropipette. C, schematic showing the method used to define the distribution of the percentage of Ca2+ puffs at the regions exposed to higher LPA concentration (a) and the regions exposed to lower LPA concentration (b) in relation to the site of the micropipette. D, the quantitative analysis of the percentage of Ca2+ puffs localized in regions a and b. Data are presented as mean ± S.E. (error bars). E, LPA gradient-induced differential intracellular Ca2+ mobilization in DKO-LPA2 MEFs, not in DKO MEFs. F, plot showing the probability of triggering a Ca2+ wave by LPA-induced Ca2+ puffs in DKO-LPA2 MEFs (n = 42). G, low frequency Ca2+ puffs (<3 Hz) failed to trigger a Ca2+ wave. Scale bar, 10 μm. H, high frequency Ca2+ puffs (>5 Hz) triggered a Ca2+ wave. Scale bar, 10 μm. In G and H, the circles show the locations of the active Ca2+ puff sites. The Ca2+ signals recorded at marked regions are depicted by the corresponding traces. The amplitude is expressed as F/F0. F is the fluorescence intensity in the marked regions where the spark appeared. F0 is fluorescence intensity of the same area in the absence of Ca2+ puffs. The red arrows indicate the site with the highest LPA concentration. IB, immunoblot.
We further investigated the spatiotemporal properties of the LPA-elicited Ca2+ puffs by dividing the cell into two regions of equal area, proximal and distal in relation to the position of the micropipette (Fig. 3C). Analysis of the occurrence of Ca2+ puffs showed higher incidences in the proximal region (nearer to the micropipette, region labeled a) than that in the distal region (away from the micropipette, region labeled b) (Fig. 3D). This observation suggested that the LPA gradient induced asymmetric LPA2 receptor activation and caused a Ca2+ puff gradient that mirrored the extracellular LPA gradient. In contrast to DKO-LPA2 MEFs, LPA treatment induced only slight Ca2+ mobilization in DKO MEFs (Fig. 3E), suggesting that the LPA-induced Ca2+ response in DKO-LPA2 MEFs was primarily mediated by activation of LPA2. During the 1-h exposure to an LPA gradient, the global [Ca2+]i increased and then decreased to create a series of Ca2+ oscillations in DKO-LPA2 MEFs (Fig. 3F). In each Ca2+ oscillation, Ca2+ puffs were silenced during and shortly after a Ca2+ wave and then arose independently with increasing frequency to trigger the subsequent Ca2+ waves. We found that low frequency Ca2+ puffs (<3 Hz) often liberated insufficient Ca2+ to act as effective triggers of the waves (Fig. 3G), and only the high frequency Ca2+ puffs (>5 Hz) triggered rapid regenerative Ca2+ responses (Fig. 3H). Spatially, we observed that Ca2+ puffs between a wave and the successive Ca2+ wave repeatedly originated in the region proximal to the LPA source. Our results are consistent with a previous finding that the subcellular distribution of Ca2+ puff sites determines the spatial patterning of Ca2+ signals (35). Thus, recurring spatiotemporal activation of intracellular Ca2+ puffs during an oscillation maintained the spatiotemporal Ca2+ puff gradient during continuous exposure to an LPA gradient.
LPA2 Mediates Gradient Sensing in Fibroblasts
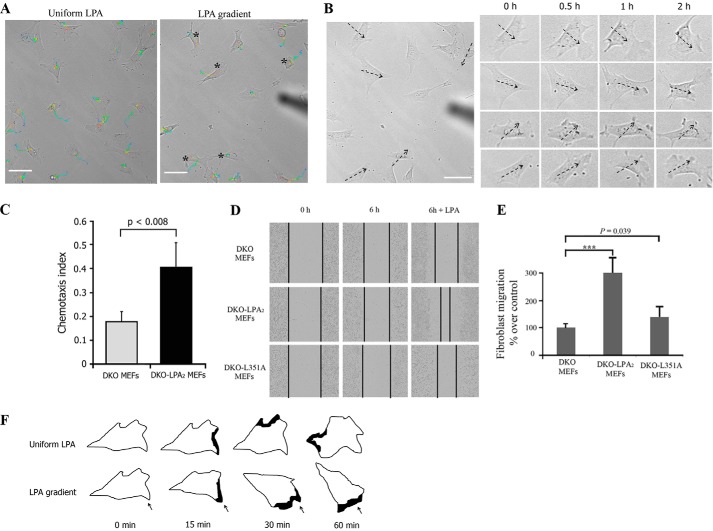
We used SPT to follow individual cells and calculate the velocity and directionality of migrating DKO-LPA2 MEFs during exposure to an LPA gradient (Fig. 4A and supplemental Video 4). A new cell membrane protrusion is the first morphological manifestation of chemotaxis (36). We observed that DKO-LPA2 MEFs extended new protrusions always toward the source of LPA gradient, regardless of whether or not there was a pre-existing protrusion (Fig. 4B). In contrast, when LPA was uniformly present in the culture medium, the cells extended protrusions with equal frequency around the entire perimeter of the cells (supplemental Video 5). As shown in Fig. 4C, DKO-LPA2 MEFs displayed a high CI, whereas DKO MEFs showed a low CI. In addition, wound healing assays indicated that reconstitution with WT-LPA2 converted DKO MEFs into a highly motile phenotype, confirming that LPA2 enhances LPA-induced fibroblast migration (Fig. 4, D and E). The spatial correlation of an LPA gradient, cell membrane protrusions, and motility results confirmed that LPA2 is responsible for gradient sensing and directional movement of fibroblasts toward LPA (Fig. 4F).
