Background: Chrysanthemyl diphosphate synthase (CDS) is known to catalyze the formation of the irregular terpenoid, chrysanthemyl diphosphate (CPP).
Results: CDS also catalyzes the next step from CPP to chrysanthemol.
Conclusion: CDS is actually a chrysanthemol synthase (CHS) with bifunctional enzyme activity.
Significance: Identification of CHS increases our understanding in terpene biosynthesis and paves the way for engineering biosynthesis of the most widely used natural pesticide, pyrethrins.
Keywords: Biosynthesis, Enzyme Kinetics, Mutagenesis, Plant Biochemistry, Terpenoid
Abstract
Chrysanthemyl diphosphate synthase (CDS) is the first pathway-specific enzyme in the biosynthesis of pyrethrins, the most widely used plant-derived pesticide. CDS catalyzes c1′-2-3 cyclopropanation reactions of two molecules of dimethylallyl diphosphate (DMAPP) to yield chrysanthemyl diphosphate (CPP). Three proteins are known to catalyze this cyclopropanation reaction of terpene precursors. Two of them, phytoene and squalene synthase, are bifunctional enzymes with both prenyltransferase and terpene synthase activity. CDS, the other member, has been reported to perform only the prenyltransferase step. Here we show that the NDXXD catalytic motif of CDS, under the lower substrate conditions prevalent in plants, also catalyzes the next step, converting CPP into chrysanthemol by hydrolyzing the diphosphate moiety. The enzymatic hydrolysis reaction followed conventional Michaelis-Menten kinetics, with a Km value for CPP of 196 μm. For the chrysanthemol synthase activity, DMAPP competed with CPP as substrate. The DMAPP concentration required for half-maximal activity to produce chrysanthemol was ∼100 μm, and significant substrate inhibition was observed at elevated DMAPP concentrations. The N-terminal peptide of CDS was identified as a plastid-targeting peptide. Transgenic tobacco plants overexpressing CDS emitted chrysanthemol at a rate of 0.12–0.16 μg h−1 g−1 fresh weight. We propose that CDS should be renamed a chrysanthemol synthase utilizing DMAPP as substrate.
Introduction
Pyrethrins are found predominantly in pyrethrum flower heads (Tanacetum cinerariifolium) and represent the economically most important natural pesticide (1, 2). They are neurotoxins effective against a wide range of insect species and are applied broadly in private homes, gardens, stables, and organic agriculture. Their low toxicity to warm-blooded animals and high degradability under sunlight gives them environmentally friendly properties (3).
Pyrethrins comprise a group of six closely related esters with either chrysanthemic acid (type I esters) or pyrethric acid (type II esters) as terpene acid moieties. Chrysanthemic and pyrethric acid share a common cyclopropane ring structure, but the latter possesses a terminal carboxymethyl group. The acid moieties have been shown to be predominantly derived from the methyl-erythritol phosphate pathway (4, 5). The methyl-erythritol phosphate pathway provides two universal C5 terpene building blocks, isopentenyl diphosphate (IPP)2 and its isomer dimethylallyl diphosphate (DMAPP), in the plastids of plants. Monoterpenes (C10), diterpenes (C20), and carotenoids (C40) are synthesized via this pathway (6). The first pathway-specific step for pyrethrin biosynthesis is the condensation of two molecules of DMAPP, yielding chrysanthemyl diphosphate (CPP) (Fig. 1A) (7). The gene responsible for this step has been cloned, and the recombinant enzyme has been characterized as chrysanthemyl diphosphate synthase (CDS, EC 2.5.1.67) (8). The genes and enzymes involved in the next steps, converting CPP into the monoterpene alcohol, chrysanthemol, and the further oxidation to chrysanthemic acid, have not been identified yet, but, recently, the reactions have been shown to occur in the glandular trichomes (9). The final step of the esterification of chrysanthemic acid with one of the lipid alcohols is performed by a recently cloned GDSL lipase from pyrethrum. This enzyme transfers the chrysanthemoyl group from the CoA thioester to pyrethrolone to produce pyrethrin I but does so in the pericarp of the seeds and not in the glandular trichomes (9, 10).
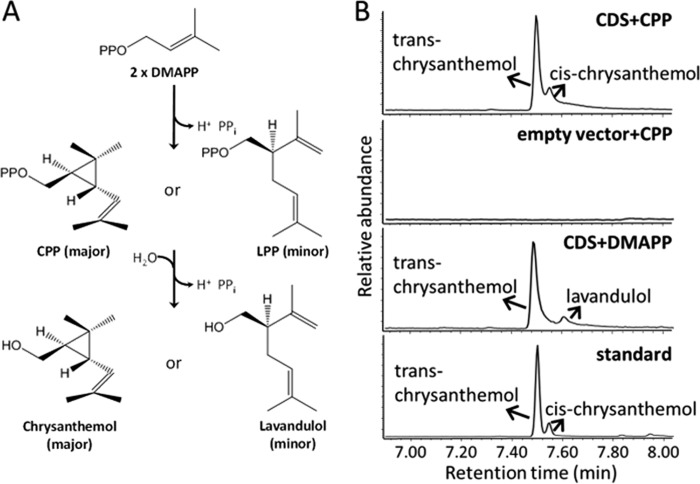
FIGURE 1.
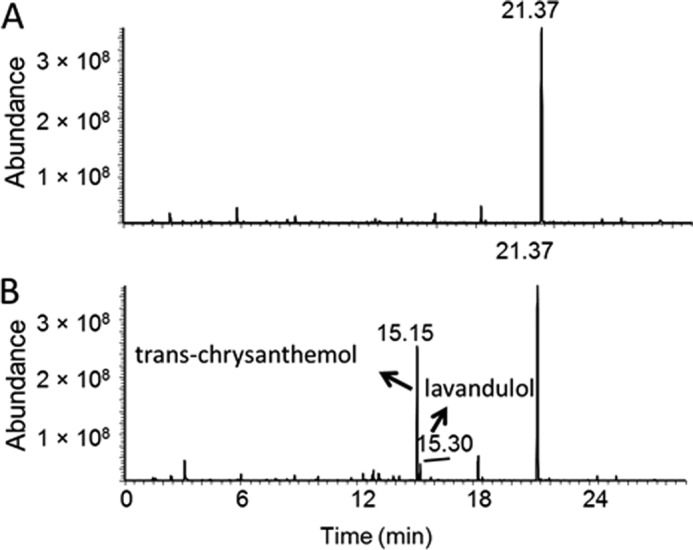
Reactions catalyzed by CDS. A, schematic of the two-step reaction catalyzed by CDS. The chirality of the molecules is shown as publication (11). Major and minor refer to the relative product quantity in each conversion. B, GC-MS chromatograms of the products of purified CDS enzyme assayed with CPP or DMAPP. Empty vector, control assay with protein purified (by eluate volume) from E. coli cells harboring empty vector; standard, a mixture of trans- and cis-chrysanthemol. For the assay with CPP, the assay time was 20 h. For the assay with DMAPP, the assay time was 96 h.
