Background: Mitochondrial dynamics play a role in maintaining energy production and metabolism in mammalian cells.
Results: Wnt-5a signaling stimulated mitochondrial dynamics in neurons, triggering the fission-fusion process through calcium mobilization.
Conclusion: Wnt-5a signaling controls mitochondrial morphology and dynamics in postsynaptic regions.
Significance: Noncanonical Wnt-5a signaling modulates mitochondrial dynamics in normal neurons, and it is a new therapeutic target for brain disease.
Keywords: Calcium, Mitochondria, Neurobiology, Signal Transduction, Wnt Signaling
Abstract
The Wnt signaling pathway plays an important role in developmental processes, including embryonic patterning, cell specification, and cell polarity. Wnt components participate in the development of the central nervous system, and growing evidence indicates that this pathway also regulates the function of the adult nervous system. In this study, we report that Wnt-5a, a noncanonical Wnt ligand, is a potent activator of mitochondrial dynamics and induces acute fission and fusion events in the mitochondria of rat hippocampal neurons. The effect of Wnt-5a was inhibited in the presence of sFRP, a Wnt scavenger. Similarly, the canonical Wnt-3a ligand had no effect on mitochondrial fission-fusion events, suggesting that this effect is specific for Wnt-5a alone. We also show that the Wnt-5a effects on mitochondrial dynamics occur with an increase in both intracellular and mitochondrial calcium (Ca2+), which was correlated with an increased phosphorylation of Drp1(Ser-616) and a decrease of Ser-637 phosphorylation, both indicators of mitochondrial dynamics. Electron microscope analysis of hippocampal tissues in the CA1 region showed an increase in the number of mitochondria present in the postsynaptic region, and this finding correlated with a change in mitochondrial morphology. We conclude that Wnt-5a/Ca2+ signaling regulates the mitochondrial fission-fusion process in hippocampal neurons, a feature that might help to further understand the role of Wnt-related pathologies, including neurodegenerative diseases associated with mitochondrial dysfunction, and represents a potentially important link between impaired metabolic function and degenerative disorders.
Introduction
Wnt proteins play essential roles in development and physiological processes by regulating vital cell functions, including proliferation, differentiation, synaptogenesis, apoptosis, cell survival, adhesion, migration, polarity, synapse formation, and neuronal plasticity (1–4). The best documented intracellular Wnt signaling pathway is the canonical Wnt/β-catenin signaling pathway, characterized by an increase and stabilization of cytoplasmic β-catenin levels, which translocates to the nucleus and co-activates transcription of Wnt target genes associated with Tcf/Lef transcription factors (1, 5). In contrast, several Wnts activate the β-catenin-independent pathways (noncanonical pathways), known as the planar cell polarity (Wnt/PCP) and the Wnt/Ca2+ pathways, and the binding of the Wnt ligand to Wnt receptor leads to a short lived increase in the concentration of certain intracellular signaling molecules, including inositol 1,4,5-triphosphate, 1,2 diacylglycerol, and Ca2+ levels. Wnt ligands also act through monomeric GTPases and c-Jun N-terminal kinase (JNK) (6, 7). In the central nervous system (CNS), Wnts have been implicated in the maintenance of synaptic plasticity, memory, and modulation of long term potentiation in mouse hippocampal slices (8–10).
The mitochondria are strategically localized at particular subcellular sites both for providing energy supply and for participating in intracellular signaling; a close association between the endoplasmic reticulum (ER)4 and the mitochondrial surface appears to be necessary for the propagation and regulation of ER-Ca2+ released into the mitochondria (11). Among other contributions, the mitochondria play a role in cell survival and death-promoting signals in many processes such as apoptosis and cellular necrosis (12). Therefore, the mitochondria are highly dynamic organelles that constantly change shape and numbers in response to different stimuli and can sense the levels of calcium gradients. The central player in mitochondrial division is the highly conserved, dynamin-related protein (Drp1 in mammals), which belongs to a large family of GTPases that self-assemble to regulate mitochondrial membrane structure (13, 14). There are several types of molecular bridges that mediate mitochondrial contacts, such as the mitochondrial fusion protein mitofusin 2 (15). These physical contacts are persistent and maintained under dynamic conditions, suggesting that the ER-mitochondrial interface is vital for the dynamic related events (16, 17).
Several extracellular signals, including change in cytosolic calcium, levels of glucose, synaptic activity, neurotransmitters, and growth factors, have been reported to regulate positioning, mitochondrial transport, and dynamics, moving these organelles to energy-demanding sites such as synapses, dendritic spines, and axons (18, 19). Wnt signaling activates mitochondrial biogenesis, which in turn produces elevated levels of reactive oxidative species (ROS) and oxidative damage (20). This effect on mitochondrial function and ROS generation may likely be responsible for some of the major biological consequences of altered Wnt signaling (21). Soluble frizzled related protein 5 (sFRP5) deficiencies stimulate the oxidative capacity of adipocytes with increased mitochondrial activity, mediated in part by PGC-1α and the mitochondrial transcription factor A (22). Other ligands such as Wnt-3a also increased oxygen consumption and the expression of mitochondrial genes. Taken together, these findings support a model of adipogenesis in which sFRP inhibits Wnt signaling to suppress oxidative metabolism and stimulate adipocyte growth during obesity (23). Wnt signaling also regulates mitochondrial physiology and insulin sensitivity (20). Regulation of mitochondrial division is critical for normal cellular function. Excess division is linked to numerous diseases, including neurodegeneration and diabetes (24, 25).
Previously, we have shown that activation of the Wnt signaling pathway with Wnt-5a induces rapid changes in the clustering of the post-synaptic density protein (PSD-95) (26). Wnt-5a modulates the trafficking and retention of GABAA receptors on the hippocampal neuronal surface, as well as the amplitude of GABA-evoked currents; these results are mediated by the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), an important serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex, which is stimulated by Wnt-5a and frizzled homologs (27). Wnt-5a-mediated signaling has been shown to stimulate dendritic spine morphogenesis in hippocampal neurons and the amplitude of NMDA spontaneous miniature currents (28). Herein, we investigate the role of the noncanonical Wnt-5a on mitochondrial fission-fusion dynamics in rat hippocampal neurons, and our results indicate that the Wnt-5a signaling pathway modulates mitochondrial fission-fusion and mitochondria traffic in rat hippocampal neurons toward the somato-dendritic region, and the post-synaptic CA1 region in particular.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant Wnt-5a and sFRP2 were purchased from R&D Systems; FK506 was from Sigma; Gö6976 was from Calbiochem. Antibody Drp1 was purchased from BD Biosciences, and p-Drp1 (serine 616 and serine 637) was purchased from Cell Signaling Technology, Inc. (Danvers, MA); pPKC was from Abcam; PKC and GAPDH were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Lipofectamine 2000 was from Invitrogen. Alexa-conjugated secondary antibodies Fluo3-AM, Rhod2-AM, Mitotracker-Orange, and the TMRM probe were purchased from Molecular Probes (Carlsbad, CA).
Animals and Ethical Standards
Experiments were performed on Sprague-Dawley rat fetuses (E18) and 2-month-old C57BL/6J mice. All mice and rats were housed in the University Facility. The experimental procedures were approved by the Bioethical and Biosafety Committee of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile.
Primary Cultured Rat Hippocampal Neurons
Rat hippocampal cultures were prepared from Sprague-Dawley rats at embryonic day 18. At 2 days in vitro (DIV), cultured neurons were treated with 2 μm cytosine arabinoside (Invitrogen) for 24 h to eliminate most of the glial cells. This method resulted in highly enriched neuronal cultures (∼95% neurons) (26, 27). Primary hippocampal neurons were cultures with Neurobasal medium supplemented with 1% B27 from Invitrogen until 14 DIV.
Hippocampal Slices
Hippocampal slices were prepared from brains of 2-month-old C57BL/6J mice as described previously (10, 28). Briefly, the animals were anesthetized with isoflurane and then sacrificed. Brains were surgically extracted, and transverse slices of the hippocampus (350 μm) were cut under cutting solution (85 mm NaCl, 75 mm sucrose, 3 mm KCl, 1.25 mm NaH2PO4, 25 mm NaHCO3, 10 mm glucose, 3.5 mm MgSO4, 0.5 mm CaCl2, 3 mm sodium pyruvate, 0.5 mm sodium ascorbate, pH 7.4) with a Leica VT 1000s vibratome. Slices were incubated in cold artificial cerebrospinal fluid (126 mm NaCl, 3 mm KCl, 1.25 mm NaH2PO4, 25 mm NaHCO3, 10 mm glucose, 2 mm MgSO4, 2 mm CaCl2, 3 mm sodium pyruvate, 0.5 mm sodium ascorbate, pH 7.4) and were maintained oxygenated (95% O2, 5% CO2) until the treatment.
Electron Microscopy from CA1 Hippocampal Slices
Rat hippocampal slices were used for electron microscopy analysis according to standard procedures (29–31). Briefly, hippocampal slices were treated and directly fixed in 3% glutaraldehyde in 50 mm cacodylate buffer (pH 7.2) for 3 days at room temperature. Then they were treated with 1% osmium tetroxide in cacodylate buffer for 90 min and then with 1% aqueous uranyl acetate, dehydrated in acetone, and embedded in Epon resin. The Epon-embedded lamina of hippocampal slices was cut in small pieces and included in the resin. Areas to be examined by electron microscopy (CA1 region of the hippocampus) were selected from 1-μm sections stained with toluidine blue for light microscopy. Ultrathin sections were cut on a Reichert Ultramicrotome (Newark, DE), placed on 300-mesh copper electron microscopy grids, stained with uranyl acetate and lead citrate, and examined using a Phillips Tecnai 12 transmission electron microscope (Philips Electron Optics, Holland) at 80 kV, from the facility of our faculty. Digital images obtained were analyzed by ImageJ (National Institutes of Health), and the quantitative analysis was performed with n = 3 using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA).
