Abstract
Toxoplasma gondii is an intracellular protozoan parasite that causes a Th1 cellular immunity. Our previous study showed that T. gondii lysate antigen (TLA) treatment in S180 tumor-bearing mice resulted in tumor reduction by suppressing CD31 expression, a marker of angiogenesis. In the present study, to investigate tumor suppressive effect of TLA under the absence of T lymphocytes, athymic nude mice were compared with euthymic mice in the anti-tumorigenic effect triggered by TLA in CT26 tumors. According to the results, intratumorally injected TLA reduced tumor growth and TIMP-1 level, a metastatic marker, in both euthymic and athymic mice. TLA treatment led to a sharp increase in IL-12 expression in serum cytokine profiling of athymic mice, and increased MyD88 signals in macrophages derived from the bone marrow, implying the activation of innate immunity. The selective induction of IL-12 by TLA treatment had an anti-tumorigenic effect.
Keywords: Toxoplasma gondii, athymic mouse, anti-tumorigenesis, IL-12, TIMP-1, MyD88, NF-κB
INTRODUCTION
Toxoplasma gondii, a protozoan parasite found across the world, causes an asymptomatic disease in healthy individuals [1,2]. However, immunocompromised conditions in humans, such as AIDS and organ transplantation patients, can result in acute toxoplasmosis [1]. Recently, T. gondii infection has been highlighted to be helpful in some diseases. In this regard, our previous study examined the favorable effects of T. gondii infection and T. gondii lysate antigen (TLA) on neurodegenerative diseases and tumor induction [3,4]. In particular, the anti-tumorigenic effect of the TLA injection was due to a decrease in CD31 for anti-angiogenesis playing a potential role in reducing the tumor mass [3]. TLA treatment also delayed the tumor formation in the early stage of tumor growth in 20-methylcholanthrene-induced tumors [5]. Th1 immune responses generally play a crucial role in reducing tumor growth in mice infected with T. gondii [6,7]. Accordingly, early cellular events causing T-cell immune responses need to be well known through animal experiments.
T. gondii infection in a lung carcinoma mouse model shows significant increases in CD8+ T cells, IFN-γ mRNA levels, serum IgG2a titers, and cytotoxic T lymphocytes [6]. Apicomplexan parasite infections, like T. gondii and malaria, also suggested the importance of Th1 immunity, cytotoxic NK cells, and tumor-specific T cells in antitumor effects [8]. In general, an antitumor effect in tumor immunotherapy can be developed through the induction of an adoptive Th1-dominant immunity [9,10]. In addition, the immunological memory suitable for the generation of tumor-specific cytotoxic T lymphocytes is induced when tumor-bearing mice receive Th1 cell therapy in the immunomodulating protocol with IL-12 [9,10].
IL-12 plays a key role in the interaction between innate and adaptive arms of immunity by regulating inflammatory responses [11]. Endogenous IL-12 is required for resistance to many pathogens and chemically induced tumors [11]. In various tumor models, IL-12 treatment employs the effector mechanism of innate as well as adaptive immunity and develops an antitumor effect in tumor-induced mice [10,11]. Based on these findings, the antitumor effect of TLA treatment may be induced by some complex mechanisms, such as innate and/or adaptive immunity and anti-angiogenesis. In this regard, the present study focuses on the immune phenotype related with cytokine profiles providing antitumor immune responses after TLA treatment, and examines the relationship of T-cells for triggering of antitumor immunity. For this, the study considers athymic BALB/c/nu/nu mice and compares the results with those for euthymic BALB/c mice. The tumor size and cytokine profiles of euthymic and athymic mice, and IL-12 induction, its down-signals, and myeloid differentiating factor 88 (MyD88) in macrophages derived from bone marrow (BMMs) after TLA treatment were analyzed to examine the antitumor effect and macrophage activation.
MATERIALS AND METHODS
Experimental animals
Six-week-old female BALB/c mice and BALB/c nu/nu mice were purchased from the ORIENT BIO Animal Center (Seongnam-si, Korea). All experiments using mice were conducted according to the ethical standards of Seoul National University's IACUC (SNU-090102-3).