FIGURE 4.
Gradient sensing of fibroblasts stimulated by an LPA gradient. A, representative moving trajectories of DKO-LPA2 MEFs in exposure to an LPA gradient or uniform LPA. The cells were tracked for 1.5 h at a temporal resolution of 1 min/frame. The black stars mark cells significantly migrated toward the LPA gradient. The trajectories are color-coded. Time information is given by the color of the trajectory changing from blue (start) to red (end). The corresponding videos are available as supplemental Videos 4 and 5. Scale bar, 50 μm. B, time lapse images of DKO-LPA2 MEFs stimulated with an LPA gradient. The dashed arrows point to the direction of higher LPA concentration in the gradient. Scale bar, 50 μm. C, chemotaxis index of DKO-LPA2 MEFs or DKO MEFs exposed to an LPA gradient. D, time lapse images of the wound healing assay in DKO, DKO-LPA2, and DKO-L351A MEF monolayers in the presence or absence of 1 μm LPA. E, quantitative analysis of fibroblast migration in a wound healing assay at 6 h postwound in the presence of 1 μm LPA. The data are expressed as the ratios of migratory distance of cells to that of DKO MEFs (mean ± S.E. (error bars), n = 4). F, schematic representations showing the formation of protrusions induced by an LPA gradient. In a gradient of LPA, fibroblasts extend new protrusions toward the LPA gradient. When exposed to uniform LPA, fibroblasts extend new protrusions around the entire perimeter of the cell. The black spot marks the formation of protrusions. The arrow indicates LPA gradient direction.
LPA Induces Assembly of a Macromolecular Complex of LPA2
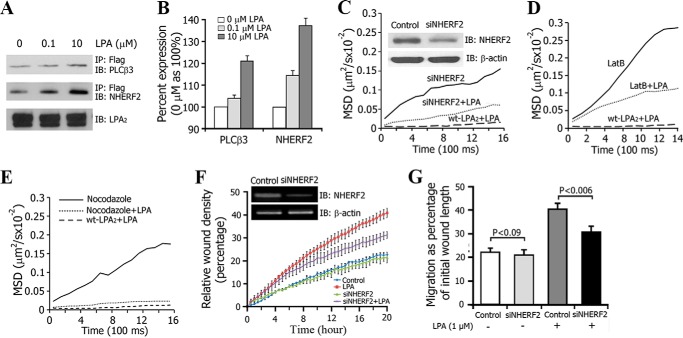
Ligand binding can reduce the mobility of cell surface receptors by successive coupling to a set of protein partners (37–39). The scaffolding protein NHERF2 specifically binds to the postsynaptic density-95/discs large/zona occludens-1 (PDZ) motif of LPA2 and recruits signaling molecules, including PLC-β3, into a macromolecular complex (40). We observed that LPA exposure increased LPA2·NHERF2·PLC-β3 interactions in a dose-dependent manner (Fig. 5, A and B). We used siRNA to knock down the endogenous NHERF2 in 3T3-LPA2 cells and monitored the lateral mobility of LPA2. The NHERF2 protein level was reduced by ∼70% with siRNA (Fig. 5C, inset), which significantly increased the lateral mobility of activated LPA2.
FIGURE 5.
LPA-induced macromolecular complex formation of LPA2 on the plasma membrane. A, co-immunoprecipitation was performed to test the macromolecular complex formation of LPA2, NHERF2, and PLC-β3. B, the bar graph depicts the average ratios of PLC-β3 and NHERF2 with LPA treatment to their corresponding controls (without LPA treatment). The data are densitometry readings from the immunoreactive bands as represented in A (mean ± S.E. (error bars), n = 3). C, MSD versus time plots for LPA2 in 3T3-LPA2 cells with or without knockdown of NHERF2 expression and in the presence or absence of LPA. The inset blots show the knocking down of NHERF2 expression in 3T3-LPA2 cells. D, MSD versus time plots of LPA2 in 3T3-LPA2 cells treated with 1 μm LatB for 30 min in the absence or presence of 1 μm LPA. E, MSD versus time plots of LPA2 in 3T3-LPA2 cells treated with 30 μm nocodazole for 30 min in the absence or presence of 1 μm LPA. F, the kinetics of the relative wound density from wound healing assays conducted in the IncuCyteTM high content imaging system. The assays were performed using DKO-LPA2 MEFs with or without transient knockdown of NHERF2 expression. The inset blots show the knocking down of NHERF2 expression in DKO-LPA2 MEFs. G, quantitative analysis of cells migration as shown in F. IB, immunoblot.
