Abstract
Aim
To determine pre- and post-dosing effects of prenatal methadone compared to buprenorphine on fetal well-being.
Design
A secondary analysis of data derived from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study, a double-blind, double-dummy, randomized clinical trial.
Setting
Six United States sites and one European site that provided comprehensive opioid-dependence treatment to pregnant women.
Participants
81 of the 131 opioid-dependent pregnant women completing the MOTHER clinical trial, assessed between 31 and 33 weeks of gestation.
Measurements
Two fetal assessments were conducted, once before and once after study medication dosing. Measures included mean fetal heart rate (FHR), number of FHR accelerations, FHR reactivity in the fetal non-stress test (NST), and biophysical profile (BPP) score.
Findings
Significant group differences were found for number of FHR accelerations, non-reactive NST, and BPP scores (all Ps < 0.05). There were no significant group differences before time of dosing. Significant decreases (all Ps < 0.05) occurred from pre- to post-dose assessment for mean FHR, FHR accelerations, reactive NST, and fetal movement. The decrease in accelerations and reactive NST were only significant for fetuses in the methadone group and this resulted in a significantly lower likelihood of a reactive NST compared to fetuses in the buprenorphine group.
Conclusion
Buprenorphine compared with methadone appears to result in less suppression of mean fetal heart rate, fetal heart rate reactivity, and the biophysical profile score after medication dosing and provide support for the relative safety of buprenorphine when fetal indices are considered as part of the complete risk-benefit ratio.
Keywords: fetal, opiates, biophysical profile, heart rate, tbc
INTRODUCTION
Although neither the U.S. Food and Drug Administration (FDA) nor the European Medicines Evaluation Agency (EMEA) has approved methadone for the treatment of opioid dependence in pregnancy, it is considered the standard of care for pregnant opioid dependent women, given over 40 years of data supporting its efficacy and safety in the mother, fetus, and the newborn. Compared to no treatment or medication-assisted withdrawal, methadone-treated pregnant women enrolled in a comprehensive treatment program have improved prenatal care and less illicit substance use, and their infants have improved neonatal outcomes [1–3].
There is growing evidence that buprenorphine, while currently approved only for the treatment of opioid dependence in non-pregnant patients, may serve as an efficacious and relatively safe medication for the expectant mother, fetus, and neonate [4–7]. We have shown previously in a randomized clinical trial (The Maternal Opioid Treatment: Human Experimental Research: MOTHER) the utility and relative maternal and neonatal safety of both methadone and buprenoprhine, with buprenorphine also demonstrating a statistically significant and clinically meaningful reduction in neonatal abstinence severity and treatment duration compared to methadone [8].
While there are numerous reports on the management of the methadone-exposed newborn, the effects of methadone on the fetus are less well understood. Both methadone and buprenorphine cross the placenta [9, 10] with concentrations that reflect maternal dose during pregnancy [11, 12] and, as such, have the potential to affect the fetus.
Several indices of fetal well-being are part of standard obstetric care and have been utilized to measure the effects of drugs on the fetus, including the external, non-invasive fetal non-stress test (NST) and the biophysical profile (BPP). The NST involves electronic monitoring of fetal heart rate (FHR) to determine if at least two FHR accelerations from baseline level occur within a 20-minute period. The absence of such FHR reactivity, usually in response to fetal movement, is predictive of intrauterine growth restriction, hypoxia, and less optimal neonatal outcomes [13, 14]. A reactive NST is one of five parameters measured in the BPP in addition to the presence of fetal movement, tone, breathing, and adequate amniotic fluid [15]; low BPP scores have been related to fetal distress, perinatal mortalities and abnormalities in the fetal central nervous system [16–18].
Anyaegbunam et al. (1997) compared measures of fetal well-being in methadone-maintained pregnancies and age, parity and gestational age-matched, non-drug exposed controls. They reported that 19.6% of the methadone-exposed fetuses compared to only 4.2% of those in the control group had a non-reactive NST (P <0.01); methadone-exposed fetuses also took longer to achieve a reactive study (35.5 v. 14.9 minutes, P = 0.0016) [19]. In another study, methadone and other opiates were associated with decreased mean fetal heart rates as early as 11 weeks of gestation relative to a comparison group of non-drug-dependent mothers matched on crown–rump length, maternal age, ethnic background and smoking status (methadone: mean FHR = 156.0, SD = 7.3, controls: mean FHR = 159.6, SD = 6.5; P = 0.02) [20]. Others have reported decreased body movements, observed fetal breathing movements, and coupling of fetal movement and heart rate accelerations after methadone administration [21–23]. Despite the suppression of the parameters used to score the BPP, the overall score was not found to differ before or after dosing when given additional time to complete the assessment and was determined to be a clinically useful indicator of fetal well-being in methadone-exposed fetuses [24].
To our knowledge, there has only been one previous report analyzing the effects of buprenorphine compared to methadone on fetal behavior. This report, which utilized participants from one MOTHER study site, demonstrated that in later fetal development (32–35 weeks) buprenorphine-exposed fetuses displayed less suppression of motor activity and longer duration of movements compared to a methadone-exposed group [25].