Generation of Control Media and Wnt Ligand-conditioned Media
Control and Wnt ligands conditioned media were prepared from L Cells (ATCC CRL-2648), L Wnt-3a (ATTC CRL-2814) cells, and L Wnt-5a (ATTC CRL-2814) cells. Cells were grown until 90% confluence, approximately, and the culture medium was replaced by Neurobasal medium without supplement or antibiotics. After 60 h of incubation, the media were recovered, centrifuged, quantified, and sterile-filtered and stored at 4 °C until use. Conditioned media were diluted 1:5 for use in cultured neurons. Recombinant Wnt-5a was used at 300 ng/ml (26, 28).
Mitochondrial Length Measurements
Hippocampal neurons were transfected with the plasmid mito-GFP for 30 min at 37 °C using Lipofectamine2000 and photographed under confocal microscopy. Mitochondrial length was made in ImageJ software (National Institutes of Health), using Feret's diameter tool. Neurons were treated with recombinant Wnt-5a. For comparative purposes, mitochondria were classified into three different categories, with length ranging from less than 0.21 to 0.54 μm (small), 0.55 to 1.59 μm (normal), and 1.6 to 10 μm (long) (32). Quantification of the neuronal population that underwent mitochondrial fission in response to Wnt-5a incubation was made considering the population of neurons treated with Wnt-5a, which presented a higher proportion of small mitochondria compared with the population of neurons that exhibit baseline mitochondria undergoing division. A minimum of 10 micrographs were made for each treatment and subsequently scored and quantified.
Three-dimensional Image Reconstruction
Mitochondrial network images obtained from the ELYRA S.1 super-resolution structured illumination microscope (SR-SIM) (Zeiss, Oberkochan, Germany) were reconstructed in three-dimensional isosurface using Imaris software, Facility of University of Concepción, Chile. Serial images (z stacks) were collected with a step of 0.2 μm to obtain between 21 and 24 images. Z-projection was performed with those images to reconstruct 4.2- and 4.8-μm thickness from the whole neuron. Sphericity of each reconstructed mitochondria was determined as described (31).
Determination of Mitochondrial Potential in Live Hippocampal Neurons
Mitochondria membrane potential was determined using the mitochondrial dye, TMRM. 15 DIV hippocampal neurons were labeled with TMRM (20 nm) for 30 min in KRH buffer supplemented with 5 mm glucose and 0.02% pluronic acid, and all procedures and experiments were made at 37 °C. Fluorescence changes in intensity were recorded using a confocal microscope (Carl Zeiss 5 LSM); region of interest fluorescence was used as a measure of mitochondrial potential; the signals from control neurons and neurons treated with different stimuli were compared using identical settings for laser power and detector sensitivity for each separate experiment. We analyzed TMRM intensity changes in 10–15 cells on average for each experiment. Estimation of fluorescence intensity of TMRM was presented as the pseudo-ratio (ΔF/Fo), which was calculated using the following formula: ΔF/Fo = (F − Fbase)/(Fbase − B), where F is the measured fluorescence intensity of the indicator; Fbase is the fluorescence intensity before the stimulation, and B is the background signal determined from the average of areas adjacent to the cells (33, 34).
Hippocampal Neuron Transfection
Hippocampal neurons were transfected using Lipofectamine 2000 (Invitrogen), 11 DIV days after seeding onto coverslips in 24-well culture plates at a density of 6 × 104 cells per well. Primary hippocampal neurons were transfected with mito-GFP. Neurons were incubated for 2 h at 37 °C, and then the media were replaced with Neurobasal/B27 growth medium until 14 DIV when the experiments were performed.
Immunofluorescence in Primary Hippocampal Neuron Cultures
Hippocampal neurons were plated onto polylysine-coated coverslips (30,000 neurons/cover). Cells were rinsed twice with PBS supplemented with 10 μm CaCl2 and 100 μm MgCl2 (PBSCM) and fixed with a freshly prepared solution of 4% paraformaldehyde and 4% sucrose in PBS for 20 min and permeabilized for 5 min with 0.2% Triton X-100 in PBS. After several rinses with PBSCM, cells were incubated in 1% BSA in PBSCM (blocking solution) for 30 min at room temperature, followed by an overnight incubation at 4 °C with the Drp1 primary antibody. Cells were washed with PBSCM and then incubated with Alexa-conjugated secondary antibodies for 30 min at 37 °C (35).
Analysis of Drp-1 by Western Blotting
Hippocampal neurons and slices were lysed in ice-cold lysis buffer (10 mm Tris-HCl (pH 7.8), 100 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, and 0.5% sodium deoxycholate) supplemented with protease and phosphatase inhibitors. Homogenates were maintained in ice for 30 min and then centrifuged at 15,000 × g for 10 min at 4 °C. The supernatant was recovered, and protein concentration was determined by the BCA protein assay kit (Pierce). Proteins were resolved in SDS-PAGE (10% polyacrylamide), transferred to a PVDF membrane, and incubated with primary antibodies. The reactions were followed by incubation with secondary antibodies peroxidase-labeled (Thermo Fisher Scientific, Inc., Rockford, IL). Primary antibodies used were as follows: anti-Drp1 (1:1000); anti-phospho-Drp1 Ser-616 (1:1000); anti-phospho-Drp1 Ser-637 (1:1000); anti-PKC (1:1000); anti-phospho-PKC (1:20,000), and anti-GAPDH (1:5000) (26)
Mitochondrial and Intracellular Calcium Imaging in Hippocampal Neurons
For in vivo cell imaging, neurons were seeded at a density of 1 × 105 cells in 25-mm coverslips and used for the experiments after 14 DIV. Hippocampal cells were loaded with 1 μm Fluo-3 (Molecular Probes, Carlsbad, CA) for 30 min at 37 °C to measure total calcium. For mitochondrial calcium, the neurons were labeled with visible wavelength calcium-sensitive dye Rhod-2 (1 μm) at 4 °C for 3 min followed by 30 min at 37 °C. Subsequently, the cultures were washed three times with Neurobasal media and imaged with a DSU IX81 Spinning Disk Confocal Microscope (Olympus, Center Valley, PA). After a basal measurement of Rhod-2 or Fluo-3 in Tyrode's buffer (135 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 10 mm Hepes, 5.6 mm glucose (pH 7.3)) for 3 min, Wnt-5a-conditioned medium was added with a micropipette to cells and recorded for 10 min. Rhod-2 λex/em = 549/578 nm; Fluo-3 λex/em = 488/526 nm. Estimation of fluorescence intensity of Fluo-3 and Rhod-2 was presented like the pseudo-ratio (ΔF/Fo) indicated by ΔF/Fo = (F − Fbase)/(Fbase), where F is the measured fluorescence intensity of the indicator; Fbase is the fluorescence intensity before the stimulation (33).
Statistical Analysis
Results are expressed as means ± S.E. Data were analyzed by one-way ANOVA, followed by Bonferroni's post hoc or Student's t test. p ≤ 0.05 was considered as statistically significant. Statistical analysis was performed using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA).
RESULTS
Wnt-5a Increases the Population of Hippocampal Neurons with Small Mitochondria in a Time-dependent Manner
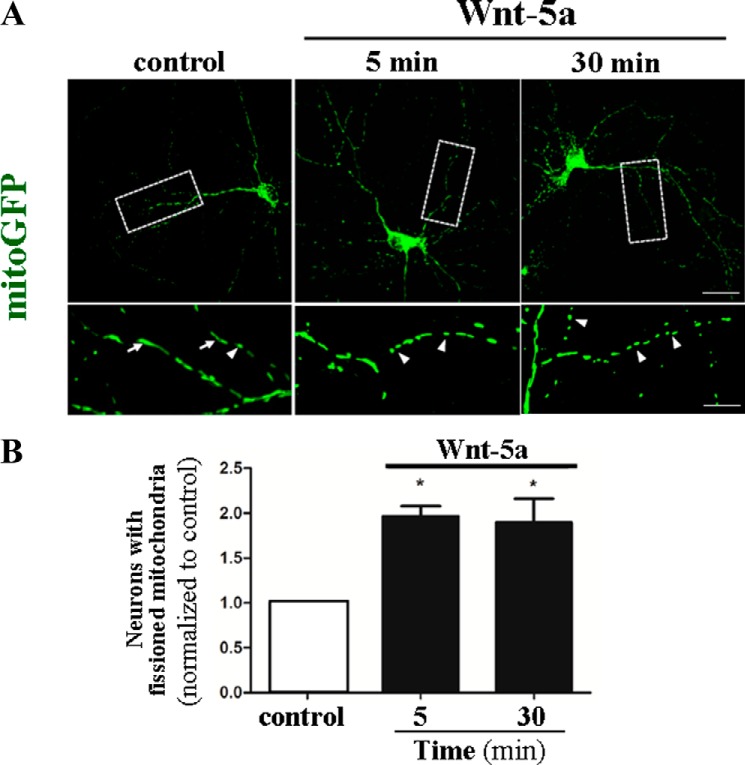
Mitochondria form a dynamic interconnected network, integrated with other cellular compartments, and under normal physiological conditions, the mitochondria perform a diverse range of functions, including the production of ATP, ROS, and lipid biosynthesis, and play a central role in metal metabolism and regulation of intracellular calcium homeostasis (36). The regulation of mitochondrial dynamics is vital in neurons, which includes the transport and localization of mitochondria where it is required to generate high levels of energy. We performed a time course experiment to evaluate whether Wnt-5a modulates mitochondrial dynamics in cultured hippocampal neurons. Neurons were transfected with mitoGFP and on 15DIV were stimulated with Wnt-5a (Fig. 1). Results indicated that around 30% of control neurons have small mitochondria at the beginning of the experiments (Fig. 1A, white arrow). Then cultured neurons were treated with Wnt-5a for several time intervals (5–30 min), and under these conditions the Wnt ligand stimulates mitochondrial division. More specifically, the number of hippocampal neurons, whose population of mitochondria had a length ranging from 0.21 to 0.54 μm, was higher than the corresponding population observed in control neurons (Fig. 1A, white arrowhead), after Wnt-5a treatment for 5 and 30 min (Fig. 1, white arrowhead). In Wnt-5a treated neurons, we observed a 2-fold increase in the number of hippocampal neurons containing small mitochondria compared with the control (Fig. 1B), and this increase was maintained until 30 min of stimulation. The morphological distribution of the increased number of small mitochondria determined after 30 min of Wnt ligand incubation suggests that these mitochondria are preferentially concentrated in the thinner dendritic processes of neurons (Fig. 1A, compare with the inset). Images obtained from control and Wnt-5a-treated neurons indicates that the transfection procedures alone did not affect the integrity or viability of the neurons and mitochondria, confirming that the effects of induction of mitochondrial fission observed at different times are due to the specific action of Wnt-5a.