Preparation of T. gondii lysate antigen (TLA)
The TLA was prepared according to the procedures in a previous study [3]. Pure parasites obtained from mice intraperitoneally infected with T. gondii RH strain were washed in 10 mM PBS (pH 7.4), and sonicated on ice. The supernatant (TLA) was filter-sterilized through a membrane with 0.22 µm pores. The protein concentration was determined using the BCA™ Protein Assay Kit (Thermo Scientific, Rockford, Illinois, USA), adjusted by concentration of 100 µg/100 µl, and stored at -70℃ until used.
Tumor induction
Tumors were generated by subcutaneously injecting 3×106 CT-26 cells containing EGFP genes inserted into a viral vector (a kind gift from Dr. Jung Weon Lee at Department of Pharmacy, Seoul National University, Seoul, Korea) in the middle portion of the dorsal subcutaneous layer of experimental mice. CT-26 cells were cultured in complete DMEM containing 10% FBS (Invitrogen, Carlsbad, California, USA) and 1% antibiotics (100 IU/ml penicillin G and 100 µg/ml streptomycin) (Invitrogen). Because CT-26 cells are stable for expressing the EGFP, the intensity of the EGFP after tumor generation can be measured by the Maestro™ imaging system (Perkin Elmer Corp, Waltham, Massachusetts, USA).
Measurement of tumor size
The tumor size was measured using a caliper and calculated by the following formula: length×width×height×0.5. The tumor weight was measured immediately after mice were sacrificed [3].
Cytokine array
The experimental group was divided into 4 groups; no tumor and tumor induction by PBS and TLA (100 µg/100 µl) treatment in both euthymic BABL/c and athymic BABL/c/nu/nu mice. Pooled sera (n=3) in each group were analyzed using a mouse cytokine kit (RayBiotech, Inc., Norcross, Georgia, USA) according to the manufacturer's instructions. Signal intensity was quantified using a densitometer (Multi Gauge, Fuji Film, Tokyo, Japan), and images were processed using a luminescent image analyzer (Fuji Film). Data were interpreted by comparing the signal intensity of the cytokine level in the no-tumor group (PBS treatment) with that in the tumor-induction group (TLA treatment) for each cytokine. The difference in the cytokine level was represented in percentage terms.
Isolation of BMMs
BMMs were prepared as follows [12]. Briefly, the femur and tibia of hind legs were removed from sacrificed BALB/c mice (8-week old, ORIENT BIO). The cavity of bone marrow was flushed with cold complete RPMI media containing 10% FBS (Invitrogen) and 1% antibiotics (Invitrogen). Isolated cells were washed 3 times with RPMI media and then subcultured with the supplementation of L929 cell culture supernatant (as a source of the macrophage colony-stimulating factor; M-CSF) to be of a 20% concentration [12]. Subcultured cells were incubated for 7 days at 37℃ and 5% CO2 in 6-well culture plates (SPL Life Sciences Co., Pocheon, Korea), and adherent cells were detached by trypsin (Gibco, Grand Island, New York, USA) and used for BMMs. To verify the purity of BMMs, cells incubated with M-CSF-conditioned media (L929 cell culture supernatant) for 2 weeks were checked with an FITC-conjugated anti-mouse F4/80 monoclonal antibody (eBioscience, San Diego, California, USA). The purity of BMMs exceeded 95%.
IL-12 induction in BMMs after TLA treatment
The level of IL-12 produced in BMMs with TLA (100 µg/100 µl) treatment for 24 hr was measured using the sandwich ELISA method. The capture antibody (IL-12) (Mabtech Inc., Nacka Strand, Sweden) was diluted to 2 µg/ml in PBS (pH 7.4), coated on a 96-well plate (Costar, New York, USA) at 100 µl/well, and incubated overnight at 4℃. After the blocking step, the culture supernatant was added to the wells, and incubated for 2 hr. Then, a biotin-conjugated anti-IL-12 antibody (Mabtech Inc.) was applied and incubated for 1 hr. Finally, streptavidin-HRP (Mabtech Inc.) was added and incubated for 1 hr. TMB solution was used for the enzymatic reaction. OD levels at 450 nm were measured using a spectrophotometer (Molecular Device, Menlo Park, California, USA).