As part of the MOTHER trial, at approximately 32 weeks of gestation, fetal assessment was scheduled prior to and following maternal double-blind opioid medication administration. The purpose of this fetal testing was to examine the differential effect of methadone and buprenorphine on fetal well-being measured by a fetal non-stress test and biophysical profile. Given a lower severity and shorter duration of NAS treatment in the buprenorphine compared to methadone-exposed neonates seen in the MOTHER trial, and based on the published data to date, we hypothesized that both medication groups would have reductions in fetal well-being parameters after receiving the study drug dose compared to before the dose, but that buprenorphine-exposed fetuses would have more optimal fetal assessments after the medication dose than methadone-exposed fetuses, including higher likelihood of having a reactive NST, more FHR accelerations, and more fetal breathing movements. We did not expect to find differences between the groups in the pre-dose assessment.
METHODS
Parent Study
Participants for this study were enrolled in and completed the MOTHER study, a multi-site randomized clinical trial to compare the safety and efficacy of buprenorphine and methadone in pregnant opioid-dependent women and their neonates [8]. All sites received local Institutional Review Board approval, and independent data and safety oversight was conducted by a Data and Safety Monitoring Board. A detailed description of site selection, study coordination, subject selection, and protocol details are published elsewhere is this Addiction issue [26]. The primary and key secondary outcomes have been previously reported [8].
Participants
Briefly, pregnant women with confirmed opiate dependence were enrolled if they were between 18 and 41 years of age, carrying a single fetus, and were between 6 and 30 weeks of gestation. A subset of the participants reported on here (N=7 in the methadone and N=3 in the buprenorphine group) were included in a separate NIDA-funded study (PI: Lauren Jansson) and subsequent published report examining measures of fetal heart rate, fetal movement and their coupling collected at expanded times including earlier and later gestational ages than this assessment [25].
Fetal Assessment
The MOTHER protocol called for maternal participants to complete two fetal assessments at 32 weeks (range 31.7 to 33.7 weeks) into their gestation; one assessment two hours prior to scheduled double-blind study medication dosing and a repeated assessment two hours after dosing. Of the 131 women enrolled in the MOTHER study, 4 delivered their infants prior to completing the assessment and 7 women did not attend their scheduled assessment session, yielding 120 participants with data potentially available for the analysis of fetal assessment data OR biophysical profile data. Subsequent to the publication of the MOTHER primary outcomes paper and the freezing of the MOTHER database, it was determined that there was a failure to enter fetal assessment data into the MOTHER database for 5 participants, due to data transmission or data entry errors. There were also another 34 participants who provided data that were not included in the MOTHER database because their data were considered by the Coordinating Center to be outside of the correct timing window for either gestational age or pre- and/or post-medication dosing. Therefore, 81 participants had complete pre- and post-dose fetal assessment data available for analysis.
The fetal assessment included fetal heart rate monitoring for a fetal Non-Stress Test (NST) and real time B-mode ultrasound imaging for a Biophysical Profile (BPP) [27]. All assessments were completed and interpreted by physicians or nurses who were trained and certified for standard fetal ultrasound and heart rate monitoring at each institution.
Non-Stress Test
The NST is an external, non-invasive monitoring of fetal heart rate (FHR) in beats per minute (bpm) using a pulsed Doppler transducer for a continuous period of at least 30 minutes. Standard definitions suggested by the National Institutes of Child Health and Human Development Workshop Report on Electronic Fetal Monitoring were used for the measures obtained from fetal heart rate patterns [28, 29]. FHR accelerations are abrupt increases in FHR from baseline of at least 15 beats per minute (bpm) for at least 15 seconds. A change of 10 bpm for 10 seconds is used as the criterion before 32 weeks GA. A “reactive” NST indicates normal autonomic function and is defined as at least two FHR accelerations in a 20-minute period, otherwise the NST was considered “non-reactive”. In the present study, the variable was re-coded based on the recorded number of fetal heart rate accelerations for each participant so that those who had 2 or more accelerations were coded as “reactive” and those who had less than 2 were coded as “non-reactive”.
Biophysical Profile
The BPP is also a non-invasive test that is traditionally used clinically to predict the presence of fetal distress and abnormalities in the fetal central nervous system [16, 17]. The BPP generates a biophysical profile score from 0–10 determined by the presence (score = 2; normal) or absence (score = 0; abnormal) of five parameters within a 30-minute period: a reactive NST, two or more gross body movements, one or more episodes of limb or hand flexion and extension (muscle tone), breathing movements lasting at least 20 seconds, and a normal amniotic fluid volume index (AFI; 5–24 cm for the 4 quadrant total). Two of the sites (Baltimore and Vienna) coded the parameters of fetal breathing, tone and movement on a 3 point scale (0, 1, and 2) rather than 0 or 2 for 7 of the participants, with a score of “1” indicating that partial criteria were met. These cases were re-coded to conform to the standard definition. Those with a score of “1” for fetal breathing or movement were coded as 0 as consistent fetal breathing and at least 2 gross body movements are required for a score of “2”. However, fetal tone is considered present if fetal movement is observed at all; therefore a score of 1 for fetal tone was re-coded to a “2”.