Anchoring to the cytoskeleton is one of the major factors that restricts the dynamic mobility of membrane proteins (41). NHERF2 contains a C-terminal ezrin-radixin-moesin (ERM) domain that can link it to the cytoskeleton (42). Disruption of the cytoskeleton using latrunculin B (which inhibits actin polymerization and disrupts microfilament-mediated processes), but not nocodazole (which disrupts microtubules), caused a significant increase in lateral mobility of LPA2 in response to directionally applied LPA (Fig. 5, D and E). Previous reports have shown that knockdown of NHERF2 decreased cell migration (26, 43). Theisen et al. (43) demonstrated that NHERF2·M-cadherin·β-catenin·PDGF receptor macromolecular complexes play an important role in cell motility. Using high content microscopic techniques, we investigated the effect of NHERF2 on the migration of LPA-stimulated DKO-LPA2 MEFs. Transient transfection of these cells with siRNA targeting NHERF2 resulted in an ∼70% decrease in the amount of NHERF2 (Fig. 5F, inset). Wounds were made in confluent monolayers of DKO-LPA2 MEFs, and images were taken at 30-min intervals for up to 24 h. The kinetics of the RWD indicated that knockdown of NHERF2 resulted in slow RWD kinetics with significant reduction of the LPA-stimulated migrations from 40.79 ± 1.79 to 31.18 ± 1.98% (p < 0.006) (Fig. 5, F and G), indicating that knockdown of NHERF2 decreased LPA-induced MEF migration. These observations suggest that the LPA gradient-induced and polarized reduction in the mobility of LPA2 was due to the spatiotemporal assembly of the macromolecular complex linking LPA2 to its binding partners and the cytoskeleton.
LPA2-dependent Cell Migration Requires PLCβ-mediated Activation of Ca2+ Microdomains
The role of PLC activation in LPA-mediated migration of intestinal epithelial cells has been demonstrated (44). We examined the functional involvement of PLCβ in LPA-induced fibroblast migration using the PLCβ inhibitor U73122. Treatment of DKO-LPA2 MEFs with U73122 significantly reduced the frequency of LPA-induced Ca2+ puffs (Fig. 6A). Using high content microscopic techniques, we investigated the regulation of fibroblast migration by PLCβ. The kinetics of the RWD is depicted in Fig. 6B. Treatment with U73122 resulted in slow RWD kinetics with significant reduction of the basal migration of DKO-LPA2 MEFs, from 29 ± 1.3 to 17.2 ± 1.1% (p = 0.002), and LPA-stimulated migration also was significantly inhibited by U73122, from 40.2 ± 1.8 to 20.6 ± 1.3% (p < 0.001) (Fig. 6C). These data suggest that generation of LPA2-mediated Ca2+ puffs and fibroblast migration require PLCβ activity.
FIGURE 6.
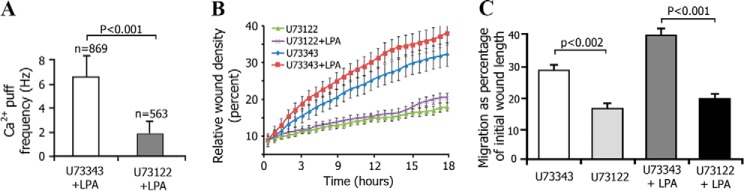
LPA2-dependent cell migration was inhibited by a PLC-β inhibitor. A, frequency of LPA-induced Ca2+ puffs in DKO-LPA2 MEFs treated with PLC-β inhibitor U73122 (2 μm) or its inactive analog U73343 (2 μm). B, kinetics of the relative wound density in the presence of U73122 (2 μm), U73343 (2 μm), or either U73122 or U73343 plus LPA (1 μm). The data were analyzed using IncuCyteTM software. C, the migration of DKO-LPA2 MEFs at the basal or LPA-activated state with U73122 (2 μm) or U73343 (2 μm) treatment. Error bars, S.E.
Disruption of the PDZ Motif Attenuates the LPA Gradient-induced Spatiotemporal Organization of Ca2+ Puffs and Impairs Gradient Sensing of Fibroblasts
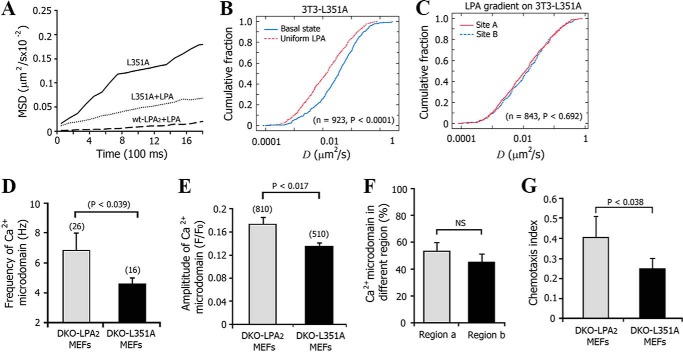
The intracellular C-terminal PDZ motif of LPA2 plays an important role in the regulation of LPA2-mediated signaling (45). We reconstituted 3T3 cells with the L351A-LPA2 mutant, which has a leucine-to-alanine mutation in the PDZ motif that renders it inactive to forming PDZ motif-dependent complexes. We found that this mutation increased the Dmed of activated LPA2 ∼5.5-fold, from 0.0023 ± 0.0012 to 0.0128 ± 0.0025 μm2/s. However, this mutant had no effect on the Dmed of the receptor in its basal state (Fig. 7, A and B). In the presence of an LPA gradient, the mobility of the L351A mutant was indistinguishable between the proximal portion and the distal portion relative to the LPA source (Fig. 7C), which suggested that the PDZ motif of LPA2 is required for the spatial macromolecular complex formation of LPA2 and tightly regulates the lateral mobility of activated LPA2.