Western blot analysis of MyD88 in BMMs treated with the TLA
BMMs of 2×105 cells were cultured in 6-well plates (SPL Life Sciences Co.), and when their confluency was 90% by observation of microscopy, the BMMs were treated with the TLA (100 µg/100 µl). After 24 hr cultivation, cells were harvested and homogenized to collect soluble proteins. The concentration of soluble proteins was measured using the BCA™ Protein Assay Kit (Thermo Scientific). Soluble proteins were subjected to SDS-PAGE and then transferred to the PVDF membrane. The nonspecific binding of the membrane was blocked with 5% skim milk for 2 hr. Primary antibodies include anti-MyD88 rabbit IgG (1:5,000, Enzo Life Science, New York, USA) and anti-β-actin rabbit IgG (1:10,000, Bethly laboratory Inc., Montgomery, Texas, USA). After incubation for 12 hr, membranes were washed using 10 mM Tris-buffered saline with Tween 20 (TBS-T, pH 7.4), and then HRP-conjugated anti-rabbit IgG (1:20,000, Bethly Inc.) was used as a secondary antibody. After incubation for 2 hr, the membrane was colored with the ECL chemiluminescence kit (DNR, Jerusalem, Israel). Images of the blotted membrane were obtained using the LAS-1000 lumino-image analyzer (GE Healthcare, Cleveland, Ohio, USA).
Statistical analysis
A statistical analysis was conducted using an unpaired t-test. P-values <0.05 were considered significant, and the results are presented as the mean±SD.
RESULTS
TLA treatment reduced tumor growth in both euthymic (BALB/c) and athymic (BALB/c nu/nu) mice
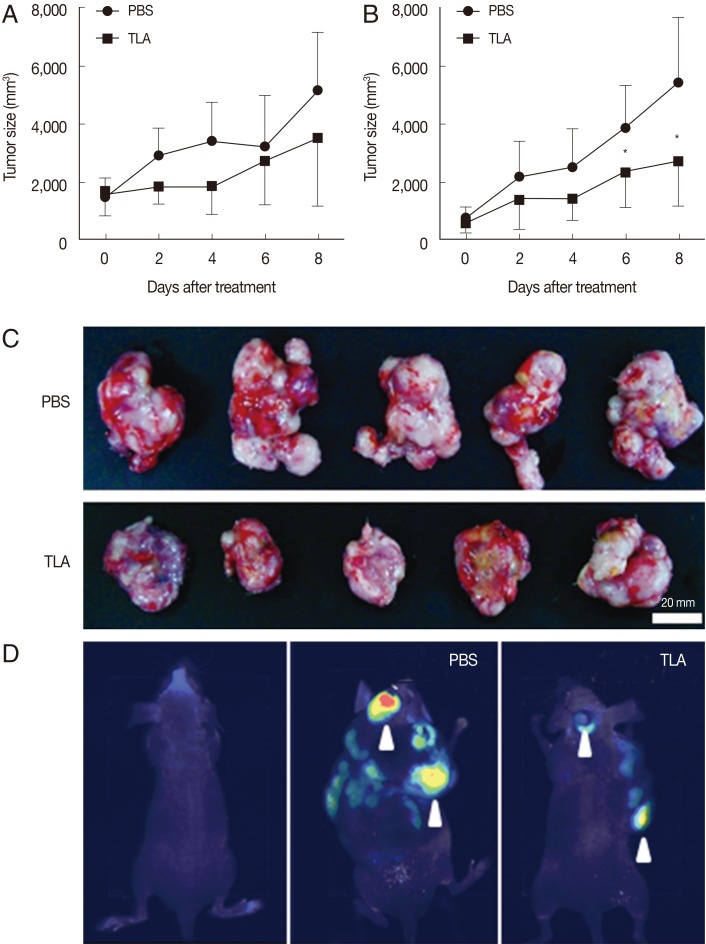
CT-26 carcinoma cells injected subcutaneously into the middle portion of the back of mice generated similar-sized tumors in both euthymic and athymic mice. Two weeks after the injection of CT-26 cells, mice were randomly assigned to the PBS- and TLA-treated groups. The TLA (100 µg/100 µl) was introduced directly by an intratumoral injection once a day over an 8-day period. In the PBS-treated group, the tumor size increased steadily over time in both euthymic and athymic mice (Fig. 1). Euthymic (Fig. 1A) and athymic (Fig. 1B) mice showed a similar trend in terms of the increase in the tumor size. However, the tumor size in TLA-treated mice did not increase over time and was smaller than that in PBS-treated mice. The difference in the tumor size on day 8 between the PBS- and TLA-treated groups was larger in athymic mice than in euthymic mice with statistical significance. Fig. 1C shows the tumor mass isolated from PBS- and TLA-treated athymic mice. The degree of metastasis was observed using an imaging system (Fig. 1D). According to the results in Fig. 1D, metastasis showed a sharp decrease in TLA-treated mice.