Outcome Measures
The outcome measures included: (1) mean fetal heart rate (beats per minute) over the course of the NST, a continuous measure assumed to follow a normal distribution; (2) the number of FHR accelerations (0, 1, 2, 3, or more than 3, which was coded as 4); (3) BPP total score, both assumed to follow a Poisson distribution; (4) reactive NST (yes v. no) and (5) observed fetal breathing (yes v. no), both assumed to follow a binomial distribution.
Statistical Analysis
Independent variables included Medication Group (buprenorphine v. methadone) and study Site [US Urban (Baltimore, MD; Philadelphia, PA: Detroit MI, Providence, RI) v. US Rural (Burlington, VT; Nashville, TN) v. European (Vienna)], pooled to minimize the potential for site heterogeneity to adversely impact the statistical analyses [8], and Assessment (pre- v. post-dosing), together with the Medication Group X Assessment interaction, which would test for differential change from pre- to post-dosing testing in the two medication groups. Covariates were included in the models to control for their potential influence on the outcomes variables: gestational age at time of the assessment [30], fetal sex [31], the number of hours before or after drug dose (1, 2, or 3) [32], and time of day (morning, afternoon, or evening) the fetal assessment occurred [33].
Baseline maternal demographic measures were compared between medication groups using one-way analysis of variance for continuous measures and χ2 tests of independence for categorical measures using exact methods. A generalized estimating equations (GEE) approach was utilized to estimate and test the parameters in the statistical model for the outcome measures. GEE is an extremely flexible statistical approach, and allows for inferential analyses for continuous, Poisson, and binomial outcomes, and allows for missing data on the outcome measures. Pairwise comparisons of the parameters estimates for any significant interaction effect were made for significant analyses using a Bonferroni correction for multiple comparisons. All analyses were conducted using SPSS 19.0 software.
RESULTS
Demographics
The demographic data of the participants with missing data did not differ from those who had complete data available. Of the 81 participants with full data available, 33 (40.7%) had been randomized to buprenorphine treatment and 48 (59.3%) to methadone treatment. Table 1 presents the demographic variables for the participants in each medication group. There were no significant differences between the groups on any of the demographic measures (all Ps > 0.05).
Table 1.
Maternal & Fetal Characteristics
| Buprenorphine (n=33) M (SD) or f(%) |
Methadone (n=48) M (SD) or f(%) |
F or χ2 | p< | |
|---|---|---|---|---|
| Maternal age in years | 25.0 (5.2) | 27.3 (5.5) | 3.4 | 0.07 |
| Number of pregnancies | 2.2 (0.3) | 2.8 (0.2) | 1.9 | 0.17 |
| Number of living children | 1.5 (0.2) | 1.7 (0.2) | 1.0 | 0.31 |
| Number of OB Visits | 8.8 (2.9) | 8.7 (4.0) | 0.0 | 0.88 |
| Maternal Weight Gain (pounds) | 20.2 (13.3) | 17.5 (17.7) | 0.5 | 0.47 |
| Body Mass Index at enrollment | 24.6 (5.6) | 26.2 (6.8) | 1.2 | 0.28 |
| Systolic Blood Pressure^ | 110.4 (11.4) | 110.7 (9.2) | 0.0 | 0.90 |
| Diastolic Blood Pressure^ | 65.2 (9.1) | 66.1 (8.7) | 0.2 | 0.68 |
| Fundal Height^ | 30.1 (7.1) | 32.0 (1.4) | 2.4 | 0.12 |