FIGURE 1.
Wnt-5a ligand activity increases the population of hippocampal neurons, which possess mitochondria in dynamic process. Hippocampal neurons were transfected with mito-GFP (green) and stimulated with Wnt-5a at 15 DIV at 37 °C. A, micrographs show a representative control neuron with a magnification of mitochondria from dendritic processes. Neurons were treated with Wnt-5a ligand for 5 and 30 min, and the inset labels dendritic processes where mitochondria show normal morphology (white arrows) and fission mitochondria (white arrowheads). B, quantification of baseline population of neurons showing small mitochondria (0.21 to 0.54 μm) as an indicator of mitochondrial dynamics compared with neurons treated with Wnt-5a ligand at 5 and 30 min. The data suggest that Wnt-5a induces an increase in the number of neurons with a higher proportion of small mitochondria distributed preferentially in thinner dendritic processes (zoom of micrographs). Results are the mean ± S.E., n = 3 experiments, one-way ANOVA test, post hoc Bonferroni's test; *, p < 0.05.
Wnt-5a Induces Morphological Changes Related to the Fission and Fusion Process in Hippocampal Neurons
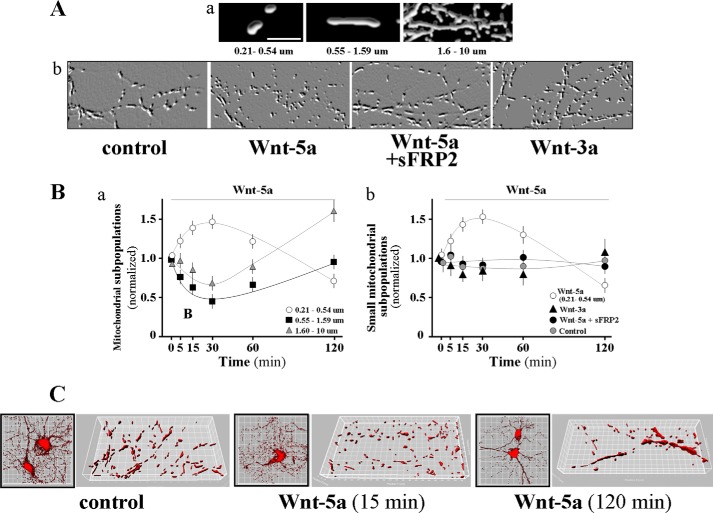
To study the change in mitochondrial dynamics in the population of neurons analyzed, we stimulated primary neuronal cultures with a canonical ligand Wnt-3a and a noncanonical ligand as Wnt-5a, and we then quantified the length and morphology of the mitochondria stained with Mitotracker and photographed in a confocal fluorescence microscope in a time-dependent procedure (see “Experimental Procedures”). Mitochondria showed morphological changes related to the fission and fusion process and were classified as small (0.21–0.54 μm), normal (0.55–1.59 μm), and large (1.6–10 μm) (Fig. 2A, panel a). Mitochondrial dynamics were specific for the Wnt-5a ligand. When we used sFRP2, a soluble scavenger, for this ligand (37), the effect was not observed. Moreover, the canonical ligand Wnt-3a did not show changes in the morphology of mitochondria (Fig. 2A, panel b). In particular, the subpopulation of mitochondria with normal length rapidly decreased at 30 min after stimulus and then recovered at 120 min (Fig. 2B, panel a, black square). On the contrary, the small subpopulation of mitochondria increased rapidly in number 30 min after treatment and began to decrease at 60 min after stimulus (Fig. 2B, panel a, white circle). Large mitochondria decreased after 30 min, and this type of mitochondria reached the maximum at 120 min (Fig. 2B, panel a, gray triangle). However, hippocampal neurons cultured under the same conditions were stimulated with a canonical Wnt-3a ligand that activated the Wnt/β-catenin pathway, and no significant change was observed in mitochondrial morphology as compared with small mitochondria stimulated with Wnt-5a (Fig. 2B, panel b, Wnt-3a, black triangle versus Wnt-5a, white circle). We also preincubated the cultured neurons with sFRP2 (37). We found that the effect of Wnt-5a on mitochondrial dynamics under these conditions was abolished (Fig. 2B, panel b, black circle). Our mitochondrial network images obtained with super-resolution confocal microscopy from 15 DIV control show a normal population of mitochondria (Fig. 2C, control neurons). 15 min after Wnt-5a treatment, the morphology of mitochondria was small and rounded (Fig. 2C); however, after 120 min of Wnt-5a stimulation, mitochondria recovered their normal morphology, and fused mitochondria are also observed (Fig. 2C). These data suggest that Wnt-5a produces a balance of opposing events, to maintain the overall morphology and metabolic stability of neuronal mitochondria.
FIGURE 2.
Wnt-5a signaling induces mitochondrial dynamics in hippocampal neurons. A, hippocampal neuron cultures from 15 DIV were labeled with Mitotracker. Photographs show subpopulations of mitochondria analyzed as follows: small, 0.21–0.54 μm; medium; 0.55–1.59 μm; and large, 01.6–10 μm (panel a). Neurons were treated with different Wnt ligands and Wnt-5a plus sFRP2 at 37 °C, and representative micrograph shows the morphology of mitochondria under different stimulus (panel b). B, different subpopulations of mitochondria were quantified in neurons stimulated with Wnt-5a in time course experiments (small, 0.21–0.54 μm; white circle, medium; 0.55–1.59 μm, black square; large, gray triangle) (panel a). Then the hippocampal neurons were stimulated with Wnt-5a ligand (white circle), Wnt-3a (black triangle), Wnt-5a plus sFRP2 (black circle), and control hippocampal neurons (gray circle), and small mitochondria were analyzed and plotted (panel b). C, hippocampal neurons stimulated with Wnt-5a ligand for 15 and 120 min, three-dimensional iso-surface images of mitochondria were generated with the Imaris software, using a 55% threshold level and 10 serial images of 0.1 μm, the reconstructed images show the control neurons and neurons treated with Wnt-5a. The analysis was performed by ANOVA. Basal condition was established as the mean of T0 obtained for each condition with Bonferroni's test. Results are the mean ± S.E. n = 4 to quantify mitochondrial subpopulations. One-way ANOVA test, post hoc Bonferroni's test; *, p < 0.05.