Fig. 1.
Effects of TLA treatment on CT26 tumor growth. When the tumor mass was about 40 mm3, mice were given PBS or TLA through an intratumoral injection in euthymic (A, n=6) and athymic (B, n=8) tumor groups. In athymic mice (B), tumor growth in TLA-treated mice was significantly lower from day 6 after TLA treatment than that in PBS-treated mice (*P<0.05). Images of tumors isolated in athymic mice on day 8 of an intratumoral injection with PBS or the TLA are shown (C). Because the EGFP gene was stably expressed in CT26 cells, the metastasis of inoculated CT26 cells was observed using an imaging system (D).
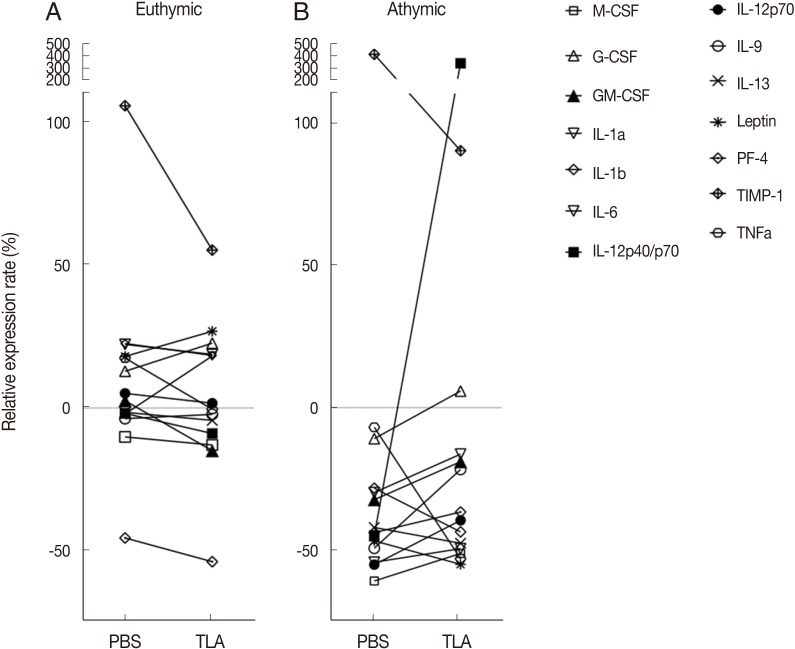
A comparison of cytokine profiles between euthymic and athymic tumor-bearing mice
Sera collected on day 8 (the end of the experiment) were pooled and applied to an assay to investigate cytokine profiles. Serum cytokine profiles of the PBS- and TLA-treated euthymic and athymic BALB/c/nu/nu mice were compared with those of non-tumor BALB/c and BABL/c/nu/nu mice based on the signal intensity of each cytokine (Fig. 2A, B). The overall pattern of the relative signal value (%) was as follows: Tumor induction (PBS treatment) in euthymic mice showed an increase below 50% at most cytokine levels except for TIMP-1, a tumor marker, in comparison to that in non-tumor mice (Fig. 2A). Tumor induction in athymic mice (PBS treatment) led to immune suppression to about -50% at most cytokine levels except for TIMP-1 in comparison to that in non-tumor athymic mice. TLA treatment in both euthymic and athymic mice induced a slight increase within 50% at most cytokine levels except for IL-12p40 and TIMP-1, suggesting an increase in immunity by TLA treatment (Fig. 2A, B). In particular, a clear outcome of TLA treatment is the sharp IL-12 induction in athymic mice. The results showing a decrease in TIMP-1 from 70.1% to 33.4% in euthymic mice and from 401.8% to 90.0% in athymic mice indicates a reduction in tumor growth and metastasis by TLA treatment. This is consistent with the results for the tumor size and metastasis based on real images in Fig. 1C and 1D and demonstrates that IL-12, which showed increases in cytokine expression in both euthymic and athymic mice, had a significant anti-tumorigenic effect.
Fig. 2.