| Gestational Age at Fetal Assessment | 31.9 (0.7) | 32.1 (0.6) | 1.2 | 0.28 |
| Gestational age at study entry | 0.1 | 0.83 | ||
| Early (6–21 weeks) | 18 (54.6%) | 25 (52.1%) | ||
| Late (22–30 weeks) | 15 (45.5%) | 23 (47.9%) | ||
| Fetal Sex=Male | 19 (57.6%) | 20 (41.7%) | 1.4 | 0.24 |
| Race | 4.3 | 0.12 | ||
| White | 31 (93.9%) | 41 (85.4%) | ||
| Black | 1 (3%) | 7 (14.6%) | ||
| Other | 1 (3%) | 0 (0%) | ||
| Education | 1.0 | 0.62 | ||
| More than High School | 5 (15.2%) | 7 (14.6%) | ||
| High School Graduate | 11 (33.3%) | 21 (43.8%) | ||
| Less than high school | 17 (51.5%) | 20 (41.7%) | ||
| Employed | 6 (18.2%) | 7 (14.6%) | 0.2 | 0.76 |
| Legal status uninvolved | 29 (87.9%) | 35 (79.9%) | 1.8 | 0.16 |
| Married | 4 (12.1%) | 7 (14.6%) | 0.1 | 0.75 |
| Gestational Diabetes | 0 (0%) | 1 (2.1%) | 0.0 | 0.85 |
| Current cigarette smoker | 31 (93.9%) | 47 (97.9%) | 0.9 | 0.56 |
| Cocaine use prior to enrollment | 7 (21.2%) | 16 (33.3%) | 0.9 | 0.35 |
| Antidepressant | 24 (72.7%) | 32 (66.7%) | 0.1 | 0.74 |
| Anxiolytics | 14 (42.4%) | 13 (27.1%) | 1.4 | 0.23 |
Mean of weeks 30–34 of pregnancy; data available for n=30 Buprenorphine and n=47 methadone
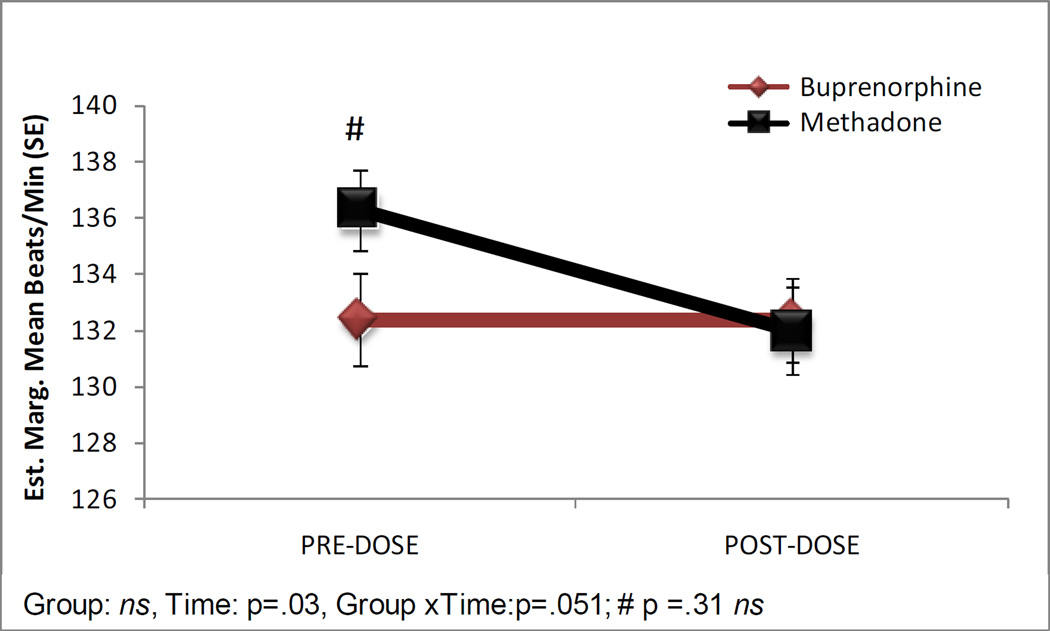
Figure 1 shows the estimated marginal means for FHR from the pre- and post-dose assessments by medication group. Two participants in the methadone group did not have values entered for post-dose FHR. The medication groups did not significantly differ with the mean FHR (SE) for fetuses in the buprenorphine group of 132.37 (1.37) compared to 134.15 (1.11) for those in the methadone group (b = .37, SE = 2.02, P = .86). There was a significant change over assessment with a mean FHR (SE) of 134.34 (1.18) in the pre-dose assessment compared to 132.18 (1.12) in the post-dose assessment (b = 4.31, SE = 1.99, P = 0.03). The medication group X assessment effect approached significance (b = −4.28 SE = 2.19, P = 0.051), as there was a non-significant trend for a decrease in mean FHR from pre- to post-dose assessment for fetuses in the methadone group only (P = 0.19). Fetuses in the buprenorphine group showed a lower mean FHR than fetuses in the methadone group in the pre-dose assessment (132.38 (1.66) v. 136.30 (1.44), respectively), however this was not a significant difference based on pair-wise comparisons with Bonferroni correction (P = 0.31); the groups did not differ at the post-dose assessment (buprenorphine=132.36 (1.47), methadone=131.99 (1.54), P = 1.0).
Figure 1.
Fetal heart rate estimated marginal means in beats per minute in the pre and post-dose assessments. #Pairwise comparison with a Bonferonni correction, P > 0.05, not significant (ns).
Overall, fetuses in the buprenorphine group had more FHR accelerations than those in the methadone group [2.92 (.17) v 2.41 (.17); b =.27 SE =.13, P = 0.04], see Figure 2). Fetuses had more FHR accelerations in the pre-dose assessment compared to the post-dose assessment [3.06 (.14) v. 2.30 (.18); b = .36 SE = .18, P = 0.002]. Although the overall group X assessment interaction was not significant (P = 0.20), the methadone group had a significant decrease from a mean (SE) of 2.89 (.18) accelerations in the pre-dose assessment to 2.01 (.23) in the post-dose assessment (P = 0.004).
Figure 2.
Exponentiated mean number of Fetal Heart Rate (FHR) Accelerations in the pre- and post-dose assessments.