CDS, the enzyme catalyzing the first pathway-specific step, evolved from farnesyl diphosphate synthase (FDS) (8, 11, 12). It takes two molecules of DMAPP as substrate to produce CPP as major product and lavandulyl diphosphate (LPP) as minor product (Fig. 1). Therefore, compared with FDS, the product size of CDS has shifted from C15 to C10, and the enzyme activity of CDS has changed from chain elongation to cyclopropanation. CDS has been cloned and characterized from pyrethrum (T. cinerariifolium) (8) and sagebrush (Artemisia tridentata ssp. spiciformis) (13), but, recently, CDS-like genes have also been reported from Tanacetum coccineum, Achillea asiatica, Chrysanthemum lavandulifolium, Aster ageratoides, Helianthus exilis, and Helianthus annuus (12). For sagebrush, the CDS protein sequence shares 75% identity (and 96% similarity) with the FDS protein sequence from the same species. FDS catalyzes the sequential c1′-4 condensation of IPP with DMAPP to form the intermediate geranyl diphosphate (GPP, C10) and, with another IPP, the product farnesyl diphosphate (FPP, C15), or it catalyzes c1′-4 condensation of GPP with IPP to yield FPP (14). CDS catalyzes the c1′-2-3 condensation of two units of DMAPP, yielding CPP (C10) (8), but the reported affinity of DMAPP for CDS is very low, with a Km value of 600 μm (8) compared with Km values for FDS of 1–20 μm for IPP, DMAPP, or GPP (15). Furthermore, DMAPP concentrations in plastids are estimated at 30 μm (see “Discussion”).
The c1′-2-3 cyclopropane ring structure in CPP is also found in the C30 and C40 terpenoids, presqualene diphosphate and prephytoene diphosphate. Presqualene diphosphate and prephytoene diphosphate are intermediates in reactions producing squalene and phytoene, which are precursors for biosynthesis of sterols and carotenoids. These reactions are catalyzed by squalene synthase (EC 2.5.1.21) and phytoene synthase (EC 2.5.1.32), respectively (16). Squalene synthase catalyzes the condensation of two molecules of FPP to give presqualene diphosphate, and subsequent reductive rearrangement of this intermediate generates squalene (17). Phytoene synthase catalyzes a similar reaction, taking geranylgeranyl diphosphate as substrate to give prephytoene diphosphate as an intermediate and phytoene as a final product (16). Notably, both these terpenoid synthases are bifunctional enzymes catalyzing consecutive prenylation and dephosphorylation steps (18, 19).
Among the very large family of short-chain prenyltransferases and terpene synthases (C10, C15, and C20) (20–22), to our knowledge, only two of them were bifunctional. α-Farnesene synthase from apple (Malus domestica) could take both FPP (C15) or GPP (C10) and IPP (C5) to produce α-farnesene (C15), and both activities were associated with the (semi)conserved DDXXD motif (23), which is required for metal ion binding (24). Myrcene synthase from bark beetle (Ips pini) was initially identified as a GDS (25) but was later discovered to take GDP to further produce myrcene (C10) (26). The authors predicted that both prenyltransferase and terpene synthase activities shared the same catalytic domain on the basis of modeling of the protein structure.
Our preliminary experiments with cell lysate of Escherichia coli expressing CDS showed that the lysate was able to produce chrysanthemol directly from DMAPP, whereas cell lysate of E. coli transformed with the empty vector was not able to convert CPP to chrysanthemol. This suggested that CDS could also be a bifunctional enzyme catalyzing two consecutive reactions from DMAPP to CPP and then to chrysanthemol (Fig. 1A). We now report the characterization of CDS to synthesize chrysanthemol from CPP and DMAPP in vitro and the confirmation of this claim in planta using transgenic tobacco plants.
EXPERIMENTAL PROCEDURES
Plant Materials and Chemicals
Pyrethrum plants (T. cinerariifolium) were grown in the field in Wageningen, The Netherlands. CPP and chrysanthemol were purchased from Isoprenoids Lc. DMAPP was obtained from Sigma. CPP was a mixture of trans- and cis-racemic forms in a ratio around 9:1, and so was the chrysanthemol.
Cloning Full-length cDNA and Genomic DNA of CDS
To clone the full-length cDNA of CDS, the following primers were used in 3′ and 5′ RACE PCRs: TcCDS_F, 5′-CATCTTCTGGACCTCTTCAATGAG-3′; TcCDS_R, 5′-GTACTGAACAATCCGACGGTTAAG-3′. RACE libraries were synthesized with 1 μg of total RNA from a pyrethrum ovary using Supercript® II reverse transcriptase (Invitrogen) and the Smart RACE kit (Clontech). In a final step on the basis of the complete sequence information, primers were designed to obtain the full-length cDNA (accession number JX913536). Eight clones were sequenced. On the basis of the full-length cDNA information, primers were designed for amplification of the genomic DNA corresponding to the gene with introns (accession number JX913537). The sequence of CDS obtained by us differs slightly from the one reported by Rivera et al. (8) (accession number I13995). The detailed differences are explained in the results. The CDS sequence used in this study is the one obtained by us (accession number JX913536).
Protein Production in E. coli and Purification
The open reading frame of CDS without the putative targeting sequence was subcloned into the pRSET-A expression vector (Invitrogen) fused to an amino-terminal histidine tag and expressed in E. coli BL21(AI) (forward primer, 5′-TTATGGATCCACTACGACATTGAGCAGCAATCTAG-3′; reverse primer, 5′-TTATGAATTCTTACTTATGTCCCTTATACATCTTTTCC-3′; restriction sites are underlined). E. coli cells were grown to an A600 of 0.6 and then induced with 0.02% l-arabinose at 18 °C for 16 h. After harvesting, the cells were resuspended in lysis buffer containing 50 mm Tris-HCl (pH 7.5), 300 mm NaCl, and 1.4 mm β-mercaptoethanol; disrupted by sonification (6 × for 10 s); and centrifuged at 13,000 × g for 10 min at 4 °C. His-tagged proteins were purified with nickel-nitrilotriacetic acid-agarose (Qiagen) according to the protocol of the manufacturer. Proteins were concentrated and desalted in 50 mm Tris-HCl (pH 7.5, containing 1.4 mm β-mercaptoethanol) using Amicon centrifugal filters for protein purification and concentration (Millipore), checked for purity by SDS-PAGE, and quantified by the Bradford method using bovine serum albumin as a standard (27). The protein purity was greater than 90%.
Reaction of CDS with CPP
The activity of CDS to produce chrysanthemol from CPP was assayed by incubating 35 μg of purified enzyme in a final volume of 100 μl of assay buffer (pH 7.0) containing 15 mm MOPSO, 2 mm dithioerythreitol, 12.5% (v/v) glycerol, 1 mm MgCl2, 1 mm ascorbic acid, 0.1% (v/v) Tween 20, and various concentrations of CPP. After addition of a 100-μl pentane overlay, the assays were incubated for 20 h at 30 °C. Then the assay mixture was extracted as described previously (28), and the organic phase was concentrated to 200 μl under nitrogen flow. A 1-μl portion of the concentrated organic phase was analyzed by GC-MS using a GC (Agilent 7890A) equipped with an HP5 MS column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness) and a mass-selective detector (Agilent 5975C, Hewlett Packard, Agilent Technologies). The injection port (splitless mode), interface, and MS source temperatures were 250 °C, 290 °C, and 180 °C, respectively. The oven was programmed at an initial temperature of 45 °C for 1 min with a ramp of 15 °C min−1 to 280 °C and a final time of 3.5 min. Proteins extracted from E. coli lysate cells harboring the empty vector were assayed with CPP as a control. For identification, the authentic standard of chrysanthemol was run under identical conditions.
To determine the apparent Km value of CDS for CPP, the Mg2+ concentration (1.0 mm) and pH value (7.0) were set at optimal levels as described previously (8), and the CPP concentration varied between 10 and 600 μm with eight different data points. Triplicate sets of assays were performed at each CPP concentration. The apparent Km value was obtained by fitting the data to the Michaelis-Menten equation using KaleidaGraph (Synergy Software).