Wnt-5a Induces Mitochondrial Dynamics in Hippocampal Neurons without Altering the Membrane Potential
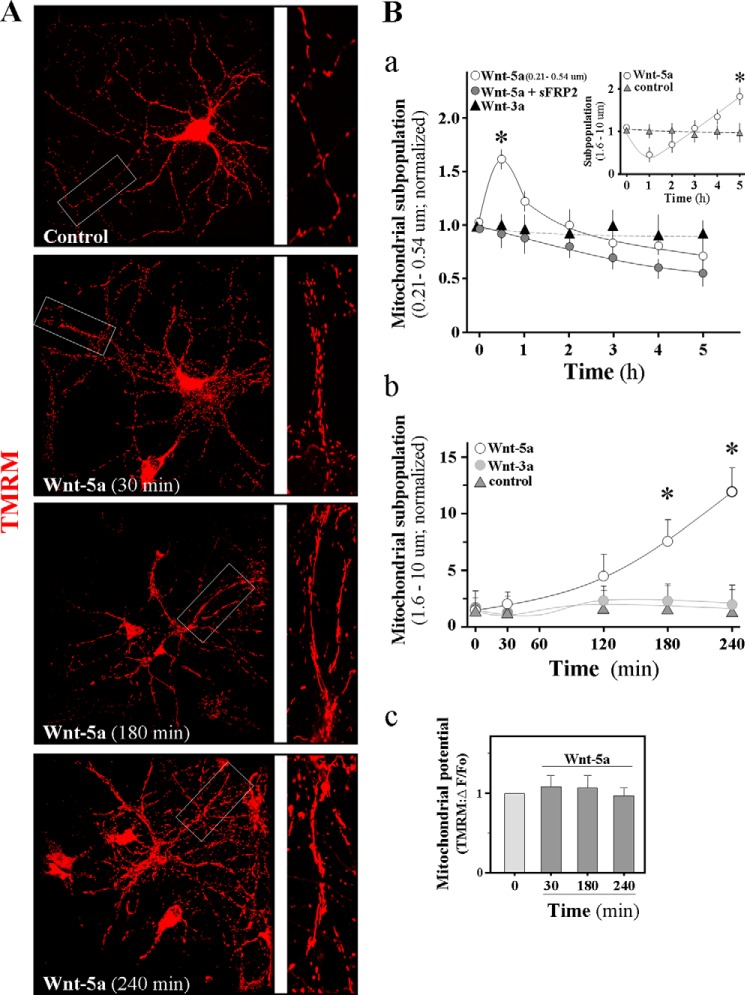
Mitochondrial distribution and dynamics are influenced by physical connections between the mitochondrial outer membrane and intracellular membranes (plasma membrane, peroxisomes, auto-phagosome, lysosomes, and mitochondrion-associated membranes; which is necessary for the ER-mediated sequestration and handling of cytosolic calcium by mitochondria (12, 17). Mitochondrial structure and function are regulated by division and fusion events, which are influenced by the potential of the mitochondrial membranes. The following experiments evaluate the membrane potential of mitochondria and calcium regulation by the Wnt-5a on mitochondrial dynamics. In that sense, mitochondrial dynamic studies were performed with an indicator of mitochondrial membrane potential such as the TMRM probe (Fig. 3). It is also apparent that Wnt-5a treatment determines clear morphological alterations in the mitochondria; in fact, our results indicated that mitochondria suffer morphological alterations after Wnt-5a treatment, which at the beginning corresponds to mitochondrial fission and later on to fusion processes. Control mitochondria show normal morphology (Fig. 3A, control neurons and inset). Afterward, this process continues for 30 min when the mitochondria enter the fission process (Fig. 3A, 30 min and inset show small mitochondria); many mitochondria recover their normal morphology at 180 min of treatment, whereas others begin the process of fusion (Fig. 3A, 180 min and inset), and the process continues until 240 min (Fig. 3A and inset show large mitochondria). Quantification of this process shows a rapid increase in the subpopulation of small mitochondria under Wnt-5a stimulus (Fig. 3B, panel a, white circle). This process was abolished with sFRP2 (Fig. 3B, panel a, black circle), and canonical ligands such as Wnt-3 show no activity on mitochondrial dynamics (Fig. 3B, panel a, black triangle). The number of large mitochondria increases after 4 h of incubation with the Wnt ligand (Fig. 3B, panel a, inset). Then, we quantify the large subpopulation of mitochondria; this population shows a significant increase in the number of fused mitochondria during stimulation with Wnt-5a, up to 240 min of stimulation, and this process was not observed in neurons incubated with the Wnt-3a ligand (Fig. 3B, panel b). All the processes analyzed with TMRM dye show normal membrane potential throughout the experiment time (Fig. 3B, panel c). We conclude that Wnt-5a signaling modulates mitochondrial dynamics, affecting the normal organelle processes by mechanisms that probably involve changes in calcium (see below). Our data provide the first in vivo evidence that the mitochondrial fission-fusion process occurs without altering the mitochondrial membrane potential during the whole experimentation time, and it is stimulated by the presence of Wnt-5a.
FIGURE 3.
Wnt-5a induces mitochondrial fission-fusion in hippocampal neurons without changes in mitochondrial membrane potential. A, primary cultures of rat embryo hippocampal neurons 15 DIV were labeled with TMRM dye to detect mitochondrial membrane potential and treated for 4 h with Wnt-5a; the representative micrographs are shown in the inset to show morphology of mitochondria. B, quantification of small mitochondria was made in presence of Wnt-5a (white circle), Wnt-5a plus sFRP (black circle), or Wnt-3a (black triangle) in time course experiments. Inset shows the large population of mitochondria that appear over time under the stimulus of Wnt-5a (white circle) as compared with the control neurons (gray triangle) (panel a). The graph shows the large mitochondrial population under the activity of Wnt-5a in a time course experiment (white circle) compared with Wnt-3a (light gray circle) and control neurons (gray triangle) (panel b). Mitochondrial micrographs were made in the Carl Zeiss LSM confocal microscope. Quantification of mitochondrial membrane potential was measured with TMRM dye under Wnt-5a stimulus until 240 min at 37 °C (panel c). Results are representative for n = 3–4 experiments. One-way ANOVA test, post hoc Bonferroni's test; *, p < 0.05.
Wnt-5a Activates Wnt/Ca2+ Signaling Pathway in Hippocampal Neurons Inducing an Increase in Intracellular and Mitochondrial Ca2+ Levels
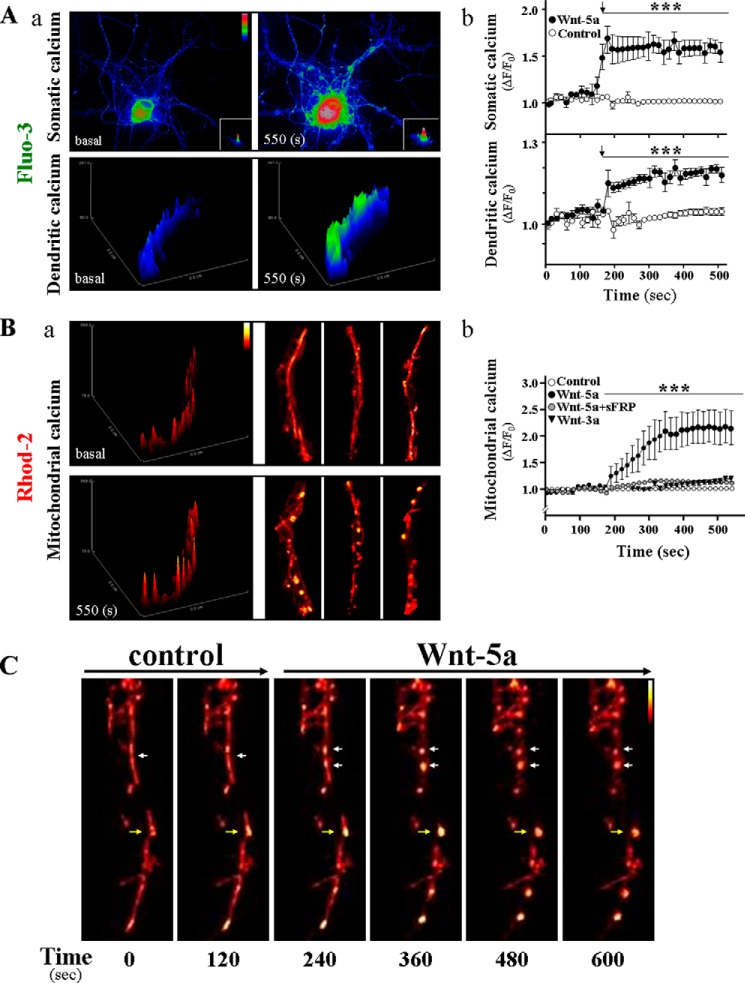
Wnt-5a is able to activate noncanonical Wnt signaling pathways in hippocampal neurons (26). It has been described that Wnt-5a induced an increase in intracellular Ca2+ levels, and whether this ligand induces Ca2+ changes in cellular organelles, such as the mitochondria, which regulates Ca2+ levels inside the cell, remains unclear (28). Therefore, we evaluated cytosolic Ca2+ levels using the Fluo3-AM, a Ca2+ probe (Fig. 4A). Addition of Wnt-5a triggered a significant and rapid increase in cytosolic Ca2+ levels, 2-fold above the basal line at 195 s of incubation with the ligand in the soma of neurons (Fig 4A, panels a and b, top graph), and 20% over the control at the level of the neurites (Fig. 4A, panels a and b, bottom graph). To evaluate whether these changes in Ca2+ levels induced by Wnt-5a also alter mitochondrial Ca2+ levels, we loaded neurons with Rhod2-AM, a cell permeant labeled Ca2+ indicator (Fig. 4B, panel a, shows neurites at basal time and after 550 s of stimulus). After Wnt-5a stimulation, neuronal neurites showed a significant increase of around 2.5-fold over the control in mitochondrial Ca2+ levels at 300 s of stimulus (Fig. 4B, panel b). Wnt-5a activity on neuritic calcium was also evaluated in the presence of sFRP2, and this treatment abolished the Wnt-5a-dependent effects over mitochondrial calcium levels (Fig 4B, panels b and c, gray circle). To further confirm whether mitochondrial Ca2+ changes induced by Wnt-5a are related to the fission events described above for this ligand, we evaluated mitochondrial fission in live cell experiments in hippocampal neurons loaded with Rhod2-AM. After a basal line (120 s), where long mitochondria were observed in binary color (Fig. 4C, one white arrow and yellow arrow), the neurons were stimulated with Wnt-5a, and fission events were observed in several points along neurites (see 240–360-480 up to 600 s) (Fig. 4C, see arrows). Interestingly, these mitochondrial morphological changes (Fig. 4C, white arrow) were associated with an increase in Ca2+ levels as observed in the yellow spots (imaging in pseudo color) (Fig. 4C, yellow arrow), where mitochondria demonstrated fission processes in response to Wnt-5a. These changes in Ca2+ homeostasis suggest that Ca2+ is likely to be functionally important in the control of mitochondrial dynamics specifically in hippocampal neurons in a Wnt-5a-dependent manner.
FIGURE 4.