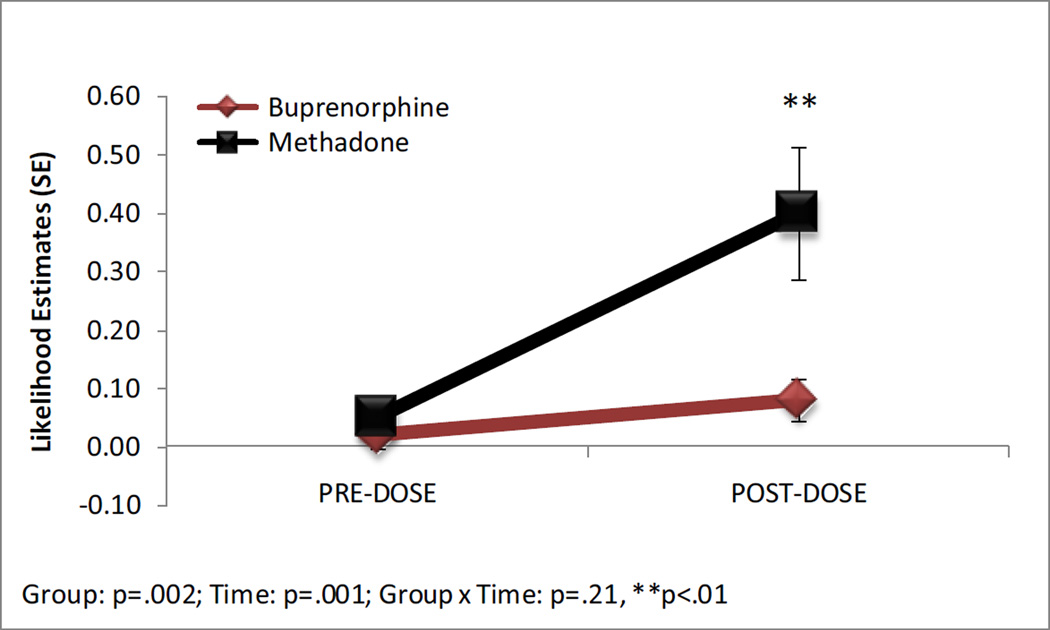
A non-reactive NST occurred less frequently in the buprenorphine group than the methadone group (b = −2.05, SE = .67; adjusted Odds Ratio (aOR) =.13, 95% CI = .04 – .48), P =0.002, see Figure 3). A non-reactive NST was less likely to occur in the pre-dose assessment compared to the post-dose assessment overall (b = −2.45, SE = .62; aOR = 0.09, 95% CI =.03 – .29, P = 0.006). This result was due to fetuses in the methadone group being less likely to have a non-reactive NST before the dose compared to after the dose (dose difference = −.35, SE = .11, P = 0.01) and more likely than fetuses in the buprenorphine group to have a non-reactive NST after receiving the medication (dose difference = −.33, SE = 0.12, P = 0.008).
Figure 3.
Likelihood estimates for a non-reactive fetal non-stress test in the pre- and post-dose assessments. ** Pairwise comparison with a Bonferonni correction, P < 0.01.
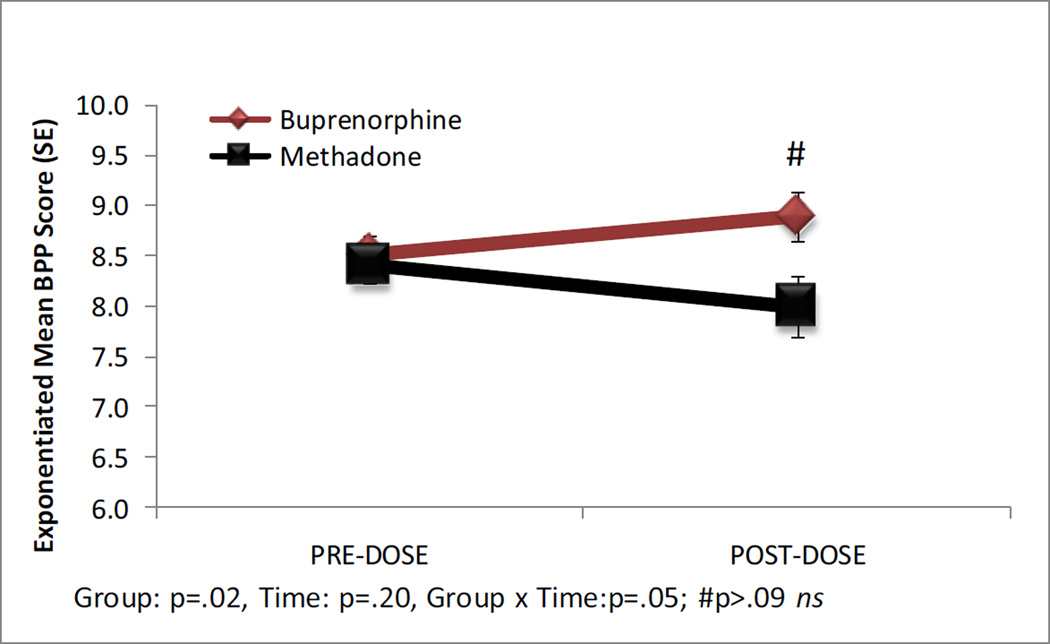
The biophysical profile scores are shown in Figure 4. Four fetuses in the methadone group (8.3%) and two (6.1%) in the buprenorphine group were missing parameter scores for the BPP and were excluded from summary score analyses. BPP scores were higher for fetuses in the buprenorphine compared to the methadone group [8.70 (.15) v. 8.20 (.20); b = .11 SE = .05, P = 0.02]; however the overall change from pre- to post- dosing was not significant (P = 0.20). There was a significant medication group X assessment interaction (b = −.09, SE = .05, P = 0.05); however, the pair-wise comparisons for each medication group over time and between groups at each assessment were not significant (all Ps > 0.05).