Reaction of CDS with DMAPP
The activity of CDS to produce diphosphates (CPP and LPP) and terpene alcohols (chrysanthemol and lavandulol) from DMAPP was assayed by incubating 35 μg of purified enzyme in a final volume of 100 μl of assay buffer, described above, but with 600 μm DMAPP instead of CPP. After addition of a 100-μl pentane overlay, the assays were incubated for 96 h at 30 °C. The chrysanthemol production was determined by analyzing the organic phase as described above. CPP production was determined by analyzing the water phase of the same reaction tube. For that, 50 μl of water phase was transferred to a fresh reaction tube and treated with calf alkaline phosphatase (Sigma). Liberated compounds were extracted as described previously (8). The extracted compounds were then analyzed by GC-MS as described above. For Km determination, assays were performed with different DMAPP concentrations from 10–600 μm with eight different data points. Triplicate sets of assays were performed at each DMAPP concentration. To check non-enzymatic conversion from CPP to chrysanthemol in this 96-h assay, control reactions without enzyme were performed with CPP concentrations ranging from 2–80 μm. This range covered a maximum concentration of 25 μm CPP produced by CDS under different DMAPP concentrations.
For identification of chrysanthemol, the authentic standard of chrysanthemol was run under identical conditions. For identification of lavandulol, mass spectra of eluted peak fractions were compared with the published mass spectrum for lavandulol (11).
Determination of DMAPP Substrate Inhibition in the Reaction of CDS with CPP
To check whether chrysanthemol production by CDS from CPP is inhibited by DMAPP, different amounts of DMAPP were added into CDS enzyme assays containing 150 μm CPP (the apparent Km value of CPP determined above). The enzyme assays were conducted as described above (20-h incubation). DMAPP concentrations were tested in a range of 0–600 μm with eight different data points. The determinations were replicated three times. Terpene alcohol and diphosphate products were analyzed by GC-MS as described above.
Site-directed Mutagenesis
Site-directed mutagenesis was performed using a QuikChange Lightning site-directed mutagenesis kit (Agilent) according to the instructions of the manufacturer. The following oligonucleotides were synthesized and used for the substitution of the residue asparagine (at position 283) by aspartate or glycine, respectively: TcCDS-N283D_F, 5′-GGTATGTATTATCAAATTCAGGATGATTATCTCGACAC-3′ and TcCDS-N283D_R, 5′-GTGTCGAGATAATCATCCTGAATTTGATAATACATACC-3′ (the aspartate residue is underlined); TcCDS-N283G_F, 5′-GGTATGTATTATCAAATTCAGGGTGATTATCTCGACAC-3′ and TcCDS-N283G_R, 5′-GTGTCGAGATAATCACCCTGAATTTGATAATACATACC-3′ (the glycine residue is underlined). The resulting mutant cDNAs were sequenced to verify mutations. Mutated proteins were then expressed and assayed as described above. For the enzyme assays, the mutants were assayed with either CPP or DMAPP, and the substrate concentration for CPP and DMAPP was 200 and 600 μm, respectively. CDS was used as positive control.
The product detection was done slightly differently compared with the other experiments because of equipment change. A 1-μl portion of the organic phase was analyzed by GC-MS coupled with a DSQ mass spectrometer using an Agilent 6890 Network detector (Thermo Fisher Scientific) and equipped with a HP5 MS column (30 m × 0.25 mm i.d., 0.25-μl film thickness, Agilent Technologies). The oven was programmed at an initial temperature of 40 °C for 3 min with a ramp of 10 °C min−1 to 280 °C and final time of 2 min.
Subcellular Localization of CDS Using GFP
The gene fragment corresponding to the first 54 amino acids of CDS or the whole CDS gene without the stop codon were cloned into the binary vector pBINPLUS-1.1eGFP (29) as an in-frame protein fusion to a GFP reporter gene using the KpnI and XbaI restriction sites. These constructs were named pCDSTAR-GFP and pCDS-GFP, respectively. Tobacco plants (Nicotiana tabacum Samsun) were transformed with these constructs as described previously (30). Transgenic tobacco mesophyll protoplasts were isolated according to Sheen (31). Images were taken using a confocal laser-scanning microscope (Zeiss) with fluorescence bandwith filters of 620–750 nm for chlorophyll imaging and 500- 530 nm for GFP.
Generation of Transgenic Tobacco
The complete cDNA of CDS was placed under the control of chrysanthemum RbcS1 promoter, and Agrobacterium tumefaciens strain AGL-0 harboring the binary vector was used to transform the tobacco plants using protocols described previously (30). Tobacco plants transformed with a vector lacking CDS were used as a control. Transgenic plants were grown in a greenhouse at 25 ± 2 °C under a 18/6 h light/dark photoperiod. The presence of CDS was checked by PCR. All PCR-positive plantlets of the T0 transgenic line were analyzed by GC-MS to check the presence of chrysanthemol in the headspace. The three plantlets with the highest production of chrysanthemol were analyzed by LC-MS. Three plantlets from the empty vector control line were also analyzed by GC-MS and LC-MS.
Volatile GC-MS and Non-volatile LC-MS Analysis of Transgenic Tobacco
Volatiles from cut leaves were collected from 4-week-old plants. The second leaf from the top was harvested for headspace trapping. The volatiles were sampled for 2 h and then analyzed by GC-MS as described previously (32). The temperature program of the gas chromatograph was 40 °C for 3 min, rising to 280 °C at 10 °C min−1, and final time for 2 min. The mass spectrometer was set to scan from 35–450 m/z. The helium flow was constant at 1.0 ml min−1. The ionization potential was set at 70 eV. For the identification of chrysanthemol, the authentic standard of chrysanthemol was run under identical conditions. For identification of lavandulol, the mass spectrum of the eluted peak was compared with the published mass spectrum of lavandulol (11). The third leaf from the top was used for non-volatile analysis according to a protocol for untargeted metabolomics of plant tissues (33) as described in detail previously (32).
Statistical analyses of GC-MS and LC-MS data were conducted as reported previously (32). The processing parameters of MetAlign for GC-MS data were set to analyze scans 1340–16,000 (corresponding to a retention time of 2.32–28.05 min) with a maximum amplitude of 3.5 × 108. The parameters for LC-MS data were set to analyze scans 70–2620 (corresponding to a retention time of 1.4–49.73 min) with a maximum amplitude of 35,000.
RESULTS
Sequence Determination of Full-length cDNA of CDS
In earlier work, the start codon of pyrethrum CDS was not determined (8). This motivated us to study the 5′ end sequence of CDS cDNA using RACE PCR. Eight 5′ end sequences obtained by us were quite different from the 5′ end sequence reported previously for CDS (accession number I13995, Ref. 8). We then determined the genomic DNA sequence of CDS (accession number JX913537) from pyrethrum to see whether alternative splicing could explain these differences. By comparing the cDNA and genomic DNA sequences, an intron was easily identified at the 5′ end. The putative start codon proposed by Rivera et al. (8) is actually in this intron region. Twenty-four nucleotides corresponding to the first eight amino acids of CDS reported by Rivera et al. (8) were the intron sequence. The rest of the coding sequence of CDS from Rivera et al. (8) is nearly 100% the same as that obtained by us, with only 1 bp difference and no effect on the protein sequence. We also found that one of our eight sequenced 5′ RACE products was not correctly spliced (data not shown). Apparently, Rivera et al. (8) cloned this low-frequency mRNA product. The CDS used in this study was our own clone isolated from pyrethrum ovaries (accession number JX913536).