Cytokine profiles of euthymic (A) and athymic (B) mice sera after PBS or TLA treatment. The results are shown as the relative expression rate (%) of each cytokine expression value in PBS- or TLA-treated mice in comparison to that in non-tumor euthymic (A) and athymic (B) mice. M-CSF, macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; PF-4, platelet factor-4; TIMP-1, tissue inhibitor of metalloproteinase-1.
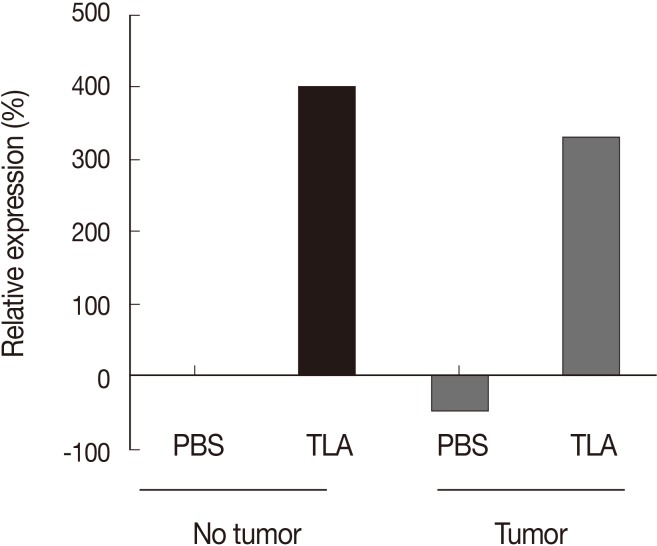
Prominent IL-12 induction as a unique function of TLA
Athymic mice deprived of mature T lymphocytes showed a marked increase in IL-12 by TLA treatment in a tumor-bearing state (Fig. 2B). To investigate whether prominent IL-12 production would be induced after TLA treatment regardless of the tumor status, PBS or TLA treatment was provided to tumor and non-tumor mice (Fig. 3). According to the results, IL-12 was produced directly by TLA treatment regardless of the tumor status. Relative IL-12 expression (e.g., the level of expression relative to the IL-12 level in non-tumor and PBS-treated mice) increased through TLA treatment by about 320-400% regardless of the presence of a tumor (Fig. 3). This indicates that the treatment of TLA alone induced IL-12 production.
Fig. 3.
An increase of IL-12 after TLA treatment regardless of the presence of a tumor in athymic mice. The results show the relative expression rate (%) between the values of IL-12p40 in each experimental group (pooled sera) based on the value of IL-12p40 in PBS-treated mice with no tumor.
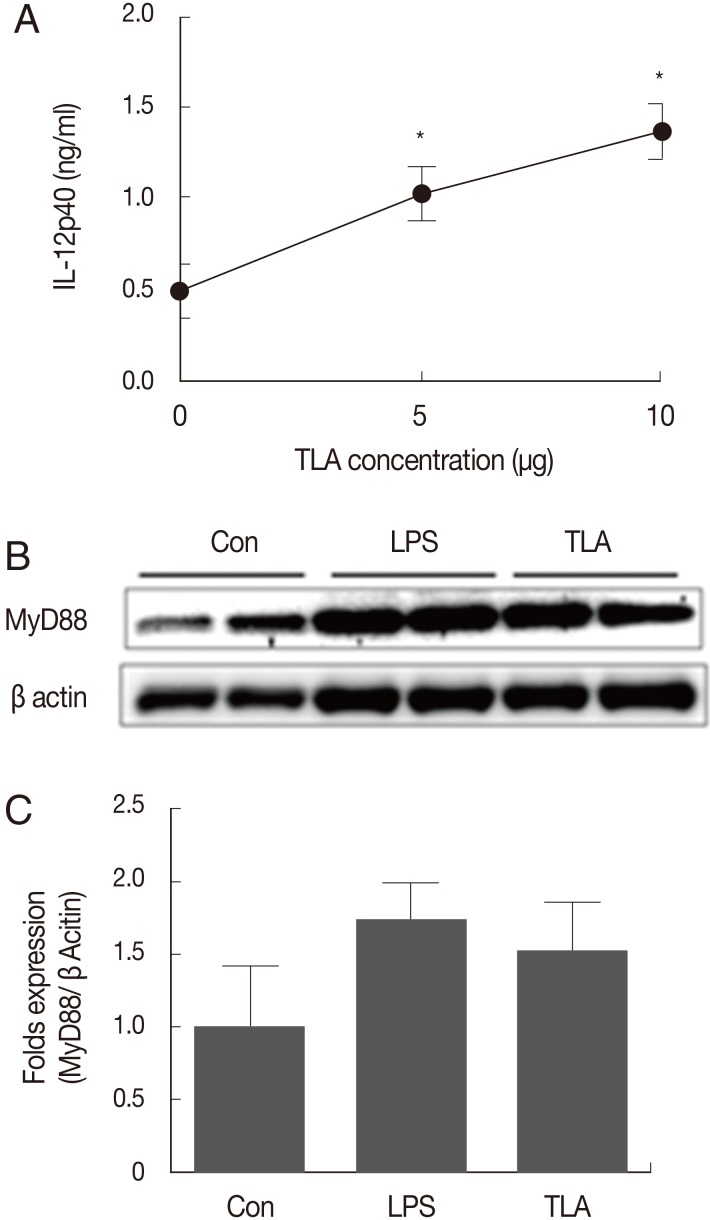
Increase of IL-12 and MyD88 cell signal induced by TLA treatment in BMMs
For athymic mice, TLA treatment directly induced IL-12 production (Figs. 2, 3). This suggests that a major candidate cell population for IL-12 production under the deprivation of mature T cells may be macrophages. Accordingly, BMMs were used to examine the relationship between TLA treatment and IL-12 expression (Fig. 4). BMMs, 2×105 cells, were loaded onto a well of a 96-well plate and cultured under 3 TLA concentrations (0, 5, and 10 µg/ml). BMMs incubated with the TLA