FIGURE 7.
Disruption of the PDZ motif of LPA2 attenuated the LPA gradient-induced spatial decrease in LPA2 mobility. A, MSD versus time plots for LPA2-L351A and WT-LPA2 expressed in 3T3 cells treated with or without 1 μm LPA. B, comparison of cumulative fraction plots from the KS-test of the difference in the diffusion coefficient of lateral L351A at basal and uniform LPA-activated state. The respective number of trajectories (n) and p value are listed. C, KS-test of the difference in the diffusion coefficient of lateral L351A mutant at sites A and B after exposure to an LPA gradient. D, frequency of Ca2+ puffs in DKO LPA2 or DKO-L351A MEFs. The number of cells tested is indicated above each bar. Data are presented as mean ± S.E. (error bars). E, mean amplitude of Ca2+ puffs in DKO-LPA2 or DKO-L351A MEFs. The number of Ca2+ puffs tested is indicated above each bar. F, quantitative analysis of the percentage of Ca2+ puffs localized in region a (close to the micropipette) and region b (away from the micropipette) in DKO-L351A MEFs. Data are presented as mean ± S.E., n = 42. G, chemotaxis index from DKO-L351A MEFs compared with DKO-LPA2 MEFs upon exposure to an LPA gradient.
We also reconstituted DKO MEFs with the L351A-LPA2 (DKO-L351A MEFs). Disruption of the PDZ motif caused a 21.3% reduction in the frequency (Fig. 7D) and a 38.2% reduction in the amplitude (Fig. 7E) of Ca2+ puffs. After exposure to an LPA gradient, The occurrence difference of Ca2+ puffs in regions a and b of DKO-L351A cells is not significant (Fig. 7F), contrary to that in DKO LPA2 MEF (Fig. 3D). Also, fewer Ca2+ puffs were initiated in region a of DKO-L351A MEFs compared with that of DKO-LPA2 MEFs. Thus, disruption of the PDZ motif attenuated the sensitivity of fibroblasts to an LPA gradient. In addition, DKO-L351A MEFs exhibited a low CI compared with DKO-LPA2 MEFs (Fig. 7G). Taken together, our data suggest that the PDZ motif-mediated asymmetric macromolecular complex assembly of LPA2 regulates the gradient sensing of fibroblasts.
DISCUSSION
Based on our findings, we propose that LPA gradient sensing in fibroblasts is mediated by spatially organized, PDZ motif-mediated assembly of macromolecular complexes of LPA2 on the plasma membrane, which causes asymmetric intracellular Ca2+ signals to govern directional migration of fibroblasts (Fig. 8).
FIGURE 8.
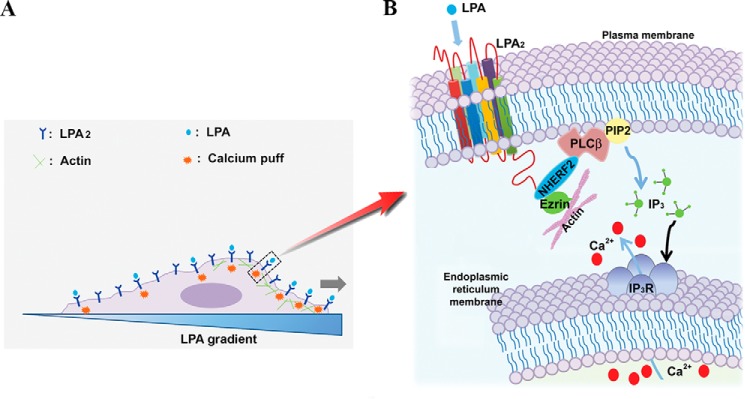
A proposed model to depict the mechanism underlying the LPA2-mediated gradient sensing and migration of fibroblasts in response to an extracellular LPA cue. A, a schematic representation of asymmetric Ca2+ puff activation in cells exposed to an LPA gradient. B, LPA-induced and LPA2-mediated gradient sensing of fibroblasts is initiated through the asymmetric formation of LPA2 complexes, which activate downstream asymmetric PLC-β3-inositol 1,4,5-trisphosphate/Ca2+ signaling to trigger Ca2+ puffs to guide the gradient sensing and migration of fibroblasts.