Enzymatic Characterization of CDS with CPP
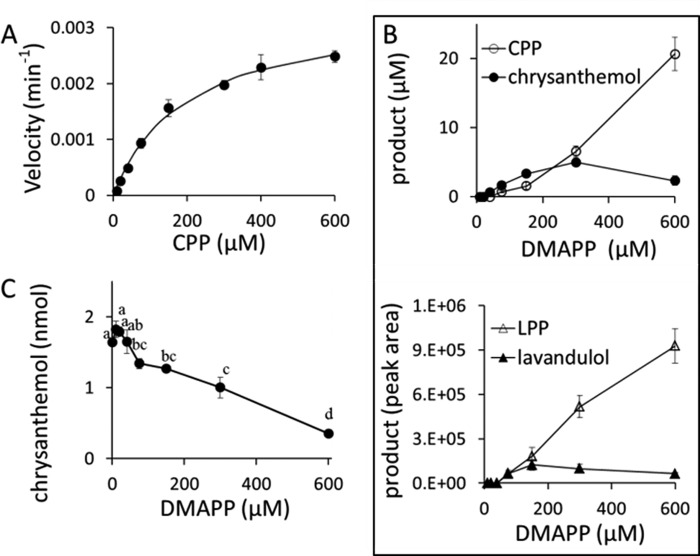
CDS takes DMAPP as substrate to produce CPP (8). Surprisingly, when CPP was provided, CDS converted CPP into chrysanthemol (Fig. 1B). The reaction followed standard Michaelis-Menten kinetics (r = 0.997) with apparent Km and kcat values of 196 ± 23 μm and 3.3 ± 0.2 × 10−3 min−1, respectively (Fig. 2A). Chrysanthemol was not detected in the control assay incubated with CPP for 20 h (Fig. 1B).
FIGURE 2.
GC-MS product analyses of reactions of purified CDS with CPP and/or DMAPP as substrate. A, CDS turnover rate to produce chrysanthemol when CPP was provided as a substrate. The incubation time was 20 h. B, product analysis of CDS incubated with different concentrations of DMAPP for 96 h measuring both the intermediate diphosphate and product alcohol. 1.E+06, 1 × exponent of 10 (6) = 1,000,000. C, inhibition of CDS chrysanthemol synthase activity by the substrate DMAPP. In these assays, both CPP and DMAPP were substrates, but CPP concentration was kept at 150 μm and DMAPP concentration varied from 0–600 μm. The incubation time was 20 h. Data points with the same letter (a, b, c, or d) are not significantly different (p < 0.05). Error bars indicate S.E. (n = 3).
Product Analysis of CDS with DMAPP
The conversion of CPP into chrysanthemol suggested that CDS supplied with DMAPP should also produce chrysanthemol. However, no formation of chrysanthemol was detected when CDS was assayed with 2 mm DMAPP for 2 h (8) or upon longer incubation for 6, 12, and 24 h.
We then decided to prolong the assay time to 96 h and lowered the DMAPP concentration. First we made sure that CDS was active during the 96-h assay. CDS was incubated with 400 μm CPP, and chrysanthemol production was checked every 24 h. A linear correlation was found between chrysanthemol production and assay hours with R2 = 0.995 (data not shown), indicating that CDS was fully active during this 96-h assay. The DMAPP concentration was then lowered from 2000 to 600 μm, which is the previously published Km value of CDS for DMAPP to produce CPP (8). Fig. 1B shows that, under those modified assay conditions, chrysanthemol, as well as lavandulol, were detected in the 96-h assay. However, by incubating the same water phase with alkaline phosphatase, significant concentrations of CPP and LPP were also found in the assays. Clearly, a quantitative approach was necessary to establish the balance in the production of chrysanthemyl and lavandulyl diphosphates and alcohols. For this, a series of DMAPP concentrations ranging from 10–600 μm were assayed with CDS for 96 h, and both terpene alcohol and phosphate production was determined (Fig. 2B). Chrysanthemol production increased with DMAPP concentration up to 300 μm (Fig. 2B, top panel). Further increase of DMAPP concentration led to a decrease of chrysanthemol but a strong further increase of CPP production. More chrysanthemol than CPP was detected, with DMAPP concentrations ranging from 40–150 μm (Fig. 2B, top panel). The DMAPP concentration required for half-maximal activity to produce chrysanthemol was ∼100 μm, and, at that concentration on a molar basis, at least twice as much chrysanthemol was formed compared with CPP. On the other hand, CPP production increased with DMAPP concentrations up to the tested maximum of 600 μm (Fig. 2B, top panel). To check non-enzymatic conversion of CPP into chrysanthemol, control reactions without enzymes were performed at CPP concentrations ranging from 2–80 μm. This range covered the CPP production in 96-h assays, which produced a maximum of 25 μm CPP with DMAPP. However, no chrysanthemol was detected in any of the control reactions.
The relationship between DMAPP substrate concentration and lavandulol or LPP production was very similar to chrysanthemol and CPP (Fig. 2B, bottom panel). Lavandulol production was highest with 150 μm DMAPP and decreased with higher concentrations of DMAPP, whereas LPP production increased with DMAPP concentrations up to the 600 μm tested maximum.
Chrysanthemol Production with CPP Is Inhibited by DMAPP
The above experiments suggested that higher concentrations of DMAPP substrate caused inhibition of chrysanthemol production. We therefore assayed CDS activity with both CPP and DMAPP as substrates in the same assay tube. In all assays, CPP concentration was kept constant at 150 μm (around the Km value of CDS for CPP), whereas DMAPP concentrations varied from 0–600 μm. In general, addition of DMAPP to the assays caused a decrease of chrysanthemol production, and this effect was significant from 75 μm upward (Fig. 2C). Chrysanthemol production was not inhibited when 10–40 μm DMAPP was added.
Subcellular Localization of CDS
The N-terminal sequence of CDS was predicted to serve as a targeting signal to plastids (8). To test the prediction, we analyzed the targeting with gene fusions to GFP. The gene fragment corresponding to the first 54 amino acids or the whole cDNA without the stop codon were fused to GFP and transferred to tobacco (N. tabacum) protoplasts. Transient expression of the fused GFP in tobacco cells showed that both the N-terminal signal peptide and the complete CDS protein targeted GFP to plastids (Fig. 3).
FIGURE 3.
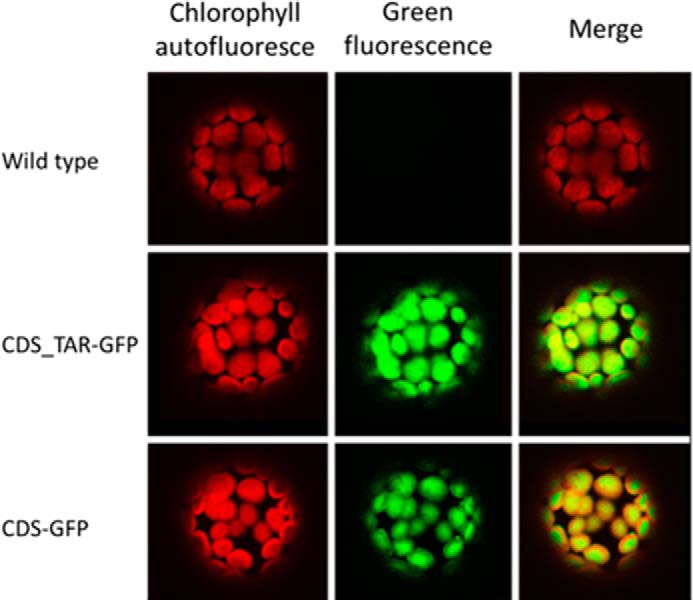
Subcellular localization of CDS using transient expression of GFP fusions in tobacco protoplasts. CDS_TAR-GFP, the putative CDS targeting signal (first 54 amino acids) fused to GFP. CDS-GFP, the complete CDS fused to GFP.