Figure 4.
Exponentiated mean Biophysical Profile Score (BPP) in the pre- and post-dose assessments. #Pairwise comparison with a Bonferonni correction, P > 0.05, not significant (ns).
Absence of observable fetal breathing movements in the pre-dose assessment had an estimated likelihood of occurrence (SE) of .18 (.07) for fetuses in the buprenorphine group and .15 (.06) in the methadone group. In the post-dose assessment these estimates increased in both groups, .24 (.08) and .28 (.09), respectively. However, the estimates were not significantly different between medication groups (P = 0.69) and the increase from pre- to post-dose assessments was not significant (P = 0.19).
All but one of the fetuses met criteria for fetal movement on the biophysical profile scoring in the pre-dose assessment; the estimated likelihood of occurrence (standard error) in the buprenorphine group was .04 (.03) and .05 (.03) for the methadone group. In the post-dose assessment, 2 (6.5%) of the buprenorphine group [.09 (.05)] and 6 (13.6%) of the methadone-group [.24 (.08)] did not meet the movement criteria for the BPP score. The group difference was not significant (P = 0.16), although the time effect was significant [aOR=.16 (.04–.69), P =0.01].
DISCUSSION
The main findings in this study were that at times estimated to be peak dosing effect, buprenorphine-exposed fetuses were more likely to have a reactive non-stress test (NST) with more fetal heart rate (FHR) accelerations and higher biophysical profiles scores than were methadone-exposed fetuses. The medication groups did not differ on these measures before the respective study medications were given. The conservative pair-wise comparisons revealed that the increased likelihood of a non-reactive NST after methadone dosing compared to buprenorphine was significant. These data indicate that buprenorphine dosing has less of a suppression of mean FHR, FHR variability, and the ability of the autonomic system to respond to and integrate responses to movement. There was an increased occurrence of fetuses not meeting movement criteria in both groups after medication dosing, indicating that both groups had suppressed movement with peak medication dose.
These findings agree with a recently published study comparing buprenorphine and methadone effects on fetal measures after once-a-day medication dosing. Using more precise measurement of fetal heart rate and FHR variability in a total sample of 17 fetuses (10 of whom were also included in these analyses), Jansson et al. (2010) found that buprenorphine-exposed fetuses had higher FHR variability with more accelerations and more integration between FHR and movement than methadone-exposed fetuses [25]. Taken together, the data presented here in a larger sample than was previously examined, provide further evidence that buprenorphine may contribute to more robust fetal well-being at least during the hours of assumed medication peak. The lack of a group difference during the apparent opiate trough (before the medication dose) suggests that the suppression of fetal well-being parameters is transient. The lack of a non-opiate-exposed comparison group prevents conclusions being drawn about pre-dose findings.
Single daily-dosing of methadone has been associated with a suppression of fetal heart rate variability and reactivity at peak levels in human fetuses [19, 20, 25, 34]. This finding is in contrast to data from fetal lambs that showed an increase in FHR and body movements (arousal) while suppressing both active and quiet sleep states [35]. Quiet sleep was also found to be suppressed after methadone administration in pregnant ewes [36] Quiet sleep is characterized by low FHR variability and few body movements; two parameters that were are often found at peak methadone doses, and was found in the current study. Sleep state has not been measured in human fetuses exposed to opiate treatment and was not measured in this study, but is the next step in understanding the effects seen with opiate administration. Newborns who had been exposed to methadone treatment in utero were found to have more active sleep and less quiet sleep than control infants in the first week after delivery [37]. It is not clear if these sleep disturbances are related to opiate withdrawal or medication exposure. Examination of the prenatal, neonatal and longer term changes in sleep and arousal in these infants is needed. Although few studies have been conducted in this area, the available data point to long term impairments in mechanisms directly related to sleep-state organization including motor inhibition, activity, and arousal mechanisms for children who were prenatally exposed to methadone and/or heroin [38].
While the strength of the study includes its multi-site, randomized, double-blind, double-dummy design, several limitations and cautions apply. Hospital medical staff specifically trained to conduct and interpret fetal testing procedures collected the data for this study; however there was no confirmation of the findings from a second clinician, and there was some variation at two institutions in how the BPPs were initially scored. BPPs were re-coded from the five individual parameters (fetal movement, tone, breathing, reactive NST, and amniotic fluid index) across all sites according to standard definitions. Values for the re-coded variable did not differ between sites; however site was used as covariate in all analyses. The use of a two-hour window for timing of assessments approximates peak and trough levels of these medications. The actual levels were not measured and there was variability in the timing of assessment within and around this window. Timing of assessment was used as a covariate in the analyses to control for its influence. Additional maternal measures could potentially affect the outcome measures, including maternal smoking, weight, and general health. However, all of these variables were strikingly similar in the study groups. Moreover, it remains unknown to what extent different dosing parameters (e.g., split-doses rather than single daily doses for methadone and/or less than daily doses of buprenorphine) may have produced a different pattern of results. Finally, it is also unknown the extent to which altered fetal behavior may be a prognostic indicator of compromised later growth and development.