Chemoattractant receptors are uniformly distributed along the plasma membrane during random or chemotactic movement, even when cells are induced to reverse direction or turn by repositioning the micropipette filled with the chemoattractant cue (7). The even distribution of the chemoattractant receptors is consistent with the fact that fibroblasts are chemotactically responsive at all points on their perimeter and, thereby, suited for gradient sensing through a spatial mechanism. To answer the question of at what point in the signaling pathway a response becomes localized, we used SPT techniques to visualize that an LPA gradient caused a spatially restricted activation of LPA2 along the plasma membrane although this receptor was uniformly distributed. Our data demonstrate that LPA affected the lateral diffusion of LPA2 within milliseconds. Although fibroblasts move far more slowly than of cells of Dictyostelium and neutrophils, we found that gradient sensing of fibroblasts is rapidly processed. Swanson et al. (46) observed that chemotaxing cells respond with an L-shaped turn of the pseudopod, instead of reorganizing the morphological polarization, when a cAMP-loaded microneedle producing the gradient is shifted to the side of the cell. FRET studies between the G-protein subunits revealed a higher GPCR-mediated activation at the leading edge of Dictyostelium cells within milliseconds (47). de Keijzer et al. (13) have reported that a spatially restricted increase in receptor mobility is involved in directional sensing during D. discoideum chemotaxis, and they observed high spatial and temporal mobility of cAMP receptor 1 within milliseconds. These observations suggest that gradient sensing occurs rapidly (within milliseconds), which strengthens our contention that the rapid changes in LPA2-mediated receptor mobility we observed provide evidence as to the mechanism of how gradient sensing occurs in fibroblasts.
Gradient sensing depends on the translation of an external gradient into an amplified intracellular signal gradient (48). This signaling asymmetry leads to an asymmetry in cell shape accomplished by cytoskeleton rearrangement. Care is needed to distinguish the differences among gradient sensing, morphological polarization, and directional migration. Morphological polarity is a passive readout for chemotaxis in eukaryotic cells (6, 49–51) but not a requirement for gradient sensing (50). Similarly, the inability to move in a given direction tells nothing about the capability to sense that direction. Experiments using latrunculin A have shown that morphologically unpolarized, immobile Dictyostelium cells can still sense a cAMP gradient (49, 52). Cells tend to maintain their once established polarity even when the direction of the gradient is altered.
Our results support the hypothesis that spatiotemporal (asymmetric) macromolecular complex formation of LPA2 initiates the translation of an external LPA gradient. The next question to be answered is what downstream process carries the signal amplification and eventually directs gradient sensing of fibroblasts. LPA induces mobilization of intracellular Ca2+ (53), and Ca2+ plays a multifunctional role in gradient sensing, cytoskeleton redistribution, and relocation of focal adhesion (19, 54, 55). The signaling and motility center, the leading lamella, contains numerous effector proteins that require high levels of calcium for activation (56, 57). However, intracellular Ca2+ displays a rear-to-front gradient, and transient increases of Ca2+ concentration have been observed in migrating cells; they are infrequent and mainly localized in the tail of the cell (20). Recently, Wei et al. (21) showed that spatially and temporally restricted flickers of Ca2+ signaling are enriched near the leading edge of motile fibroblasts. Also, the difference in the integrated intensity of Ca2+ flickers on one side of the leading edge versus the other correlates strongly with the turning behavior of the cells (58). We visualized that an LPA gradient induced a spatial-temporal distribution of Ca2+ puff sites, which determined the spatial patterning of Ca2+ signals and then steered cell migration. These observations suggest that localized Ca2+ elevation may be responsible for the local Ca2+-dependent events at the front of cells. In addition, we also observed that the slight initial asymmetric Ca2+ puff activity resulted in significantly higher Ca2+ puffs and then global Ca2+ mobilization within minutes, which may be the positive feedback loop for LPA-induced migration. Moreover, it will be important to investigate the immediate signaling events activated locally by a Ca2+ puff, such as local polymerization and depolymerization of cytoskeletal filaments or activation of Ca2+ signaling proteins in these Ca2+ microdomains. Although there have been several prior studies focusing on intracellular signaling pathways that positively or negatively regulate cell migration, few studies have addressed mechanisms regulating the gradient sensing and chemotactic response to LPA in fibroblasts. Our observations unveil a molecular mechanism to explain which factors act upstream to transmit extracellular chemoattractant signals into intracellular signals and which factors act downstream to transduce these signals into mechanical forces.
The next question to be answered is how LPA-induced rapid changes in LPA2 mobility and rapid Ca2+ dynamics (i.e. within milliseconds) in defined regions of the cells lead to the observed rather slower migration of fibroblasts (i.e. within hours). When we maintained the LPA gradient, the asymmetric pattern of receptor mobility was observed. During the 1-h exposure to an LPA gradient, the global [Ca2+]i increased and then decreased to create a series of Ca2+ oscillations. In each Ca2+ oscillation, Ca2+ puffs were silenced during and shortly after a Ca2+ wave and then arose independently with increasing frequency to trigger the subsequent Ca2+ waves, and thereby, the spatiotemporal patterns of Ca2+ puffs were maintained. Thus, recurring spatiotemporal activation of intracellular Ca2+ puffs during an oscillation can maintain the spatiotemporal Ca2+ puff gradient during continuous exposure to an LPA gradient. These local events would interact with self-organizing systems that drive motility through global polarization and locally propagating waves of actin nucleation to bias cell migration toward the source of the chemoattractant.