Live cell imaging measurement of total intracellular and mitochondrial Ca2+ levels in hippocampal neurons in response to Wnt-5a. A, representative pseudo-color images of total calcium levels measured at the soma (upper micrograph) and dendrites (lower micrograph) of hippocampal neurons loaded with the Fluo-3 probe, under time-dependent treatment with Wnt-5a (panel a). Graphs show the intracellular Ca2+ changes induced by Wnt-5a in neuronal soma and dendrites (panel b). B, hippocampal neurons were loaded with Rhod-2 probe to measure the levels of mitochondrial Ca2+ in dendritic zone (panel a). Quantification represents an average of total regions of interest of individual dendritic network for mitochondrial calcium (panel b). C, pseudo-color images obtained from time-lapse experiments in hippocampal neurons loaded with Rhod2-AM. Representative neurite shows mitochondrial dynamics, a long time after Wnt-5a treatment. White arrows indicate those dividing mitochondria under Wnt-5a stimulus; yellow arrows show mitochondria that present calcium overload and fission events at the same time, in response to Wnt-5a. Results are the mean ± S.E. n = 3 independent experiments, one-way ANOVA test, post hoc Bonferroni; ***, p < 0.001.
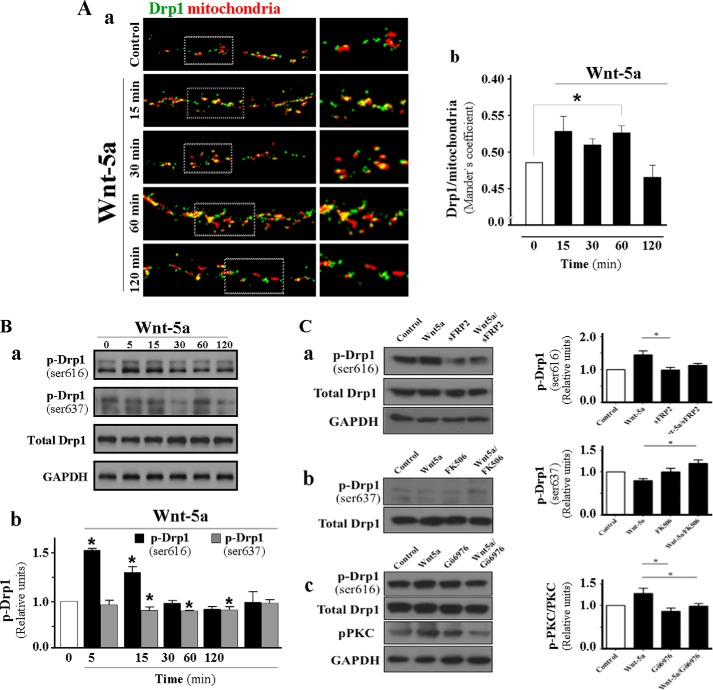
Wnt-5a Induces Drp1 Translocation to the Mitochondria in Hippocampal Neurons
Mammalian dynamin-related protein 1 (Drp1) is a GTPase that plays a key role in regulating mitochondrial fission. This protein is regulated by post-translational modifications, such as phosphorylation on Ser-616 and Ser-637. These modifications lead to the activation of Drp1, and its translocation from the cytosol to the mitochondria, to induce fission events (38, 39, 42, 58). The phosphorylation on Ser-637 inhibits Drp1 GTPase activity and mitochondrial fission (6, 68), and dephosphorylation of this residue by calcineurin promotes Drp1 translocation to the mitochondria and mitochondrial fission (13). To evaluate whether Wnt-5a modulates Drp1 activities, we analyzed both phosphorylation and translocation to the mitochondria in response to Wnt-5a treatment. Hippocampal neurons were stained with MitoTracker-Orange to detect mitochondria and immunostained with a specific antibody against total Drp1. The resulting micrographs show representative neurites in a time course of Drp1 mitochondria association until 120 min of stimulus (Fig. 5A, panel a, magnification in square). The Drp1 overlapping with mitochondria was analyzed using Mander's coefficient (40, 41) and indicates that Wnt-5a induces a significant increase in Drp1 translocation at 15 min and until 60 min (Fig. 5A, panel b). This effect was correlated with a 50% increase in the phosphorylation of Ser-616 in Drp1 in response to Wnt-5a treatment neurons at 5–15 min compared with the control (Fig. 5B, panel a, black bar). When we evaluate the state of phosphorylation of Ser-637 in Drp1, this phosphorylation significantly decreased, already 20–25%, after 15 min of stimulation with Wnt-5a and remained low until 60 min after stimulation with Wnt-5a (Fig. 5B, panel b, gray bar). Mitochondria are highly dynamic structures, able to change their morphology in response to different stimulus, such as Ca2+ (35, 42). Our results indicate that Wnt-5a stimulates Wnt/Ca2+ signaling, events correlated with phosphorylation and dephosphorylation on Drp1 to induce or initiate the phenomenon of mitochondrial dynamics in mature hippocampal neurons.
FIGURE 5.
Wnt-5a induces changes in the phosphorylation state of Drp1 protein in hippocampal neurons. A, confocal images show representative neurites from 15 DIV hippocampal neurons loaded with Mitotracker Orange (red) and that co-localized with Drp1 protein (green) from time course treatment with Wnt-5a (panel a), measured by Mander's coefficient (panel b). B, hippocampal neurons were treated until 120 min with Wnt-5a, and total p-Drp levels in Ser-616 (black bar) and Ser-637(gray bar) epitopes were detected by Western blot (panel a and graph in panel b). C, hippocampal neurons were co-incubated with Wnt-5a and sFRP2 by 30 min (panel a) or preincubated with the FK506 calcineurin inhibitors (panel b) and PKC inhibitors Gö6976 (panel c) by 30 min, and then the neurons were stimulated with Wnt-5a ligand by others for 30 min. The total and p-Drp1 levels (Ser-616 and Ser-637) and pPKC levels were detected by Western blot. The results were normalized to total Drp1 and loading control GAPDH. Representative blots show p-Drp1 (Ser-616) followed by Wnt-5a stimulation, p-Drp-1 (Ser-637) in hippocampal neurons stimulated with Wnt-5a, p-Drp1 (Ser-616) followed by Wnt-5a plus sFRP2 treatment, p-Drp1 (Ser-637) followed by Wnt-5a plus FK506 treatment, and p-Drp1 (Ser-616) followed by Wnt-5a plus Gö6976 treatment are shown. Results are the mean ± S.E. n = 3 independent experiments, analyzed with Student's t test; *, p < 0.05.
Activation of the Wnt/Ca2+ pathway leads to activation of various calcium-dependent enzymes as calcium/calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), and calcineurin. Previously in our laboratory, we have shown that Wnt-5a through CaMKII activation was able to induce the clustering and insertion of GABAA receptor in the post-synaptic region (27). Recently, it was shown that activation of the noncanonical Wnt/PKC pathway induces the formation of aggregates of mitochondria in HEK cells, but it is unknown whether this pathway can regulate the activation of Drp1 through its phosphorylation. To test whether the effects observed with Wnt-5a are specific and carried out through activation of these enzymes, hippocampal neurons were incubated in the presence of sFRP2 (Fig. 5C, panel a), the calcineurin inhibitor FK506 (Fig 5C, panel b), or the specific inhibitor for PKC-dependent Ca2+, Gö6976 (Fig. 5C, panel c); subsequently, we evaluated the phosphorylation levels of Ser-616 and Ser-637 of Drp1. Hippocampal neurons were incubated with Wnt-5a previously co-incubated for 30 min with sFRP2 (1 μg/ml). The levels of Ser-616 of Drp1 were significantly reduced in the presence of sFRP2 and reached basal levels when they were incubated with Wnt-5a/sFRP2, indicating that the increased phosphorylation of Ser-616 Drp1 is a specific effect of the ligand Wnt-5a (Fig. 5C, panel a, see blot and graph). Previously, we determined that Wnt-5a ligand generated increased nitric oxide (NO) levels in hippocampal neurons (43), which were decreased to below basal levels of the control in the presence of calcineurin inhibitor FK506 (49); this phosphatase activates neuronal NOS through its dephosphorylation and induces the production of NO. To assess whether calcineurin activates Drp1 through its dephosphorylation at Ser-637, we analyzed the levels of phosphorylation of this residue in the presence of FK506. Neurons were preincubated for 30 min with 2.5 μm FK506 and then stimulated with Wnt-5a. The levels of Drp1 Ser-637 were significantly higher than those observed in the presence of the Wnt-5a ligand, indicating that activation of Drp1 at Ser-637 would be mediated by activation of calcineurin as one of the effects of Wnt-5a (Fig. 5C, panel b, see blot and graph). Subsequently, we assessed whether Ser-616 Drp1 activation would be regulated by the PKC signaling pathway; therefore, neurons were preincubated for 30 min with the specific inhibitor of the PKC-dependent Ca2+, 200 nm Gö6976, and then were stimulated with Wnt-5a. The data indicate that in the presence of Gö6976, the levels of Drp1 Ser-616 were significantly decreased to baseline control levels, indicating that activation of Drp1 Ser-616 was induced by Wnt-5a, and it would be regulated by the activation of the PKC signaling pathway by Wnt-5a (Fig. 5B, panel c: see blot and graph).