Headspace Emissions of Transgenic Tobacco Overexpressing CDS
To verify the function of CDS in vivo, its cDNA was cloned under the regulation of the chrysanthemum RbcS1 promoter into a binary vector and used to transform tobacco plants. Strong emissions of chrysanthemol and lower emissions of lavandulol, similar to the in vitro ratios of volatile products of CDS, were detected in the headspace of transgenic tobacco plants expressing CDS (n = 9) but not in control tobacco plants (n = 3), which were transformed with the empty vector lacking CDS (Fig. 4). Chrysanthemol was emitted at levels of 0.12–0.16 μg h−1 g−1 FW. Transgenic tobacco plants were shorter and leaf color was lighter compared with empty vector control plants.
FIGURE 4.
Shown are GC-MS chromatograms obtained by dynamic headspace trapping of cut leaves of empty vector control (A) and CDS-expressing (B) tobacco plants.
Non-volatile Profile of Transgenic Tobacco Overexpressing CDS
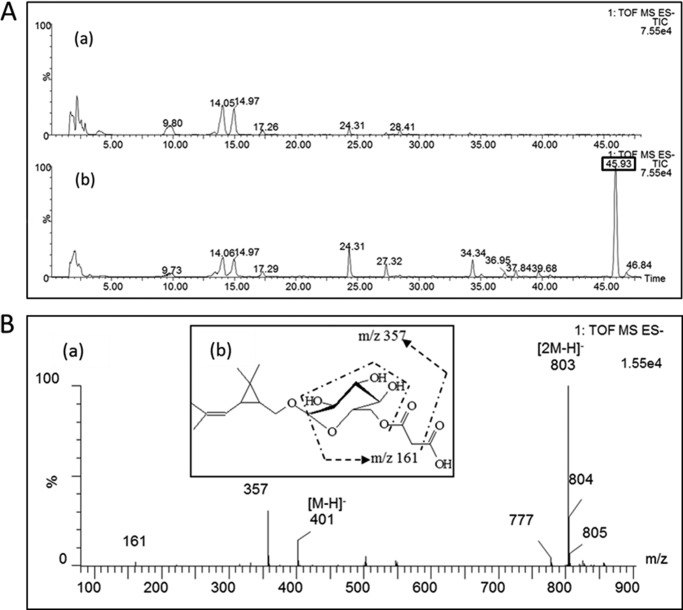
To study whether there is also accumulation of non-volatile derivative(s) of chrysanthemol and/or lavandulol, we analyzed the non-volatile metabolites in transgenic (n = 3) and control (n = 3) plants using a non-targeted approach (Fig. 5A). The newly produced compound that was most abundant, as determined by UV absorption (220 nm) resulting from the double bond of the compounds, showed up as m/z of 803.3498 and eluted at a retention time of 45.93 min. In control plants, this compound was not detected. The non-volatile product was putatively identified as chrysanthemol conjugated to malonyl glucose by comparing its mass spectrum to that of geranoyl-6-O-malonyl-β-d-glucopyranoside, which was identified by NMR in geraniol synthase-expressing maize (Fig. 5B and Ref. 32).
FIGURE 5.
LC-MS analysis of leaf extracts of empty vector and CDS-expressing tobacco plants. A, negative mode LC-MS chromatograms of aqueous methanol extract of leaves of an empty vector tobacco plant (a) and a CDS-expressing tobacco plant (b). The most significantly different compound eluting at 45.93 min is indicated in the box, and it was only detected in CDS-expressing tobacco plants but not in empty vector control plants. B, the MS spectrum of this significantly different compound (a) and the inset (b) show the schematic of its collision-induced fragmentation. The compound was putatively identified as chrysanthemol conjugated to malonyl glucose.
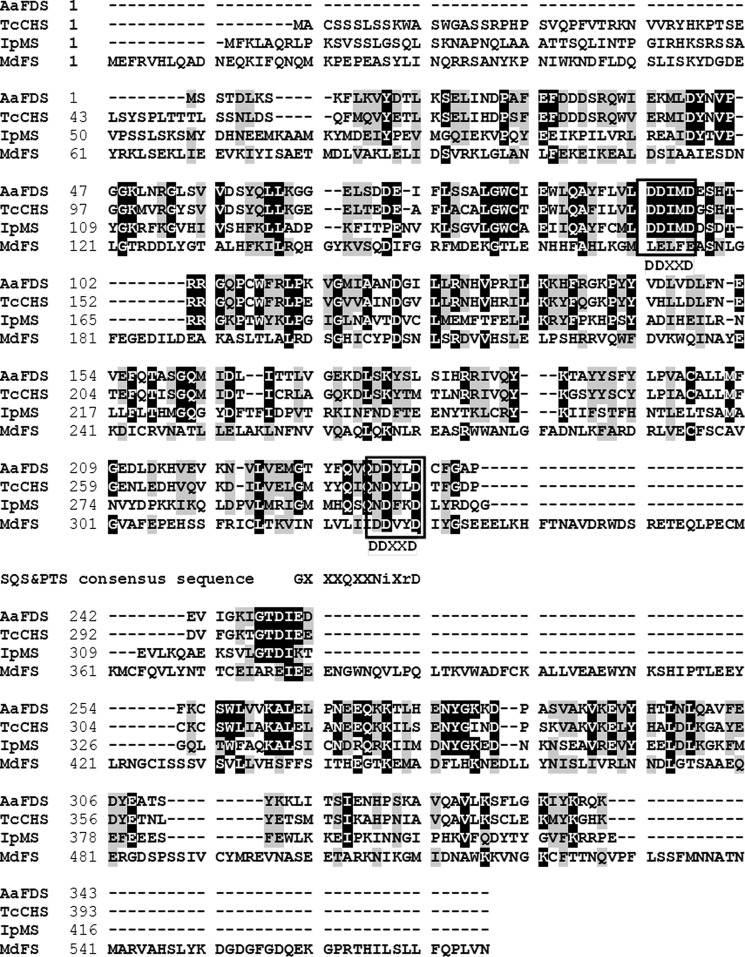
Sequence Alignment of CDS and Related Bifunctional Enzymes
To indicate amino acid sequences potentially responsible for hydrolysis activity in CDS, we compared the amino acid sequence among CDS, FDS, and the other two bifunctional short-chain terpene synthases known so far, myrcene synthase from bark beetle (I. pini) (IpMS) and farnesene synthase from apple (M. domestica) (MdFS) (Fig. 6). For FDS, CDS, and IpMS, there are two conserved DDXXD domains, whereas MdFS only contains one. However, the sequence for the second aspartate-rich motif in CDS contains the rare mutation NDXXD, with asparagine replacing the first aspartate of the DDXXD motif. Interestingly, this Asp → Asn replacement also occurs in IpMS, SQS, and PTS. For SQS and PTS it is the only “conserved” domain with short-chain terpene synthases (Fig. 6).
FIGURE 6.
Alignment of chrysanthemol synthase with two other bifunctional short-chain terpene synthases and FDS, the ancestor of CHS. AaFDS, FDS from sagebrush (Artemisia annua), which is also in the Asteraceae family as pyrethrum (T. cinerariifolium); TcCHS, bifunctional chryanthemyl diphosphate/chrysanthemol synthase from pyrethrum; IpMS, bifunctional geranyl diphosphate/myrcene synthase from bark beetle (I. pini); MdFS, bifunctional FDS/farnesene synthase from apple (M. domestica). The boxes indicate the conserved DDXXD domain of prenyltransferases and terpene synthases. SQS and PTS have very limited similarity to short-chain prenyltransferases or terpene synthases, with only the motif GXXXQXXDDXXD at the second DDXXD region in FDS partially conserved (DD = Ni) (8).