The observed spatially restricted mobility of LPA2 was a result of PDZ motif-mediated coupling with downstream signaling partners to form an LPA2·NHERF2·PLC-β3 macromolecular complex that anchors to the cytoskeleton (59). LPA gradient-induced spatial (asymmetric) activation of LPA2 could account for the primary event, which then elicits the downstream asymmetric signaling pathway needed for gradient sensing of fibroblasts. The SPT data provide the first evidence that the mobility of the chemoattractant receptor is directly involved in gradient sensing in fibroblasts. Thus, spatially localized activation of LPA2 restricts its membrane mobility via the PDZ-mediated recruitment of NHERF2·PLC-β3-coupled Ca2+ microdomains and cytoskeletal interaction that in turn govern directional migration of fibroblasts.
We observed that a macromolecular complex, including PLC-β3 assembled with LPA2, is required for triggering asymmetric distribution of Ca2+ puffs. Disruption of this PDZ motif-mediated complex of LPA2 attenuated the intracellular Ca2+ puff gradient, impaired fibroblast gradient sensing, and consequently reduced directional fibroblast migration. Our data demonstrate that treatment of DKO-LPA2 MEFs with PLCβ inhibitor U73122 significantly inhibited LPA-induced Ca2+ puff frequency as well as basal and LPA-stimulated cell migration. Frequency of Ca2+ puffs >5 Hz and spatially organized by the macromolecular complex triggered Ca2+ waves leading to directional activation of Ca2+-dependent downstream migratory signaling in the cell. Our data indicate the frequency of Ca2+ microdomain signaling as the dynamic process of signal amplification when a cell encounters an LPA gradient. Ca2+ influx during growth cone attraction is mediated by transient receptor potential channels, a class of Ca2+-permeable receptor-operated channels (60, 61). A recent study showed that NHERF2 and TRPC4 (transient receptor potential channel 4) influence Ca2+ signaling (62), making it possible that Ca2+ influx contributes in part to the amplification of local Ca2+ levels. Further experiments will be needed to confirm this hypothesis.
Gradient sensing is a crucial step during cell chemotaxis, and LPA has been shown to contribute to fibroblast recruitment induced by lung injury (63). Recent studies also have established that LPA2 mediates the migration of several types of cancer cells (26–28). Because most mammalian cell types are LPA-responsive, our findings may unveil a more general role for LPA2 in different pathological events, including cancer invasion and metastasis.
Supplementary Material
Acknowledgments
We thank Dr. David L. Armbruster for critical reading of the manuscript. We are grateful to J. Denise Wetzel (Cincinnati Children's Hospital Medical Center) for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK080834 and DK093045 (to A. P. N.) and CA092160 (to G. T.).

This article contains supplemental Videos 1–5.
- GPCR
- G-protein-coupled receptor
- LPA
- lysophosphatidic acid
- PDZ
- postsynaptic density-95/discs large/zona occludens-1
- PLC-β3
- phospholipase C-β3
- QD
- conjugated quantum dot
- SPT
- single-particle tracking
- TIRF
- total internal reflection fluorescence
- DKO
- double knock-out
- MEF
- mouse embryo fibroblast
- CI
- chemotactic index
- RWD
- relative wound density
- MSD
- mean square displacement
- KS-test
- Kolmogorov-Smirnov test
- ERM
- ezrin-radixin-moesin
- EGFP
- enhanced green fluorescent protein.
REFERENCES
- 1. Cha I., Lee S. H., Jeon T. J. (2010) Chemoattractant-mediated Rap1 activation requires GPCR/G proteins. Mol. Cells 30, 563–567 [DOI] [PubMed] [Google Scholar]
- 2. Postma M., Bosgraaf L., Loovers H. M., Van Haastert P. J. (2004) Chemotaxis: signalling modules join hands at front and tail. EMBO Rep. 5, 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iijima M., Huang Y. E., Devreotes P. (2002) Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469–478 [DOI] [PubMed] [Google Scholar]
- 4. Jasuja R., Lin Y., Trentham D. R., Khan S. (1999) Response tuning in bacterial chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 96, 11346–11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devreotes P., Janetopoulos C. (2003) Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 [DOI] [PubMed] [Google Scholar]
- 6. Jin T., Zhang N., Long Y., Parent C. A., Devreotes P. N. (2000) Localization of the G protein betagamma complex in living cells during chemotaxis. Science 287, 1034–1036 [DOI] [PubMed] [Google Scholar]
- 7. Xiao Z., Zhang N., Murphy D. B., Devreotes P. N. (1997) Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J. Cell Biol. 139, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarz-Romond T., Gorski S. A. (2010) Focus on the spatial organization of signalling. EMBO J. 29, 2675–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parent C. A., Devreotes P. N. (1999) A cell's sense of direction. Science 284, 765–770 [DOI] [PubMed] [Google Scholar]
- 10. Xu X., Meier-Schellersheim M., Jiao X., Nelson L. E., Jin T. (2005) Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol. Biol. Cell 16, 676–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X., Meckel T., Brzostowski J. A., Yan J., Meier-Schellersheim M., Jin T. (2010) Coupling mechanism of a GPCR and a heterotrimeric G protein during chemoattractant gradient sensing in Dictyostelium. Sci. Signal. 3, ra71. [DOI] [PubMed] [Google Scholar]