for 24 hr were examined on the concentration of IL-12p40 (Fig. 4). The expression of IL-12p40 was increased with an increase in the TLA concentration (Fig. 4A). As a down signal of IL-12 production in BMMs, changes in MyD88 expression were verified through a western blot analysis. The expression of MyD88 was increased with an increase in the TLA concentration (Fig. 4B). As a result, the change of MyD88 signals under the TLA treatment was consistent with the result for IL-12 expression.
Fig. 4.
The induction of IL-12p40 and MyD88/NF-κB signals in BMMs after TLA treatment. There was a significant increase in IL-12p40 after TLA treatment in a dose-dependent manner (A) (*P<0.05). The activation signal of macrophages for IL-12 induction, namely MyD88, also increased after TLA treatment (B). "Con" refers to control with no treatment. Images from lanes 1 to 2 show the results for signals in control; lanes 3 to 4 in LPS treatment; lanes 5 to 6 in TLA treatment. The bar graph shows the mean fold values for signals after each stimulant treatment compared to β-actin signals.
DISCUSSION
Recent studies have provided potential therapeutic results underlying tumor growth reductions immunologically regulated by microorganisms [3,5,6,7,8,13,14,15]. In particular, bacteria and viruses show anti-tumorigenesis by mechanisms with cytolytic activity through their intracellular replication [13,14]. Besides, the anti-tumorigenic effect of T. gondii has been verified in several in vivo and in vitro experiments [3,5,6,7,8]. For example, there is a decrease in CD31, a marker for angiogenesis, after an intratumoral injection of TLA, suggesting some molecular intervention in anti-angiogenesis [3]. T. gondii infection triggers a Th1 cellular immune response for the inhibition of angiogenesis [6]. Because a therapeutic measure for the inhibition of tumors may be induced by Th1-dominant immunity [9], the antitumor effect of T. gondii infection reported in various tumors, including sarcomas, lung carcinoma, and ependymoblastomas, was also expected by cellular immune responses triggered by T. gondii [3,6,7].