Wnt-5a Signaling Increases Mitochondrial Fission Events in the Hippocampal CA1 Region
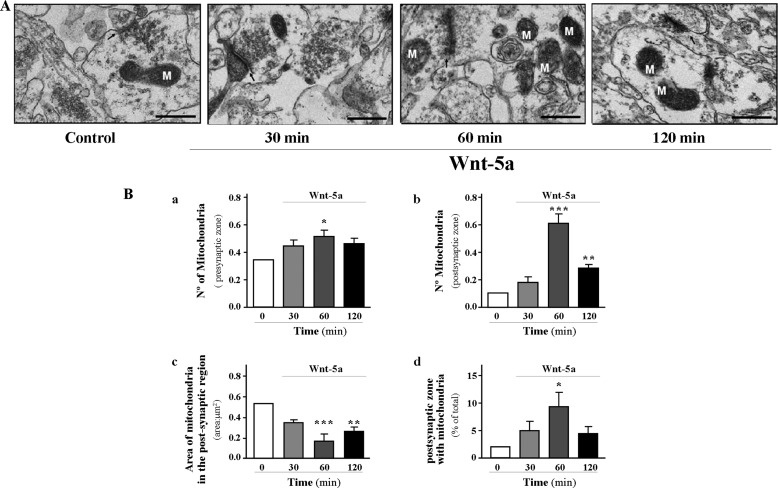
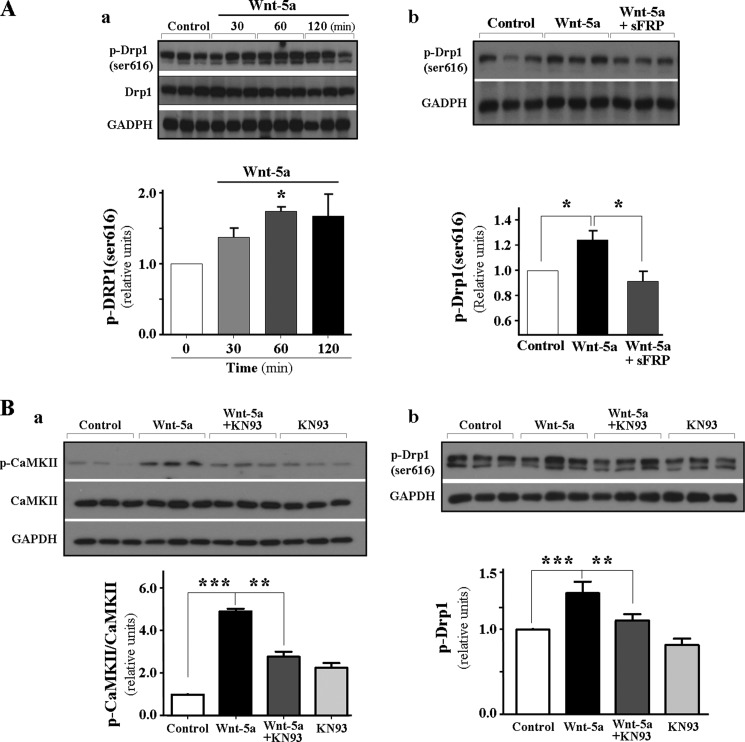
The mitochondria play an important role in maintaining synaptic function through their ability to regulate synaptic Ca2+ levels and the energy demand required for this process (44, 45). For this reason, mitochondrial position at the synapse and its trafficking to both pre- and postsynaptic regions are essential for its optimal function at the synapses (46, 47). To determine whether Wnt-5a-dependent fission events observed in the mitochondria are related to its ability to position themselves at the synapse, we performed electron microscopy analysis of the hippocampal CA1 region of the rat brains to detect whether morphological changes agree with fission events in a highly metabolic area and examined the presence and number of mitochondria at the pre- and postsynaptic regions (Fig. 6A, representative micrographs of CA1 region). Time course stimulation of hippocampal slices of the CA1 region with Wnt-5a induced a significant increase (∼45%) in the number of mitochondria present in the presynaptic terminal at 60 min of treatment (Fig. 6B, panel a). In addition, the number of mitochondria at the post-synaptic region presents a 6-fold increase at 60 min and a 3-fold increase at 120 min of incubation as compared with slide control (Fig. 6B, panel b). These changes are correlated with a decrease in mitochondrial area at these time points with Wnt-5a treatment in the postsynaptic terminal and suggest that the change in mitochondrial morphology could correspond to the fission of mitochondria (Fig 6B, panel c). Then, we quantified the number of postsynaptic terminals having mitochondria after stimulation with Wnt-5a, and this percentage of terminals increases to 10% at 60 min when compared with the control without stimulus (Fig. 6B, panel d). We also evaluated in these hippocampal slices, stimulated with Wnt-5a, the phosphorylation of Ser-616 in Drp1 protein (Fig. 7) using Western blotting. We observed a significant increase in this modification at 60 min when slices were incubated with Wnt-5a, indicating that, at this time point of treatment, mitochondria are going through fission events (Fig. 7A, panel a). At the same time, preincubation with sFRP2 abolished the Wnt-5a effect over mitochondrial fission, because p-Drp1(Ser-616) significantly decreases to control levels following co-incubation of Wnt-5a plus sFRP2 (Fig. 7A, panel b). The noncanonical Wnt/Ca2+ signaling cascade induces the release of Ca2+ from the endoplasmic reticulum, which then induces the activation of CaMKII (Fig. 7B, panel a) (49), which could be responsible for the phosphorylation of Drp protein, observed after stimulation with Wnt-5a. Results obtained using a CaMKII inhibitor, such as KN93, determined a decreased in Drp1 Ser-616 phosphorylation with respect to control levels (Fig. 7B, panel b). Under these experimental conditions, we did not observe neuronal cell death in the treated hippocampal slices, evaluated using the Hoechst staining (data not shown). It is well known that mitochondria have differential distributions in pre- and post-synaptic regions. In fact, mitochondria selectively reside at the presynaptic region for long time periods rather than at the postsynaptic region (46). Because most of the synaptic effects described for Wnt-5a are at the postsynaptic level (26, 28), all the experiments examined show that the number of postsynaptic regions increase significantly the mitochondria numbers (5-fold over control).
FIGURE 6.
Wnt-5a induces mitochondrial dynamics on CA1 region of the hippocampus of rat brain. A, micrographs from electron microscopy show representative slices of CA1 region of hippocampus treated with Wnt-5a in a time course experiment (M, mitochondria) (panel a). B, number of mitochondria in pre- (panel b) and postsynaptic terminals (panel c), respectively, was quantified in response to Wnt-5a treatment; the area of mitochondria from the postsynaptic terminals was measured (panel d), and the percentage of post-synaptic zones containing mitochondria after Wnt-5a stimulus was quantified and compared with baseline (panel e). Quantitative analysis was performed with n = 3, one-way ANOVA, post hoc Bonferroni; *, p < 0.05; **, p < 0.005; ***, < 0.0005. Bar, 500 nm.
FIGURE 7.
Noncanonical Wnt/Ca2+ signaling cascade induces the activation of CaMKII. A, CA1 hippocampal slices were treated for until 120 min with Wnt-5a, and p-Drp1(Ser-616) levels were detected by Western blot and normalized to total Drp1 and to the loading control GAPDH (panel a). Hippocampal slices were incubated with Wnt-5a and co-incubated with Wnt-5a plus 250 nm sFRP2 for 60 min, as a control of the specificity of Wnt-5a effect over p-Drp1 levels (panel b). B, pCaMKII was evaluated under Wnt-5a stimulus, and the inhibitor KN93 was used (panel a). Under the same condition, the phosphorylation of Drp1-Ser-616 was evaluated (panel b). The experiments were made in triplicate. *, p < 0.05; **, p < 0.005; ***, < 0.0005.
DISCUSSION
Wnt signaling is highly conserved among species and regulates numerous cellular processes, including cell proliferation, differentiation, cell fate decisions, and migration, all of which play crucial roles during embryonic development and in the adult nervous system (1). Wnt signaling also regulates the formation and function of neuronal circuits by controlling neuronal differentiation, axon outgrowth and guidance, dendrite development, synaptic function, and neuronal plasticity and may be reactivated or suppressed in aging or disease (48). In addition, deregulation of Wnt signaling has been associated with the pathogenesis of several neurodegenerative diseases, including Alzheimer disease (49, 50). Here, we describe that a noncanonical Wnt pathway activated by Wnt-5a controls mitochondrial dynamics in cultured hippocampal neurons and the CA1 region in the adult rat brain. The first studies were directed to determine the population of neurons exhibiting a change in the dynamics of mitochondria, positioning or movement of mitochondria within the different compartments of the neurons. These changes are essential to preserve the proper morphology and functioning of neurons. Mitochondria recruitment is critical for subcellular compartment exchange, control of shape, number, and movements of mitochondria to delivery from the site of biogenesis to the sites where energy is in high demand (11). In the brain, neuronal mitochondria supply copious amounts of ATP and modulate Ca2+ homeostasis, necessary for controlling neurotransmitter release, neurogenesis, and neuronal plasticity. In addition, mitochondria provide the tricarboxylic acid intermediates that serve as an intermediate for the synthesis of GABA and glutamate neurotransmitters (51). Neurons are sensitive to changes in mitochondrial dynamics, due to higher energy demand required to maintain optimal synaptic neurotransmission by generating adequate amounts of ATP and buffering Ca2+ (52).
Mitochondria are dynamic organelles specially in neurons that are continuously moving, fusing, and dividing in response cellular demands, and evidence suggests that the balance between the rates of fission and fusion contributes to mitochondrial morphology, quality control in mDNA content, influence in neuronal survival, response to stress, and death while maintaining calcium mitochondrial homeostasis (52, 53). Mitochondrial shape and function and cellular homeostasis is regulated by the balance between fission and fusion events in the neurons (54). The complexity of cellular signaling pathways involved in this process is under constant study. Several signaling pathways that regulate intracellular calcium levels are involved in regulating the mobility of different pools of mitochondria; the motility of mitochondria is mediated by kinesin in a calcium-dependent manner (55), and mitochondrial Rho GTPase (Miro1) is a calcium sensor for localization of mitochondria at synapses (56, 57), including its relationship with PINK1 and Parkin to arrest mitochondrial motility (58).