- 12. Ueda M., Sako Y., Tanaka T., Devreotes P., Yanagida T. (2001) Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science 294, 864–867 [DOI] [PubMed] [Google Scholar]
- 13. de Keijzer S., Sergé A., van Hemert F., Lommerse P. H., Lamers G. E., Spaink H. P., Schmidt T., Snaar-Jagalska B. E. (2008) A spatially restricted increase in receptor mobility is involved in directional sensing during Dictyostelium discoideum chemotaxis. J. Cell Sci. 121, 1750–1757 [DOI] [PubMed] [Google Scholar]
- 14. Funamoto S., Meili R., Lee S., Parry L., Firtel R. A. (2002) Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 [DOI] [PubMed] [Google Scholar]
- 15. Ferguson G. J., Milne L., Kulkarni S., Sasaki T., Walker S., Andrews S., Crabbe T., Finan P., Jones G., Jackson S., Camps M., Rommel C., Wymann M., Hirsch E., Hawkins P., Stephens L. (2007) PI(3)Kγ has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9, 86–91 [DOI] [PubMed] [Google Scholar]
- 16. Loovers H. M., Postma M., Keizer-Gunnink I., Huang Y. E., Devreotes P. N., van Haastert P. J. (2006) Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol. Biol. Cell 17, 1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu H., Goel V., Haluska F. G. (2003) PTEN signaling pathways in melanoma. Oncogene 22, 3113–3122 [DOI] [PubMed] [Google Scholar]
- 18. Chen L., Iijima M., Tang M., Landree M. A., Huang Y. E., Xiong Y., Iglesias P. A., Devreotes P. N. (2007) PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev. Cell 12, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 20. Brundage R. A., Fogarty K. E., Tuft R. A., Fay F. S. (1991) Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254, 703–706 [DOI] [PubMed] [Google Scholar]
- 21. Wei C., Wang X., Chen M., Ouyang K., Song L. S., Cheng H. (2009) Calcium flickers steer cell migration. Nature 457, 901–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henley J. R., Huang K. H., Wang D., Poo M. M. (2004) Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44, 909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blaser H., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F. L., Kawakami K., Solnica-Krezel L., Heisenberg C. P., Raz E. (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell 11, 613–627 [DOI] [PubMed] [Google Scholar]
- 24. Evans J. H., Falke J. J. (2007) Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 16176–16181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C. W., Mutoh T., Lin M. E., Teo S. T., Park K. E., Mosley A. N., Chun J. (2010) LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50, 157–186 [DOI] [PubMed] [Google Scholar]
- 26. Lee S. J., Ritter S. L., Zhang H., Shim H., Hall R. A., Yun C. C. (2011) MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology 140, 924–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen M., Towers L. N., O'Connor K. L. (2007) LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am. J. Physiol. Cell Physiol. 292, C1927–C1933 [DOI] [PubMed] [Google Scholar]
- 28. Hasegawa Y., Murph M., Yu S., Tigyi G., Mills G. B. (2008) Lysophosphatidic acid (LPA)-induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells. Mol. Oncol. 2, 54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. E S., Lai Y. J., Tsukahara R., Chen C. S., Fujiwara Y., Yue J., Yu J. H., Guo H., Kihara A., Tigyi G., Lin F. T. (2009) Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J. Biol. Chem. 284, 14558–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinha C., Ren A., Arora K., Moon C. S., Yarlagadda S., Zhang W., Cheepala S. B., Schuetz J. D., Naren A. P. (2013) Multi-drug resistance protein 4 (MRP4)-mediated regulation of fibroblast cell migration reflects a dichotomous role of intracellular cyclic nucleotides. J. Biol. Chem. 288, 3786–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wessels D., Lusche D. F., Kuhl S., Heid P., Soll D. R. (2007) PTEN plays a role in the suppression of lateral pseudopod formation during Dictyostelium motility and chemotaxis. J. Cell Sci. 120, 2517–2531 [DOI] [PubMed] [Google Scholar]
- 32. Wang D. A., Lorincz Z., Bautista D. L., Liliom K., Tigyi G., Parrill A. L. (2001) A single amino acid determines lysophospholipid specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. J. Biol. Chem. 276, 49213–49220 [DOI] [PubMed] [Google Scholar]
- 33. Meyer Zu Heringdorf D. (2004) Lysophospholipid receptor-dependent and -independent calcium signaling. J. Cell. Biochem. 92, 937–948 [DOI] [PubMed] [Google Scholar]
- 34. Contos J. J., Ishii I., Fukushima N., Kingsbury M. A., Ye X., Kawamura S., Brown J. H., Chun J. (2002) Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22, 6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marchant J. S., Parker I. (2001) Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO J. 20, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mouneimne G., Soon L., DesMarais V., Sidani M., Song X., Yip S. C., Ghosh M., Eddy R., Backer J. M., Condeelis J. (2004) Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J. Cell Biol. 166, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaaf M. J., Cidlowski J. A. (2003) Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol. Cell. Biol. 23, 1922–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ali G. S., Prasad K. V., Day I., Reddy A. S. (2007) Ligand-dependent reduction in the membrane mobility of FLAGELLIN SENSITIVE2, an Arabidopsis receptor-like kinase. Plant Cell Physiol. 48, 1601–1611 [DOI] [PubMed] [Google Scholar]