In conclusion, the present results suggest that the risk of altered fetal well-being in the third trimester is no worse for buprenorphine-exposed fetuses than the risk posed with methadone. Thus, these fetal data are consistent with previous published results with methadone and also provide support for the relative safety of buprenorphine when fetal indices are considered as part of the complete risk-benefit ratio. As more data becomes available regarding the safety and utility of buprenorphine as an alternative to methadone for pregnant opioid dependence, it will be important to better understand the fetal effects of not only methadone but also buprenorphine in this population.
Acknowledgments
This study was funded by the National Institute on Drug Abuse. None of the authors have any connections with the tobacco, alcohol, pharmaceutical, or gaming industries, or anyone substantially funded by one of these organizations. No contractual constraints on publishing were imposed by the funding body.
The clinical trial was registered with ClinicalTrials.gov (Identifier: NCT00271219; Title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
Footnotes
Declaration of interest:
H.J. discloses that she has received reimbursement for time and travel from Reckitt Benckiser.
KEO’G discloses that he has received reimbursement for time from Reckitt Benckiser.
Contributor Information
Amy L. Salisbury, Department of Pediatrics and Department of Psychiatry and Human Behavior, The Warren Alpert Medical School of Brown University
Mara G. Coyle, Department of Pediatrics, The Warren Alpert Medical School of Brown University
Kevin E. O’Grady, Department of Psychology, University of Maryland, College Park
Sarah H. Heil, Departments of Psychiatry and Psychology, University of Vermont
Peter R. Martin, Departments of Psychiatry and Pharmacology and Addiction Center, Vanderbilt University School of Medicine
Susan M. Stine, Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine.
Karol Kaltenbach, Department of Pediatrics, Jefferson Medical College, Thomas Jefferson University.
Manfred Weninger, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Pediatric Intensive Care and Neuropediatrics, Medical University of Vienna
Hendrée E. Jones, Department of Psychiatry and Behavioral Sciences and Department of Obstetrics and Gynecology, Johns Hopkins University School of Medicine, Substance Abuse Treatment Evaluations and Interventions Program, RTI International
Bibliography
- 1.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fisher G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103(9):1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- 2.Jones HE, O'Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17(5):372–386. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- 3.Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- 4.Jones HE, Johnson RE, Jasinski DR, O'Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79(1):1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95(2):239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 6.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S Groupe d'Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82(3):250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanovskaya TN, Nekhayeva IA, Hankins GD, Ahmed MS. Transfer of methadone across the dually perfused preterm human placental lobule. Am J Obstet Gynecol. 2008;198(1):126, e1–e4. doi: 10.1016/j.ajog.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 10.Zharikova OL, Deshmukh SV, Nanovskaya TN, Hankins GD, Ahmed MS. The effect of methadone and buprenorphine on human placental aromatase. Biochem Pharmacol. 2006;71(8):1255–1264. doi: 10.1016/j.bcp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Concheiro M, Jones HE, Johnson RE, Choo R, Shakleya DM, Huestis MA. Maternal buprenorphine dose, placenta buprenorphine, and metabolite concentrations and neonatal outcomes. Ther Drug Monit. 2010;32(2):206–215. doi: 10.1097/FTD.0b013e3181d0bd68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Castro A, Jones HE, Johnson RE, Gray TR, Shakleya DM, Huestis MA. Maternal Methadone Dose, Placental Methadone Concentrations, and Neonatal Outcomes. Clin Chem. 2011;57(3):449–458. doi: 10.1373/clinchem.2010.154864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn AM, Kelly J, Mansfield H, Needham P, O'Conor M, Viegas O. A randomized controlled trial of non-stress antepartum cardiotocography. Br J Obstet Gynaecol. 1982;89(6):427–433. doi: 10.1111/j.1471-0528.1982.tb03631.x. [DOI] [PubMed] [Google Scholar]
- 14.Lenstrup C, Haase N. Predictive value of antepartum fetal heart rate non-stress test in high-risk pregnancy. Acta Obstet Gynecol Scand. 1985;64(2):133–138. doi: 10.3109/00016348509154706. [DOI] [PubMed] [Google Scholar]
- 15.Lyons ER, Bylsma-Howell M, Shamsi S, Towell ME. A scoring system for nonstressed antepartum fetal heart rate monitoring. Am J Obstet Gynecol. 1979;133(3):242–246. doi: 10.1016/0002-9378(79)90673-2. [DOI] [PubMed] [Google Scholar]