Our previous study reported that T. gondii infection as well as TLA treatment showed a similar anti-tumorigenic effect suggesting that the immune environment elicited by TLA is similar to that by T. gondii infection. Accordingly, the present study addressed the topic of early triggering mechanisms underlying the antitumor effect of TLA treatment, such as early innate or adaptive immune responses. For this, the immune characteristics of euthymic BALB/c mice were compared with those of athymic nu/nu BALB/c mice. EGFP-inserted CT-26 cells were subcutaneously injected into mice to induce a tumor mass. The results after an intratumoral injection of PBS or the TLA indicate that the tumor size was smaller in TLA-treated mice than in PBS-treated mice, and this pattern was similar between euthymic (BALB/c) and athymic (BALB/c nu/nu) mice with a significant difference in athymic mice. Our data shows that the TLA has anti-tumorigenic effect regardless of the type of tumor, such as S180 sarcoma and CT26 colon carcinoma, and without engaging T cells. Another important finding is that, according to image data on EGFP fluorescence intensity and cytokine profiling data, TLA treatment clearly reduced the metastasis of locally introduced tumor cells and serum TIMP-1 level, a metastatic marker. Tissue inhibitor of metalloproteinase-1 (TIMP-1) has been suggested as a marker for both the treatment prognosis and therapeutic responses and was clinically demonstrated in breast cancer [16]. In the present study, the TIMP-1 level showed a marked increase in the tumor-induced state in both euthymic and athymic mice (70.1% and 401.8%, respectively) in comparison to non-tumor mice. After TLA treatment, however, the TIMP-1 level decreased sharply to 33.4% and 90.0%, respectively, suggesting metastasis suppression.
As mentioned earlier, Th1-type immune responses triggered by microorganisms (some types of viruses, bacteria, and parasites) are partially similar to antitumor immune mechanisms [3,5,6,7,8]. The key role played by Th1-dominant immunity in tumor immunology has been well documented for IL-12, NK cells, and other Th1 cytokines, and the induction of these cytokines has been based on the development of tumor-specific immunotherapy [9]. In this respect, T. gondii infection can selectively produce IL-12, which is also important in tumor immunology [17], and this raises the question of the extent to which the antitumor effect of TLA treatment depends on T cells. However, because our results showed the reduction of tumor mass under the state of a sharp increase of IL-12 and deprivation of mature T cells, this study highlights that IL-12 induced by TLA treatment plays the important role in the induction of anti-tumorigenic effects.
In general, an increase in IL-12, CD8+ T-cells, IFN-γ, serum IgG2a, or NK cells suggests an increase in the Th1 immune response and indicates the induction of antitumor effects [6,9,11,18,19]. Although IL-12 induction has an anti-tumorigenic effect through early innate immune responses, further cellular immunity is essential for some immune synergy in the reduction of tumor growth. However, other proinflammatory cytokines except for IL-12 (IL-12p40) showed no remarkable increases in athymic nude mice. In general, NK cells are a major producer of IFN-γ, which is related to an IL-12-induced antitumor effect [19]. The increase in IL-12 observed in the present study may be linked to further cellular immunity in both euthymic and athymic mice. Because nude mice show the appearance of marked granulocytosis instead of lymphocytes [20], our results strongly suggest that the antitumor effect of TLA treatment is related to not T-cell immunity but enforced innate immunity.
To examine whether TLA treatment initiates innate immunity by macrophages, this study considered BMMs and investigated the increase of TLR signaling in macrophage activation. Macrophages and NK cells are major immune cells against tumors, and their antitumor immunity depends on IL-12 production and cell activation [21]. During an innate immune response, the activation of macrophages can be determined by TLR signaling through MyD88 [11,22,23]. TLA treatment induced IL-12 production in BMMs, which is consistent with the results for euthymic and athymic mice. In addition, MyD88 signals were increased in a dose-dependent manner with the TLA concentration. MyD88-triggering IL-12 synthesis results from the stimulation of the Toll-like receptors (TLR) [11,17,22]. Recent studies have identified some proteins that selectively induce IL-12 production from T. gondii [24,25,26,27]. Among these, profilin triggers MyD88 signaling [24,25]. Although the present study does not investigates the role of profilin in IL-12 production after TLA treatment, the results suggest that a certain TLA component, like T. gondii profilin, may induce resistance to tumor growth through mechanisms related to early and selective IL-12 induction.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program of the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT, and Future Planning (NRF-2011-0013824) and by the Seoul National University Bundang Hospital Research Fund (SNUBH Grant no. 03-2011-016).
Footnotes
The authors report no conflict of interest related to this study.