- 39. Plested A. J., Mayer M. L. (2009) AMPA receptor ligand binding domain mobility revealed by functional cross-linking. J. Neurosci. 29, 11912–11923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh Y. S., Jo N. W., Choi J. W., Kim H. S., Seo S. W., Kang K. O., Hwang J. I., Heo K., Kim S. H., Kim Y. H., Kim I. H., Kim J. H., Banno Y., Ryu S. H., Suh P. G. (2004) NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-β3 activation. Mol. Cell. Biol. 24, 5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saxton M. J., Jacobson K. (1997) Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 26, 373–399 [DOI] [PubMed] [Google Scholar]
- 42. Shenolikar S., Weinman E. J. (2001) NHERF: targeting and trafficking membrane proteins. Am. J. Physiol. Renal Physiol. 280, F389–F395 [DOI] [PubMed] [Google Scholar]
- 43. Theisen C. S., Wahl J. K., 3rd, Johnson K. R., Wheelock M. J. (2007) NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol. Biol. Cell 18, 1220–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee S. J., Leoni G., Neumann P. A., Chun J., Nusrat A., Yun C. C. (2013) Distinct phospholipase C-β isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol. Cell. Biol. 33, 2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin F. T., Lai Y. J. (2008) Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein-protein interactions. Biochim. Biophys. Acta 1781, 558–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swanson J. A., Taylor D. L. (1982) Local and spatially coordinated movements in Dictyostelium discoideum amoebae during chemotaxis. Cell 28, 225–232 [DOI] [PubMed] [Google Scholar]
- 47. Xu X., Brzostowski J. A., Jin T. (2009) Monitoring dynamic GPCR signaling events using fluorescence microscopy, FRET imaging, and single-molecule imaging. Methods Mol. Biol. 571, 371–383 [DOI] [PubMed] [Google Scholar]
- 48. von Philipsborn A., Bastmeyer M. (2007) Mechanisms of gradient detection: a comparison of axon pathfinding with eukaryotic cell migration. Int. Rev. Cytol. 263, 1–62 [DOI] [PubMed] [Google Scholar]
- 49. Servant G., Weiner O. D., Herzmark P., Balla T., Sedat J. W., Bourne H. R. (2000) Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rubin H., Ravid S. (2002) Polarization of myosin II heavy chain-protein kinase C in chemotaxing Dictyostelium cells. J. Biol. Chem. 277, 36005–36008 [DOI] [PubMed] [Google Scholar]
- 51. Parent C. A., Blacklock B. J., Froehlich W. M., Murphy D. B., Devreotes P. N. (1998) G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91 [DOI] [PubMed] [Google Scholar]
- 52. Janetopoulos C., Ma L., Devreotes P. N., Iglesias P. A. (2004) Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 101, 8951–8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hildebrandt J. P. (1995) Lysophosphatidic acid induces inositol phosphate and calcium signals in exocrine cells from the avian nasal salt gland. J. Membr. Biol. 144, 49–58 [DOI] [PubMed] [Google Scholar]
- 54. Van Haastert P. J., Devreotes P. N. (2004) Chemotaxis: signalling the way forward. Nat. Rev. Mol. Cell Biol. 5, 626–634 [DOI] [PubMed] [Google Scholar]
- 55. Lee J., Ishihara A., Oxford G., Johnson B., Jacobson K. (1999) Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, 382–386 [DOI] [PubMed] [Google Scholar]
- 56. Stossel T. P., Fenteany G., Hartwig J. H. (2006) Cell surface actin remodeling. J. Cell Sci. 119, 3261–3264 [DOI] [PubMed] [Google Scholar]
- 57. Franco S. J., Rodgers M. A., Perrin B. J., Han J., Bennin D. A., Critchley D. R., Huttenlocher A. (2004) Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977–983 [DOI] [PubMed] [Google Scholar]
- 58. Wei C., Wang X., Chen M., Ouyang K., Zheng M., Cheng H. (2010) Flickering calcium microdomains signal turning of migrating cells. Can. J. Physiol. Pharmacol. 88, 105–110 [DOI] [PubMed] [Google Scholar]
- 59. Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401, 286–290 [DOI] [PubMed] [Google Scholar]
- 60. Li Y., Jia Y. C., Cui K., Li N., Zheng Z. Y., Wang Y. Z., Yuan X. B. (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 [DOI] [PubMed] [Google Scholar]
- 61. Shim S., Goh E. L., Ge S., Sailor K., Yuan J. P., Roderick H. L., Bootman M. D., Worley P. F., Song H., Ming G. L. (2005) XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 8, 730–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee-Kwon W., Wade J. B., Zhang Z., Pallone T. L., Weinman E. J. (2005) Expression of TRPC4 channel protein that interacts with NHERF-2 in rat descending vasa recta. Am. J. Physiol. Cell Physiol. 288, C942–C949 [DOI] [PubMed] [Google Scholar]
- 63. Tager A. M., LaCamera P., Shea B. S., Campanella G. S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B. A., Kim N. D., Hart W. K., Pardo A., Blackwell T. S., Xu Y., Chun J., Luster A. D. (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.