This work shows that the noncanonical Wnt/Ca2+ signaling pathway through Wnt-5a induces an increase in mitochondrial dynamics in both fission and fusion processes and motility especially at the post-synaptic region. These changes were correlated with an increase in Ca2+ levels in those mitochondria that present fission events, without producing deleterious effects or apoptosis in neurons, as had been previously described for this process (59). The increase in mitochondrial Ca2+ induced by Wnt-5a and the consequent fission of those mitochondria could be physiologically relevant in neurons, because it has been described that the traffic and movement of mitochondria are modulated by Ca2+-mediated changes inside neurons, through reduction of the mobility of mitochondria that produce a redistribution of this organelle to the synapses (56). Although most measurements were conducted in cultured cells, our data also show that mitochondrial size is relevant for this positioning especially at the post-synaptic region, as was shown in experiments in the CA1 region of the hippocampus treated with Wnt-5a and analyzed with electron microscopy, where we showed an increase in the number of mitochondria into dendritic spines, indicating that the pathway also functions in vivo. These morphological changes of mitochondria, related to fission processes, have been attributed to the phosphorylation of the fission protein Drp1 by CaMKIα (42, 61). Interestingly, there are several downstream proteins from the Wnt-5a signaling that could also be mediating the fission events through phosphorylation of Drp1, such as, CaMKII, protein kinase C (PKC), and calcineurin (61, 62).
The mammalian Drp1 protein plays a key role in regulating mitochondrial fission and shifts the balance between opposing processes that occur rapidly, indicating that modifications of these proteins may regulate mitochondrial membrane dynamics. Drp1 undergoes a number of steps to mediate mitochondrial fission, including translocation from the cytoplasm to the mitochondrial outer membrane, higher -order assembly into spirals, GTP hydrolysis associated with a conformational change and membrane deformation, and ultimately disassembly (63, 64). All these features suggested that an increased mitochondrial fission rate is required for the specific function of synapses or neuronal plasticity. Determination of protein levels of Drp1in mature neurons compared with immature neurons indicated an increase in the levels of the fission protein (46); moreover, overexpression of Drp1 produces an increase of dendritic spines (18). A molecular link between Drp1 and cell death was first suggested by the fact that Drp1-containing fission foci also contain pro-apoptotic members of the Bcl-2 family, such as BAK and BAX protein (64). In fact, mitochondrial fission is necessary for the execution of apoptosis. Indeed, suppression of Drp1 activity reduces mitochondrial fission and inhibits mitochondrial translocation of BAX, which is essential for the execution of apoptosis. Conversely, the priming of mitochondrial fragmentation by Drp1 overexpression augments the extent of apoptosis (66). However, dephosphorylation of Drp1 in Ser-637 has been shown to promote translocation of the cytosolic Drp1 to mitochondria and the concurrent fission events (62). In our experiments, mitochondrial dynamics begin with a fission process, produced by Drp1 phosphorylation in Ser-616, followed by Drp1 phosphorylation at Ser-637, which generates changes in mitochondrial morphology, to finally activate mitochondrial fusion under Wnt-5a stimulation. Throughout this dynamic process, neurons did not present apoptotic nuclei during the exposure time with the Wnt-5a ligand.
Over the last few years, compelling evidence has accumulated highlighting the relevance of the Wnt/Ca2+ pathway in several neuronal processes (2). In the Wnt/Ca2+ pathway, Wnt-5a can activate both PKC and CaMKII through the release of intracellular Ca2+ from internal stores (11, 28). Considering that the endoplasmic reticulum is the main cellular Ca2+ reserve, the observed increase in Ca2+ release triggered in the cell soma and dendrites suggests that Ca2+ certainly comes from the endoplasmic reticulum. In fact, it must be considered that Wnt-5a induces endoplasmic reticulum-Ca2+ efflux through the inositol triphosphate receptor and ryanodine receptors. The abolishment of mitochondrial dynamics achieved by the use of endoplasmic reticulum and Ca2+-specific inhibitors, directed against inositol triphosphate receptor and ryanodine receptor in the presence of Wnt-5a (67), offers further support to the notion that the endoplasmic reticulum-related Ca2+ release is dependent on downstream Wnt-5a/Ca2+ signaling. Our result through live cell imaging indicates that the division and further fusion of mitochondria along neuronal projections is strictly associated with the endoplasmic reticulum in the presence of Wnt-5a. These finding are consistent with the idea that mitochondrial fission-fusion is an endoplasmic reticulum-regulated mechanism, which could be modulated through extracellular signals such as the Wnt-5a ligand.
Neurons are highly dependent on mitochondrial function for their normal energy supply. The migration and positioning of mitochondria in neurons facilitates energy distribution throughout neuronal projections and to sites of high energy demand such as the synapses (36, 68). This is crucial to maintain optimal cellular bioenergetics. During development, the formation of dendritic and axon branching provides basal trafficking into dendrites and axons independent of synaptic activity. However, the number of mitochondria present in the dendrites is increased by synaptic activity (36, 60, 65). Our results indicate that Wnt-5a can induce and/or regulate the remodeling of the postsynaptic region, as shown by our electron microscopy analysis. Our previous study indicates that Wnt-5a induces and increases the number of synaptic terminals, specifically at the postsynaptic region (26, 28). Therefore, our results provide new insights into Wnt signaling mechanisms in mature neurons and are the first to identify an additional pathway involved in the movements of mitochondria within the neurons and mitochondrial dynamics process. Future work in this field will eventually allow us to develop novel therapeutic agents that preserve mitochondrial dynamic function and may be useful for the treatment of neurodegenerative disease in which mitochondrial function is severely compromised.
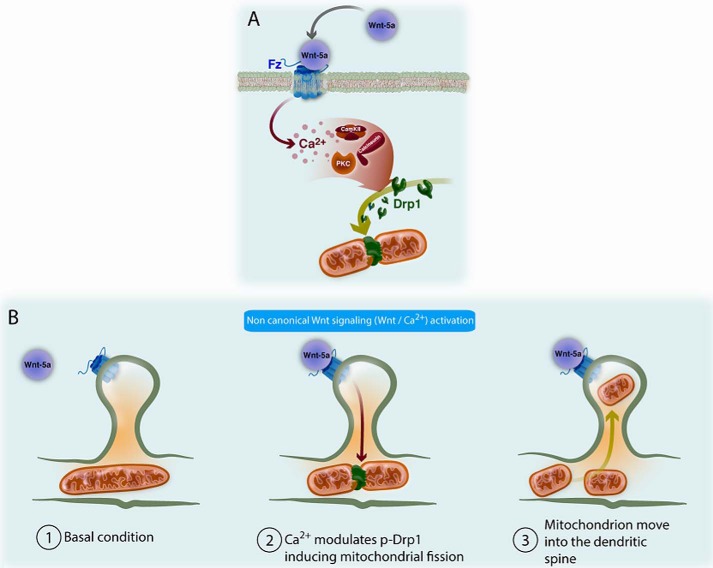
In conclusion, our studies indicate that Wnt-5a induces a significant increase of the numbers of mitochondria in the postsynaptic region. These results indicate that Wnt/Ca2+ signaling activation through Wnt-5a induces an increase in total calcium into the neuron followed by an increase in mitochondrial calcium, with a concomitant phosphorylation and dephosphorylation of Drp1 protein, which initiates the movement of fission mitochondria toward the postsynaptic region (Fig. 8).
FIGURE 8.
Wnt-5a ligand activates Wnt/Ca2+ pathway and induces mitochondrial dynamics in post-synaptic regions. The synaptic architecture of the post-synaptic region is important for synaptic transmission; remodeling modifications and signaling pathways are fundamental for synaptic transmission and plasticity, and therefore the synaptic processes require an amount of energy. A, activation of the noncanonical Wnt signaling pathway through Wnt-5a ligand results in the release of intracellular calcium with the consequent activation of kinases (CaMKII and PKC) and phosphatases (calcineurin), calcium-dependent, followed by the modulation (phosphorylation-dephosphorylation steps) of Drp-1 protein and its accumulation on mitochondria, initiating the processes of mitochondrial fission and mobilization to sites of energy demand. B, increased mitochondrial dynamics involve fission-fusion process and mobilization of mitochondria to the dendritic spines in the post-synaptic region. The normal function of mitochondria ensure adequate energy levels for different cell processes, such as postsynaptic transduction. Wnt-5a has proven to be able to activate the Wnt/Ca2+ pathway in the post-synaptic terminals of the CA1 region in rat hippocampus, and this activation appears now to be linked with an increased mitochondrial fission event plus the mobilization of small mitochondria to the required dendritic spine sites.
This work was supported in part by Grants PFB 12/2007 from the Basal Centre for Excellence in Science and Technology and FONDECYT 1120156 (to N. C. I.) and FONDECYT 11130529 (to C. S. A.).
- ER
- endoplasmic reticulum
- CaMKII
- Ca2+/calmodulin dependent kinase II
- DIV
- day in vitro
- ROS
- reactive oxygen species
- ANOVA
- analysis of variance
- TMRM
- tetramethylrhodamine methyl ester.