Mutagenesis of the Asparagine Abolishes Terpene Synthase and Prenyltransferase Activities
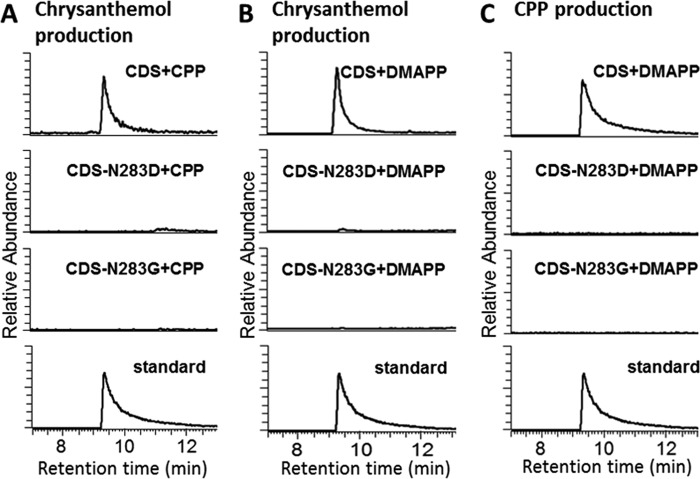
To evaluate the importance of the asparagine in the aspartate-rich motif mentioned above, we generated two site-directed mutants of CDS, N283D, and N283G, replacing the asparagine with either aspartate or glycine. Both the terpene synthase and prenyltransferase activities of CDS were completely lost in both mutants. Neither CPP nor chrysanthemol were detected in the enzyme assays when the mutants were used (Fig. 7).
FIGURE 7.
Reactions catalyzed by CDS and its mutants CDS-N283D and CDS-N283G. A, GC-MS chromatograms of the products of purified enzymes assayed with CPP. B, GC-MS chromatograms of the products in the organic phase (for chrysanthemol production) of the assays when DMAPP was provided as substrate. C, GC-MS chromatograms of the products in the water phase (for CPP production) of the assays when DMAPP was provided as substrate.
DISCUSSION
Although pyrethrins represent the economically most important and widely used natural pesticide, not much is known about their biosynthesis at the genetic or enzymatic levels. Only the genes involved in the first and last step of the biosynthetic pathway have been cloned (8, 10). CDS has been reported to catalyze the first committed step of pyrethrin biosynthesis by joining two molecules of DMAPP to produce CPP (8), but the recently evolved enzyme also has roles in other composite plant species that do not produce pyrethrins (12, 13). Interestingly, our in vitro and in vivo results demonstrate that CDS also catalyzes the subsequent conversion of CPP to chrysanthemol, which furthers our understanding of the monoterpene branch of pyrethrin biosynthesis that occurs in pyrethrum glandular trichomes (9).
CDS was active with DMAPP and CPP as substrates. In both cases, the production of the monoterpene alcohols chrysanthemol and lavandulol was detected. Rivera et al. (8) reported earlier that CPP and LPP were the final products of CDS. We used a typical protocol for the activity analysis of terpene synthases, which produce volatile and hydrophobic products (28, 34), whereas earlier protocols were on the basis of the activity analysis of diphosphate synthases, which produce non-volatile and hydrophilic products (13, 35). Although we used similar reaction conditions, the combination of the following factors in our assays could contribute to the observed differences: a pentane overlay to capture the volatile alcohol; the addition of detergent, Tween 20, to promote the release of the hydrophobic product; a low substrate concentration, common to plants; and a long incubation time to observe products in this notoriously slow reaction in vitro. In former assays, the lack of an organic layer to capture the volatile product may have prevented detecting chrysanthemol formation. The standard presence of Tween 20 in our assays may have increased the CDS activity to produce chrysanthemol, as reported for SQS and PTS. For SQS, Tween 80 stimulated its activity 10- to 20-fold, and Tween 20 showed similar stimulating effects (36). PTS also requires a detergent for maximal activity because the presence of Tween 80 has been reported to result in almost 20,000-fold higher activity compared with PTS activities reported previously (19).
Although the turnover from CPP (3.3 × 10−3 min−1) or DMAPP (∼ 1 × 10−4 min−1) to chrysanthemol was highly inefficient in the in vitro assays, the production of chrysanthemol by CDS was highly efficient in vivo. Chrysanthemol was emitted from leaves of transgenic tobacco overexpressing CDS (Fig. 4) at rates of 120–160 ng h−1 g−1 FW. This rate was on the high end of the emission rates of other overexpressed terpenes in tobacco leaves. In tobacco plants overexpressing three monoterpene synthases (CaMV35S promoter), the total level of additional monoterpenes emitted from leaves reached up to 30 ng h−1 g−1 FW (37), and plants overexpressing patchoulol synthase emitted patchoulol at levels of 50–100 ng h−1 g−1 FW (6).
The high efficiency of chrysanthemol production in plants suggests the possible presence of an endogenous phosphatase that efficiently hydrolyzes CPP or the presence of an endogenous cofactor that potentiates CDS activity (38, 39). Our experiments and existing literature seem to best support the latter possibility for the reasons given below.
First, any free aspecific phosphatase in the chloroplast stroma would be expected to also lead to a hydrolysis of DMAPP, GPP, and geranylgeranyl diphosphate. The fact that plants possess pools of these substrates freely accessible to other enzymes and do not have nonspecific production/emission of prenol, geraniol, or geranylgeraniol suggests that such phosphatases are normally not in the same cellular compartment with prenylphosphate synthases and their products.
Second, our in vitro results showed that the in vivo precursor diphosphate concentrations of DMAPP in plants are too low to generate free CPP as a main product. CDS favored the synthesis of chrysanthemol over CPP when the DMAPP concentrations were lower than 300 μm (Fig. 2B). This low DMAPP concentration range is most likely the range encountered by CDS in vivo. DMAPP concentrations in leaves have been determined for several plant species. Compared with the isoprene-emitting species cottonwood (40) and oak (41), leaf DMAPP concentrations in non-emitting species, such as Arabidopsis, are 6–10 times lower (42). Considering that tobacco is also a non-emitting species (43), leaf DMAPP concentrations in tobacco are expected be similar to those in Arabidopsis at an estimated concentration of around 10 pmol mg−1 FW (42). Then, the DMAPP level in tobacco plastids, the cell compartment to which CDS is targeted (Fig. 3 and Ref. 13), can be estimated to be around 30 μm, assuming a regression coefficient of 1 g ml−1 for leaf fresh weight to leaf volume (44), a chloroplast volume of 20% of total cell volume in mature leaf cells (45), and 60% of DMAPP to be found in the chloroplast (46).
Third, inactive GDSs have been shown before to be activated by the formation of a heterodimer with geranylgeranyldiphospate synthase (39, 47, 48). The small subunits are usually inactive in the in vitro assays, and the big subunits are either inactive (35, 49) or function as well as homodimeric geranylgeranyl diphosphate synthases (39, 47, 48). Two of four geranylgeranyl diphosphate synthase-like enzymes of tobacco could interact in tobacco with the small subunit from snapdragon (Antirrhinum majus) (48). Therefore, we propose that CDS may possibly also form heterodimers with geranylgeranyl diphosphate synthase-like large subunits of tobacco (and pyrethrum) that significantly promote its activity.
Fourth, our tests on bacterial lysates also suggested the possible presence of a cofactor. The enzyme activity of the lysate was about 10- to 40-fold higher than that of purified CDS, but this activity could not be explained by a nonspecific phosphatase because the lysate from empty vector cells did not convert CPP into chrysanthemol under the same assay conditions. This suggests that the enzyme loses activity or an activity-promoting component during purification. Copurified bands are not visible on SDS-PAGE gels, however (data not shown). We then tested the effects of adding protein (BSA), all known mineral cofactors (Murashige and Skoog salts), and NADPH to the enzyme assays (18), but the activity of purified CDS was not improved.