References
- 1.Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:11423–11428. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim H, Lee SE, Jung BK, Kim MK, Lee MY, Nam HW, Shin JG, Yun CH, Cho HI, Shin EH, Chai JY. Serologic survey of toxoplasmosis in Seoul and Jeju-do, and a brief review of its seroprevalence in Korea. Korean J Parasitol. 2012;50:287–293. doi: 10.3347/kjp.2012.50.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyo KH, Jung BK, Chai JY, Shin EH. Suppressed CD31 expression in sarcoma-180 tumors after injection with Toxoplasma gondii lysate antigen in BALB/c mice. Korean J Parasitol. 2010;48:171–174. doi: 10.3347/kjp.2010.48.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung BK, Pyo KH, Shin KY, Hwang YS, Lim H, Lee SJ, Moon JH, Lee SH, Suh YH, Chai JY, Shin EH. Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memory impairments in a murine model of Alzheimer's disease. PLoS One. 2012;7:e33312. doi: 10.1371/journal.pone.0033312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyahara K, Yokoo N, Sakurai H, Igarashi I, Sakata Y, Yoshida Y, Saito A, Hirose T, Suzuki N. Antitumor activity of Toxoplasma lysate antigen against methylcholanthrene-induced tumor-bearing rats. J Vet Med Sci. 1992;54:221–228. doi: 10.1292/jvms.54.221. [DOI] [PubMed] [Google Scholar]
- 6.Kim JO, Jung SS, Kim SY, Kim TY, Shin DW, Lee JH, Lee YH. Inhibition of Lewis lung carcinoma growth by Toxoplasma gondii through induction of Th1 immune responses and inhibition of angiogenesis. J Korean Med Sci. 2007;22(suppl):S38–S46. doi: 10.3346/jkms.2007.22.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibbs JB, Jr, Lambert LH, Jr, Remington JS. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971;124:587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, Chen L, Zhong N, Chen X. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS One. 2011;6:e24407. doi: 10.1371/journal.pone.0024407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(suppl):S52–S61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 10.Egilmez NK, Kilinc MO. Tumor-resident CD8+ T-cell: the critical catalyst in IL-12-mediated reversal of tumor immune suppression. Arch Immunol Ther Exp (Warsz) 2010;58:399–405. doi: 10.1007/s00005-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 11.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 12.Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008:pdb.prot5080. doi: 10.1101/pdb.prot5080. (doi: 10.1101/pdb.prot5080) [DOI] [PubMed] [Google Scholar]
- 13.Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ring CJ. Cytolytic viruses as potential anti-cancer agents. J Gen Virol. 2002;83:491–502. doi: 10.1099/0022-1317-83-3-491. [DOI] [PubMed] [Google Scholar]
- 15.Silobrcic V, Fredrickson G, Sedlacek R, Suit HD, Wolberg G. Immune reaction of tumor-bearing mice to Propionibacterium acnes and the antitumor effect of the bacteria. Cancer Immunol Immunother. 1982;14:10–12. doi: 10.1007/BF00199425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Würtz SO, Schrohl AS, Mouridsen H, Brünner N. TIMP-1 as a tumor marker in breast cancer-an update. Acta Oncol. 2008;47:580–590. doi: 10.1080/02841860802022976. [DOI] [PubMed] [Google Scholar]
- 17.Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, Aliberti J. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol Res. 2003;27:521–528. doi: 10.1385/IR:27:2-3:521. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Yu H, Xu TF, Li JH, Sun YF, Zhang WQ. Interleukin-12 gene modification exerts anti-tumor effects on murine mammary sarcoma cell line in vivo. Cell Mol Immunol. 2008;5:225–230. doi: 10.1038/cmi.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uemura A, Takehara T, Miyagi T, Suzuki T, Tatsumi T, Ohkawa K, Kanto T, Hiramatsu N, Hayashi N. Natural killer cell is a major producer of interferon gamma that is critical for the IL-12-induced anti-tumor effect in mice. Cancer Immunol Immunother. 2010;59:453–463. doi: 10.1007/s00262-009-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wortis HH. Immunological responses of 'nude' mice. Clin Exp Immunol. 1971;8:305–317. [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 22.Del Rio L, Butcher BA, Bennouna S, Hieny S, Sher A, Denkers EY. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J Immunol. 2004;172:6954–6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- 23.Whitworth JM, Alvarez RD. Evaluating the role of IL-12 based therapies in ovarian cancer: a review of the literature. Expert Opin Biol Ther. 2011;11:751–762. doi: 10.1517/14712598.2011.566854. [DOI] [PubMed] [Google Scholar]
- 24.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 25.Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 2005;26:509–511. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Aliberti J, Reise Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 27.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]