REFERENCES
- 1. Nusse R., Varmus H. (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 31, 2670–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inestrosa N. C., Arenas E. (2010) Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 11, 77–86 [DOI] [PubMed] [Google Scholar]
- 3. Budnik V., Salinas P. C. (2011) Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol. 21, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park M., Shen K. (2012) WNTs in synapse formation and neuronal circuitry. EMBO J. 31, 2697–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon M. D., Nusse R. (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 281, 22429–22433 [DOI] [PubMed] [Google Scholar]
- 6. Montcouquiol M., Crenshaw E. B., 3rd, Kelley M. W. (2006) Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29, 363–386 [DOI] [PubMed] [Google Scholar]
- 7. Simons M., Mlodzik M. (2008) Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 42, 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oliva C. A., Vargas J. Y., Inestrosa N. C. (2013) Wnt signaling: role in LTP, neural networks and memory. Ageing Res. Rev. 12, 786–800 [DOI] [PubMed] [Google Scholar]
- 9. Chen J., Park C. S., Tang S. J. (2006) Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J. Biol. Chem. 281, 11910–11916 [DOI] [PubMed] [Google Scholar]
- 10. Cerpa W., Gambrill A., Inestrosa N. C., Barria A. (2011) Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J. Neurosci. 31, 9466–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yi M., Weaver D., Hajnóczky G. (2004) Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H. J., Guay G., Pogan L., Sauvé R., Nabi I. R. (2000) Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J. Cell Biol. 150, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., Bernardi P., Scorrano L. (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. U.S.A. 105, 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Brito O. M., Scorrano L. (2010) An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29, 2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Brito O. M., Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 [DOI] [PubMed] [Google Scholar]
- 16. Friedman J. R., Webster B. M., Mastronarde D. N., Verhey K. J., Voeltz G. K. (2010) ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 190, 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman J. R., Lackner L. L., West M., DiBenedetto J. R., Nunnari J., Voeltz G. K. (2011) ER tubules mark sites of mitochondrial division. Science 334, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Z., Okamoto K., Hayashi Y., Sheng M. (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 [DOI] [PubMed] [Google Scholar]
- 19. Chada S. R., Hollenbeck P. J. (2003) Mitochondrial movement and positioning in axons: the role of growth factor signaling. J. Exp. Biol. 206, 1985–1992 [DOI] [PubMed] [Google Scholar]
- 20. Yoon J. C., Ng A., Kim B. H., Bianco A., Xavier R. J., Elledge S. J. (2010) Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 24, 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gurney A., Axelrod F., Bond C. J., Cain J., Chartier C., Donigan L., Fischer M., Chaudhari A., Ji M., Kapoun A. M., Lam A., Lazetic S., Ma S., Mitra S., Park I-K., Pickell K., Sato A., Satyal S., Stroud M., Tran H., Yen W.-C., Lewicki J., Hoey T. (2012) Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. U.S.A. 109, 11717–11722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holmström M. H., Iglesias-Gutierrez E., Zierath J. R., Garcia-Roves P. M. (2012) Tissue specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am. J. Physiol. Endocrinol. Metab. 302, E731–E739 [DOI] [PubMed] [Google Scholar]
- 23. Mori H., Prestwich T. C., Reid M. A., Longo K. A., Gerin I., Cawthorn W. P., Susulic V. S., Krishnan V., Greenfield A., Macdougald O. A. (2012) Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Invest. 122, 2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho D. H., Nakamura T., Lipton S. A. (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci. 67, 3435–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon Y., Galloway C. A., Jhun B. S., Yu T. (2011) Mitochondrial dynamics in diabetes. Antioxid. Redox Signal. 14, 439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farías G. G., Alfaro I. E., Cerpa W., Grabowski C. P., Godoy J. A., Bonansco C., Inestrosa N. C. (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J. Biol. Chem. 284, 15857–15866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cuitino L., Godoy J. A., Farías G. G., Couve A., Bonansco C., Fuenzalida M., Inestrosa N. C. (2010) Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J. Neurosci. 30, 8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varela-Nallar L., Alfaro I. E., Serrano F. G., Parodi J., Inestrosa N. C. (2010) Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc. Natl. Acad. Sci. U.S.A. 107, 21164–21169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inestrosa N. C., Godoy J. A., Quintanilla R. A., Koenig C. S., Bronfman M. (2005) Peroxisome proliferator-activated receptor γ is expressed in hippocampal neurons and its activation prevents β-amyloid neurodegeneration: role of Wnt signaling. Exp. Cell Res. 304, 91–104 [DOI] [PubMed] [Google Scholar]
- 30. Perkins E. M., McCaffery J. M. (2007) Conventional and immunoelectron microscopy of mitochondria. Methods Mol. Biol. 372, 467–483 [DOI] [PubMed] [Google Scholar]
- 31. Picard M., White K., Turnbull D. M. (2013) Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J. Appl. Physiol. 114, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zolezzi J. M., Silva-Alvarez C., Ordenes D., Godoy J. A., Carvajal F. J., Santos M. J., Inestrosa N. C. (2013) Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One 8, e64019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quintanilla R. A., Jin Y. N., von Bernhardi R., Johnson G. V. (2013) Mitochondrial permeability transition pore induces mitochondria injury in Huntington disease. Mol. Neurodegener. 10.1186/1750-1326-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slater P. G., Ramirez V. T., Gonzalez-Billault C., Varela-Nallar L., Inestrosa N. C. (2013) Frizzled-5 receptor is involved in neuronal polarity and morphogenesis of hippocampal neurons. PLoS One 8, e78892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brustovetsky T., Li V., Brustovetsky N. (2009) Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium 46, 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheng Z. H., Cai Q. (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruciat C. M., Niehrs C. (2013) Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 5, a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Losón O. C., Song Z., Chen H., Chan D. C. (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smirnova E., Griparic L., Shurland D. L., van der Bliek A. M. (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costes S. V., Daelemans D., Cho E. H., Dobbin Z., Pavlakis G., Lockett S. (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manders E. M., Verbeek F. J., Aten J. A. (1993) Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 169, 375–382 [DOI] [PubMed] [Google Scholar]
- 42. Han X.-J., Lu Y.-F., Li S.-A., Kaitsuka T., Sato Y., Tomizawa K., Nairn A. C., Takei K., Matsui H., Matsushita M. (2008) CaM kinase I α-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182, 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muñoz F. J., Godoy J. A., Cerpa W., Poblete I. M., Huidobro-Toro J. P., Inestrosa N. C. (2014) Wnt-5a increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochem. Biophys Res. Commun. 444, 189–194 [DOI] [PubMed] [Google Scholar]
- 44. Billups B., Forsythe I. D. (2002) Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 22, 5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicholls D. G., Budd S. L. (2000) Mitochondria and neuronal survival. Physiol. Rev. 80, 315–360 [DOI] [PubMed] [Google Scholar]
- 46. Vos M., Lauwers E., Verstreken P. (2010) Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front. Synaptic Neurosci. 2, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang D. T., Honick A. S., Reynolds I. J. (2006) Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 26, 7035–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oliva C. A., Vargas J. Y., Inestrosa N. C. (2013) Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front. Cell. Neurosci. 7, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosso S. B., Inestrosa N. C. (2013) WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Inestrosa N. C., Varela-Nallar L. (2014) Wnt signaling in the nervous system and in Alzheimer's disease. J. Mol. Cell Biol. 6, 64–74 [DOI] [PubMed] [Google Scholar]
- 51. Sibson N. R., Dhankhar A., Mason G. F., Rothman D. L., Behar K. L., Shulman R. G. (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. U.S.A. 95, 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen H., Chan D. C. (2010) Physiological functions of mitochondrial fusion. Ann. N.Y. Acad. Sci. 1201, 21–25 [DOI] [PubMed] [Google Scholar]
- 53. Saxton W. M., Hollenbeck P. J. (2012) The axonal transport of mitochondria. J. Cell Sci. 125, 2095–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liesa M., Palacín M., Zorzano A. (2009) Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89, 799–845 [DOI] [PubMed] [Google Scholar]
- 55. Wang X., Schwarz T. L. (2009) The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Macaskill A. F., Rinholm J. E., Twelvetrees A. E., Arancibia-Carcamo I. L., Muir J., Fransson A., Aspenstrom P., Attwell D., Kittler J. T. (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. MacAskill A. F., Kittler J. T. (2010) Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 20, 102–112 [DOI] [PubMed] [Google Scholar]
- 58. Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y. L., Selkoe D., Rice S., Steen J., LaVoie M. J., Schwarz T. L. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., Youle R. J. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 [DOI] [PubMed] [Google Scholar]
- 60. Chang C. R., Blackstone C. (2010) Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N.Y. Acad. Sci. 1201, 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qi X., Disatnik M.-H., Shen N., Sobel R. A., Mochly-Rosen D. (2011) Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Mol. Biol. Cell 22, 256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cribbs J. T., Strack S. (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 8, 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cho B., Choi S. Y., Cho H. M., Kim H. J., Sun W. (2013) Physiological and pathological significance of dynamin-related protein 1 (Drp1)-dependent mitochondrial fission in the nervous system. Exp. Neurobiol. 22, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brooks C., Cho S. G., Wang C. Y., Yang T, Dong Z. (2011) Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am. J. Physiol. Cell Physiol. 300, C447–C455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dedov V. N., Dedova I. V., Armati P. J. (2000) Transport of mitochondria during axonogenesis. IUBMB Life 49, 549–552 [DOI] [PubMed] [Google Scholar]
- 66. Su B., Wang X., Bonda D., Perry G., Smith M., Zhu X. (2010) Abnormal mitochondrial dynamics–a novel therapeutic target for Alzheimer's disease? Mol. Neurobiol. 41, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silva-Alvarez C., Arrázola M. S., Godoy J. A., Ordenes D., Inestrosa N. C. (2013) Canonical Wnt signaling protects hippocampal neurons from Aβ oligomers: role of non-canonical Wnt-5a/Ca2+ in mitochondrial dynamics. Front. Cell. Neurosci. 7, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cai Q., Davis M. L., Sheng Z. H. (2011) Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neurosci. Res. 70, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]