CDS shares several characteristics with the long-chain terpene synthases SQS and PTS, which both catalyze intermolecular c1′-2-3 cyclopropanation reactions. All three synthases are bifunctional enzymes, functioning as prenyltransferases in the first reaction and as terpene synthases in the second reaction. However, the cyclopropane ring structure remains in the final product of CDS but not in those of SQS or PTS. The overall enzyme activities of all three enzymes are inhibited by high substrate concentrations but the production of the intermediate diphosphates is not. For example, the production of squalene by SQS has been shown to be inhibited by FPP concentrations higher than 100 μm (18, 36), but the production of the intermediate presqualene diphosphate is not inhibited by high FPP concentrations (18). Similarly, PTS has been reported to be significantly inhibited at geranylgeranyl diphosphate concentrations above 100 μm (19), although each of the separate reactions of PTS showed Michaelis-Menten behavior (16). In our experiments, DMAPP concentrations of higher than 300 μm reduced total chrysanthemol production in favor of CPP (Fig. 2B, top panel), whereas lavandulol production was reduced by DMAPP at concentrations of higher than 150 μm (Fig. 2B, bottom panel). CPP production was not inhibited by DMAPP concentrations of up to of 600 μm (Fig. 2B, top), in good agreement with earlier data (8). This indicates that DMAPP inhibits the overall CDS activity by inhibiting the second but not the first reaction. This was confirmed by providing both DMAPP and CPP to CDS, resulting in a significant reduction of chrysanthemol production when DMAPP concentration was higher than 75 μm (Fig. 2C). These results suggest that DMAPP and CPP are converted in the same active site. This was subsequently confirmed by the loss of both enzyme activities after mutagenesis of the NDXXD motif into either DDXXD or GDXXD. The bifunctional AaFDS from apple similarly uses the same active site for both activities, as demonstrated by the loss of both enzyme activities when the first and last aspartate of DDXXD was mutated into ADXXA/D (23).
The alignment of amino acid sequences of FDS, CDS, and the other bifunctional terpene synthases (Fig. 6) showed that the Asp → Asn replacement in the second aspartate-rich motif in CDS is also present in another short-chain bifunctional enzyme, myrcene synthase, as well as in SQS and PTS. This replacement has been suggested to be not essential for cyclopropanation, the reaction shared by CDS, SQS, and PTS, because casbene synthase, an enzyme that catalyzes an intramolecular cyclopropanation of the distal double bond in geranylgeranyl diphosphate, has the typical DDXXD motif (8). We find, however, that mutating the Asn back to Asp leads to the loss of both the CPP and chrysanthemol synthase activities. Therefore, it is clear that the Asp → Asn replacement in CDS is crucial for both the prenyltransferase and terpene synthase activities.
Previously, chimeric enzymes have been made by Poulter and co-workers (11) that showed the evolution between the four fundamental coupling reactions (chain elongation, cyclopropanation, branching, and cyclobutanation) using FDS and CDS as building blocks. In those experiments, the bifunctional activity of the CDS enzyme was not known, but our findings allow an additional perspective on the evolution of FDS, with diphosphate synthase activity only, into a chrysanthemol synthase with bifunctional activity. Future research may further reveal the structural and mechanical basis of the two-step reaction and also verify this process in the other members of this new terpene synthase gene family, which potentially yields not only chrysanthemol, but also lavandulol, maconelliol, or more ancestral C15 alcohols in other plant species (11, 12).
In summary, we have shown both in vitro and in vivo that the previously reported prenyltransferase CDS also functions as a terpene synthase. Considering its overall ability in vitro to preferentially catalyze both consecutive reactions at the low substrate concentrations that prevail in vivo, we propose to rename CDS a chrysanthemol synthase. This identification moves forward our understanding of a recently evolved branch of irregular monoterpene biosynthesis that is not involved in primary metabolism like phytoene and squalene synthases. Normally, there is an ancient and strict task division in terpene biosynthesis between synthases of terpene diphosphates and terpenoid products. Terpene synthases, at the expense of the diphosphate, can modify the skeleton in highly diverse ways to accommodate the required chemical variation in both the primary and secondary metabolism of organisms. As an example, geranyl diphosphate synthases exist separately from geraniol synthases and are evolutionarily selected not to hydrolyze their own GPP product. CDS, however, evolved only very recently, and there is an evolutionary bottleneck if two enzymes are needed simultaneously to generate a useful product like chrysanthemol. The bifunctional activity solves this issue and serves the needs of a plant that has not yet evolved a secondary purpose for CPP substrates.
Acknowledgments
We thank Prof. Willem J. H. van Berkel, Prof. Marcel Dicke, and Prof. Harro J. Bouwmeester for critical and instructive comments on the manuscript.
This work was supported by Technological Top Institute Green Genetics (Stichting TTI Groene Genetica) of The Netherlands Grant 1C001RP and by National Natural Science Foundation of China Project 31372103.
- IPP
- isopentenyl diphosphate
- DMAPP
- dimethylallyl diphosphate
- CPP
- chrysanthemyl diphosphate
- CDS
- chrysanthemyl diphosphate synthase
- FDS
- farnesyl diphosphate synthase
- LPP
- lavandulyl diphosphate
- GPP
- geranyl diphosphate
- GDS
- geranyl diphosphate synthase
- FPP
- farnesyl diphosphate
- SQS
- squalene synthase
- PTS
- phytoene synthase
- FW
- fresh weight
- RACE
- rapid amplification of cDNA ends
- MOPSO
- 3-(N-morpholinyl)-2-hydroxypropanesulfonic acid.