- 16.Manning FA, Platt LD, Sipos L. Antepartum fetal evaluation: development of a fetal biophysical profile. Am J Obstet Gynecol. 1980;136(6):787–795. doi: 10.1016/0002-9378(80)90457-3. [DOI] [PubMed] [Google Scholar]
- 17.Manning FA, Snijders R, Harman CR, Nicolaides K, Menticoglou S, Morrison I. Fetal biophysical profile score. VI. Correlation with antepartum umbilical venous fetal pH. Am J Obstet Gynecol. 1993;169(4):755–763. doi: 10.1016/0002-9378(93)90002-z. [DOI] [PubMed] [Google Scholar]
- 18.Bardakci M, Balci O, Acar A, Colakoglu MC. Comparison of modified biophysical profile and doppler ultrasound in predicting the perinatal outcome at or over 36 weeks of gestation. Gynecol Obstet Invest. 2010;69(4):245–250. doi: 10.1159/000274488. [DOI] [PubMed] [Google Scholar]
- 19.Anyaegbunam A, Tran T, Jadali D, Randolph G, Mikhail MS. Assessment of fetal well-being in methadone-maintained pregnancies: abnormal nonstress tests. Gynecol Obstet Invest. 1997;43(1):25–28. doi: 10.1159/000291812. [DOI] [PubMed] [Google Scholar]
- 20.Schmid M, Kuessel L, Klein K, Metz V, Fischer G, Krampl-Bettelheim E. First-trimester fetal heart rate in mothers with opioid addiction. Addiction. 2010;105(7):1265–1268. doi: 10.1111/j.1360-0443.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 21.Jansson LM, Dipietro JA, Velez M, Elko A, Knauer H, Kivlighan KT. Maternal methadone dosing schedule and fetal neurobehaviour. J Matern Fetal Neonatal Med. 2009;22(1):29–35. doi: 10.1080/14767050802452291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittmann BK, Segal S. A comparison of the effects of single- and split-dose methadone administration on the fetus: ultrasound evaluation. Int J Addict. 1991;26(2):213–218. doi: 10.3109/10826089109053183. [DOI] [PubMed] [Google Scholar]
- 23.Wouldes TA, Roberts AB, Pryor JE, Bagnall C, Gunn TR. The effect of methadone treatment on the quantity and quality of human fetal movement. Neurotoxicol Teratol. 2004;26(1):23–34. doi: 10.1016/j.ntt.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Cejtin HE, Mills A, Swift EL. Effect of methadone on the biophysical profile. J Reprod Med. 1996;41(11):819–822. [PubMed] [Google Scholar]
- 25.Jansson LM, Dipietro JA, Velez M, Elko A, Williams E, Milio L, et al. Fetal neurobehavioral effects of exposure to methadone or buprenorphine. Neurotoxicol Teratol. 2011;33(2):240–243. doi: 10.1016/j.ntt.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones HE, Fischer MD, Heil SH, Kaltenbach K, Martin PR, Coyle MG, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER): Approach, Issues, and Lessons Learned. Addiction. doi: 10.1111/j.1360-0443.2012.04036.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning FA. Dynamic ultrasound-based fetal assessment: the fetal biophysical profile score. Clin Obstet Gynecol. 1995;38(1):26–44. doi: 10.1097/00003081-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177(6):1385–1390. Review. [PubMed] [Google Scholar]
- 29.Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666. doi: 10.1097/AOG.0b013e3181841395. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Koh SK, Hoh JK, Park MI. Difference of fetal heart rate accelerations based on 10 and 15 beats per minute. J Obstet Gynaecol Res. 2010;36(2):291–295. doi: 10.1111/j.1447-0756.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 31.Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, et al. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85(10):633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999;65(6):685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 33.Petrikovsky BM, Kaplan GP. Diurnal non-stress test variations in healthy term fetuses. A call for evening appointments for fetal testing. Early Hum Dev. 1996;44(2):127–130. doi: 10.1016/0378-3782(95)01699-6. [DOI] [PubMed] [Google Scholar]
- 34.Archie CL, Lee MI, Sokol RJ, Norman G. The effects of methadone treatment on the reactivity of the nonstress test. Obstet Gynecol. 1989;74(2):254–255. [PubMed] [Google Scholar]
- 35.Szeto HH. Effects of narcotic drugs on fetal behavioral activity: acute methadone exposure. Am J Obstet Gynecol. 1983;146(2):211–216. doi: 10.1016/0002-9378(83)91056-6. [DOI] [PubMed] [Google Scholar]
- 36.Umans JG, Szeto HH. Effects of opiates on fetal behavioral activity in utero. Life Sci. 1983;33(Suppl 1):639–642. doi: 10.1016/0024-3205(83)90584-2. [DOI] [PubMed] [Google Scholar]
- 37.Dinges DF, Davis MM, Glass P. Fetal exposure to narcotics: neonatal sleep as a measure of nervous system disturbance. Science. 1980;209(4456):619–621. doi: 10.1126/science.7190326. [DOI] [PubMed] [Google Scholar]
- 38.Hutchings DE. Methadone and heroin during pregnancy: a review of behavioral effects in human and animal offspring. Neurobehav Toxicol Teratol. 1982;4(4):429–434. [PubMed] [Google Scholar]