REFERENCES
- 1. Brewer J. G. (1973) Microhistological examination of the secretory tissue in pyrethrum florets. Pyrethrum Post 12, 17–22 [Google Scholar]
- 2. Casida J. E. (1973) Pyrethrum, the Natural Insecticide, Academic Press, New York [Google Scholar]
- 3. Casida J. E., Quistad G. B. (1995) Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses, Oxford University Press, New York [Google Scholar]
- 4. Matsuda K., Kikuta Y., Haba A., Nakayama K., Katsuda Y., Hatanaka A., Komai K. (2005) Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry 66, 1529–1535 [DOI] [PubMed] [Google Scholar]
- 5. Crowley M. P., Inglis H. S., Snarey M., Thain E. M. (1961) Biosynthesis of the pyrethrins. Nature 191, 281–282 [DOI] [PubMed] [Google Scholar]
- 6. Wu S., Schalk M., Clark A., Miles R. B., Coates R., Chappell J. (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat. Biotechnol. 24, 1441–1447 [DOI] [PubMed] [Google Scholar]
- 7. Epstein W. W., Poulter C. D. (1973) A survey of some irregular monoterpenes and their biogenetic analogies to presqualene alcohol. Phytochemistry 12, 737–747 [Google Scholar]
- 8. Rivera S. B., Swedlund B. D., King G. J., Bell R. N., Hussey C. E., Jr., Shattuck-Eidens D. M., Wrobel W. M., Peiser G. D., Poulter C. D. (2001) Chrysanthemyl diphosphate synthase: isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc. Natl. Acad. Sci. U.S.A. 98, 4373–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramirez A. M., Stoopen G., Menzel T. R., Gols R., Bouwmeester H. J., Dicke M., Jongsma M. A. (2012) Bidirectional secretions from glandular trichomes of pyrethrum enable immunization of seedlings. Plant Cell 24, 4252–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kikuta Y., Ueda H., Takahashi M., Mitsumori T., Yamada G., Sakamori K., Takeda K., Furutani S., Nakayama K., Katsuda Y., Hatanaka A., Matsuda K. (2012) Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium: a new target for plant protection. Plant J. 71, 183–193 [DOI] [PubMed] [Google Scholar]
- 11. Thulasiram H. V., Erickson H. K., Poulter C. D. (2007) Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 316, 73–76 [DOI] [PubMed] [Google Scholar]
- 12. Liu P.-L., Wan J.-N., Guo Y.-P., Ge S., Rao G.-Y. (2012) Adaptive evolution of the chrysanthemyl diphosphate synthase gene involved in irregular monoterpene metabolism. BMC Evol. Biol. 12, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hemmerlin A., Rivera S. B., Erickson H. K., Poulter C. D. (2003) Enzymes encoded by the farnesyl diphosphate synthase gene family in the big sagebrush Artemisia tridentata ssp. Spiciformis. J. Biol. Chem. 278, 32132–32140 [DOI] [PubMed] [Google Scholar]
- 14. Szkopińska A., Płochocka D. (2005) Farnesyl diphosphate synthase: regulation of product specificity. Acta Biochim. Pol. 52, 45–55 [PubMed] [Google Scholar]
- 15. Ogura K., Koyama T. (1998) Enzymatic aspects of isoprenoid chain elongation. Chem. Rev. 98, 1263–1276 [DOI] [PubMed] [Google Scholar]
- 16. Dogbo O., Laferriére A., D'Harlingue A., Camara B. (1988) Carotenoid biosynthesis: isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc. Natl. Acad. Sci. U.S.A. 85, 7054–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakashima T., Inoue T., Oka A., Nishino T., Osumi T., Hata S. (1995) Cloning, expression, and characterization of cDNAs encoding Arabidopsis thaliana squalene synthase. Proc. Natl. Acad. Sci. U.S.A. 92, 2328–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radisky E. S., Poulter C. D. (2000) Squalene synthase: steady-state, pre-steady-state, and isotope-trapping studies. Biochemistry 39, 1748–1760 [DOI] [PubMed] [Google Scholar]
- 19. Iwata-Reuyl D., Math S. K., Desai S. B., Poulter C. D. (2003) Bacterial phytoene synthase: molecular cloning, expression, and characterization of Erwinia herbicola phytoene synthase†. Biochemistry 42, 3359–3365 [DOI] [PubMed] [Google Scholar]
- 20. Chen F., Tholl D., Bohlmann J., Pichersky E. (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. The Plant Journal 66, 212–229 [DOI] [PubMed] [Google Scholar]
- 21. Tholl D. (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 9, 297–304 [DOI] [PubMed] [Google Scholar]
- 22. Tholl D., Lee S. (2011) Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book 9, e0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green S., Friel E. N., Matich A., Beuning L. L., Cooney J. M., Rowan D. D., MacRae E. (2007) Unusual features of a recombinant apple α-farnesene synthase. Phytochemistry 68, 176–188 [DOI] [PubMed] [Google Scholar]
- 24. Christianson D. W. (2006) Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 106, 3412–3442 [DOI] [PubMed] [Google Scholar]
- 25. Gilg A. B., Bearfield J. C., Tittiger C., Welch W. H., Blomquist G. J. (2005) Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 102, 9760–9765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilg A. B., Tittiger C., Blomquist G. J. (2009) Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften 96, 731–735 [DOI] [PubMed] [Google Scholar]
- 27. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 28. Bouwmeester H. J., Kodde J., Verstappen F. W., Altug I. G., de Kraker J. W., Wallaart T. E. (2002) Isolation and characterization of two germacrene a synthase cDNA clones from chicory. Plant Physiol. 129, 134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seiichi F., De Jong J., Rademaker W. (1995) Efficient genetic transformation of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura) using stem segments. Breed Sci. 45, 179–184 [Google Scholar]
- 30. Jongsma M. A., Bakker P. L., Peters J., Bosch D., Stiekema W. J. (1995) Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. U.S.A. 92, 8041–8045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheen J. (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127, 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- 32. Yang T., Stoopen G., Yalpani N., Vervoort J., de Vos R., Voster A., Verstappen F. W., Bouwmeester H. J., Jongsma M. A. (2011) Metabolic engineering of geranic acid in maize to achieve fungal resistance is compromised by novel glycosylation patterns. Metab. Eng. 13, 414–425 [DOI] [PubMed] [Google Scholar]
- 33. De Vos R. C., Moco S., Lommen A., Keurentjes J. J., Bino R. J., Hall R. D. (2007) Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2, 778–791 [DOI] [PubMed] [Google Scholar]
- 34. Aharoni A., Giri A. P., Verstappen F. W., Bertea C. M., Sevenier R., Sun Z., Jongsma M. A., Schwab W., Bouwmeester H. J. (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16, 3110–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burke C. C., Wildung M. R., Croteau R. (1999) Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer. Proc. Natl. Acad. Sci. U.S.A. 96, 13062–13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang D., Jennings S. M., Robinson G. W., Poulter C. D. (1993) Yeast squalene synthase: expression, purification, and characterization of soluble recombinant enzyme. Arch. Biochem. Biophys. 304, 133–143 [DOI] [PubMed] [Google Scholar]
- 37. Lücker J., Schwab W., van Hautum B., Blaas J., van der Plas L. H., Bouwmeester H. J., Verhoeven H. A. (2004) Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from lemon. Plant Physiol. 134, 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao Y., Honzatko R. B., Peters R. J. (2012) Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat. Prod. Rep. 29, 1153–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang G., Dixon R. A. (2009) Heterodimeric geranyl)geranyl)diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 106, 9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fisher A. J., Rosenstiel T. N., Shirk M. C., Fall R. (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal. Biochem. 292, 272–279 [DOI] [PubMed] [Google Scholar]
- 41. Brüggemann N., Schnitzler J.-P. (2002) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiol. Plant. 115, 190–196 [DOI] [PubMed] [Google Scholar]
- 42. Loivamäki M., Gilmer F., Fischbach R. J., Sörgel C., Bachl A., Walter A., Schnitzler J.-P. (2007) Arabidopsis, a model to study biological functions of isoprene emission? Plant Physiol. 144, 1066–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vickers C. E., Possell M., Laothawornkitkul J., Ryan A. C., Hewitt C. N., Mullineaux P. M. (2011) Isoprene synthesis in plants: lessons from a transgenic tobacco model. Plant Cell Environ. 34, 1043–1053 [DOI] [PubMed] [Google Scholar]
- 44. Huxley P. A. (1971) Leaf volume: a simple method for measurement and some notes on its use in studies of leaf growth. J. Appl. Ecol. 8, 147–153 [Google Scholar]
- 45. Ellis J. R., Leech R. M. (1985) Cell size and chloroplast size in relation to chloroplast replication in light-grown wheat leaves. Planta 165, 120–125 [DOI] [PubMed] [Google Scholar]
- 46. Rosenstiel T. N., Fisher A. J., Fall R., Monson R. K. (2002) Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol. 129, 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tholl D., Kish C. M., Orlova I., Sherman D., Gershenzon J., Pichersky E., Dudareva N. (2004) Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. The Plant Cell 16, 977–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Orlova I., Nagegowda D. A., Kish C. M., Gutensohn M., Maeda H., Varbanova M., Fridman E., Yamaguchi S., Hanada A., Kamiya Y., Krichevsky A., Citovsky V., Pichersky E., Dudareva N. (2009) The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. The Plant Cell 21, 4002–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang T.-H., Hsieh F.-L., Ko T.-P., Teng K.-H., Liang P.-H., Wang A. H.-J. (2010) Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. The Plant Cell 22, 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]