Abstract
This study analyzed 42 Acinetobacter baumannii strains collected between 2009–2012 from different hospitals in Beyrouth and North Lebanon to better understand the epidemiology and carbapenem resistance mechanisms in our collection and to compare the robustness of pulsed field gel electrophoresis (PFGE), multilocus sequence typing (MLST), repetitive sequence-based PCR (rep-PCR) and bla OXA-51 sequence-based typing (SBT). Among 31 carbapenem resistant strains, we have detected three carbapenem resistance genes: 28 carried the bla OXA-23 gene, 1 the bla OXA-24 gene and 2 strains the bla OXA-58 gene. This is the first detection of bla OXA-23 and bla OXA-24 in Lebanon. PFGE identified 11 types and was the most discriminating technique followed by rep-PCR (9 types), bla OXA-51 SBT (8 types) and MLST (7 types). The PFGE type A'/ST2 was the dominant genotype in our collection present in Beyrouth and North Lebanon. The clustering agreement between all techniques was measured by adjust Wallace coefficient. An overall agreement has been demonstrated. High values of adjust Wallace coefficient were found with followed combinations: PFGE to predict MLST types = 100%, PFGE to predict bla OXA-51 SBT = 100%, bla OXA-51 SBT to predict MLST = 100%, MLST to predict bla OXA-51 SBT = 84.7%, rep-PCR to predict MLST = 81.5%, PFGE to predict rep-PCR = 69% and rep-PCR to predict bla OXA-51 SBT = 67.2%. PFGE and MLST are gold standard methods for outbreaks investigation and population structure studies respectively. Otherwise, these two techniques are technically, time and cost demanding. We recommend the use of bla OXA-51 SBT as first typing method to screen isolates and assign them to their corresponding clonal lineages. Repetitive sequence-based PCR is a rapid tool to access outbreaks but careful interpretation of results must be always performed.
Introduction
Acinetobacter baumannii is an opportunistic gram negative pathogen involved in a wide number of nosocomial infections like ventilator-associated pneumonia, bloodstream, urinary tract, wound and meningitis infections frequently associated with a high rate of mortality and morbidity [1]. Outbreaks have been intensively documented worldwide and are usually caused by multidrug resistant strains and more and more carbapenem resistant strains [2], [3]. These outbreaks strains mainly belonged to three international clones I, II and III (previously called as European clones [4], [5]), but also to different clonal lineages. Karah et al. [2] analyzed the MLST-based global population structure of A. baumannii on 496 isolates. They showed the presence of 26 clones and among them, 18 were international clones and 8 European or Asian restricted clones. The International clone II was the major clone reported in 34 countries in Europe, Asia, Africa Australia, USA, and South America.
To track and monitor these outbreaks, denote strain relatedness and assign an outbreak strain to its corresponding clonal lineage, many typing methods with different intrinsic degrees of resolution are proposed [3] such as pulsed field gel electrophoresis (PFGE) [6], repetitive-sequence-based PCR (rep-PCR) [7], amplified fragment length polymorphism (AFLP) [8], multilocus sequence typing (MLST) [9], [10], 3-locus sequence typing (3-LST) [11], bla OXA-51 sequence-based typing (SBT) [12] or Multiple-Locus Variable number of tandem repeat Analysis (MLVA) [13]. Selection of an appropriate genotyping technique is not always easy and depends to the studied objectives. Many authors emphasized the great need to validate the application of each method as well as to harmonize different typing methods by reference networks [3], [14]. Among these methods, PFGE is still considered the current gold standard for A. baumannii outbreak investigation at local scale [3]. MLST has a discriminatory power lesser than PFGE and is regarded as the gold standard for large epidemiological and population structure studies. For A. baumannii, two MLST schemes have been proposed: Bartual's MLST [9] and Pasteur's MLST [10]. DiversiLab is a semi-automated form of rep-PCR with a comparable discriminatory power to PFGE [15]. The identification of eight international clones is one of the remarkable advantages of this system [7]. bla OXA-51 SBT has been proposed as a single-locus based typing [12] with a similar discriminatory power to rep-PCR [16] and Bartual and Pasteur's MLST [12], [17].
In Lebanon, there have been limited reports studying only local outbreaks in Beyrouth between 2004 and 2007 where bla OXA-58 was the only carbapenem resistance gene identified [18]–[20]. Recently, we have detected four bla NDM-1 producing A. baumannii isolated in Tripoli, Northern Lebanon from Syrian civilians wounded during Syrian war [21].
The present study has a double aim: firstly to compare the performance and effectiveness of four epidemiological typing methods (PFGE, rep-PCR, MLST and bla OXA-51-like SBT), and secondary to get primary results on circulating clones and carbapenem resistance mechanisms in Lebanon by analysis of 42 non duplicate strains conserved on Azm center for research on biotechnology and its application (Lebanese university) and collected between 2006–2012 from different hospitals in Beyrouth and North Lebanon.
Results
Identification
Forty-two strains were confirmed as A. baumannii by molecular techniques. These strains were isolated in different hospitals in Beyrouth (24 strains) and North Lebanon (18 strains) from various clinical specimens between 2009 and 2012 except one strain isolated in 2006 (Table 1). Beyrouth strains were isolated during epidemiological contexts.
Table 1. Origin and repartition of strains used in this study.
| Strain ID | Hospital | City | Department | Gender | Age | Period study | Sample origin |
| 8 | Nini | Tripoli | ICU | M | 70 | 2009 | tracheal aspirate |
| 9 | RHH | Beyrouth | ICU | M | 79 | 2011 | blood |
| 13 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 15 | RHH | Beyrouth | ICU | M | 68 | 2011 | blood |
| 17 | Nini | Tripoli | Cardiology | M | 48 | 2009 | urine |
| 19 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 20 | RHH | Beyrouth | ICU | F | 69 | 2011 | blood |
| 21 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 22 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 23 | AWH | Beyrouth | NA | M | 89 | 2011 | rectum |
| 24 | RHH | Beyrouth | ICU | M | 54 | 2011 | blood |
| 25 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 28 | Nini | Tripoli | Outpatient | F | 51 | 2011 | urine |
| 29 | Nini | Tripoli | NA | F | 22 | 2011 | urine |
| 30 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 31 | Nini | Tripoli | ICU | M | 72 | 2011 | tracheal aspirate |
| 34 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 35 | Nini | Tripoli | Outpatient | M | 37 | 2011 | bedsore |
| 36 | Nini | Tripoli | ICU | M | 74 | 2012 | tracheal aspirate |
| 37 | AWH | Beyrouth | NA | M | 89 | 2011 | throat |
| 38 | Nini | Tripoli | Cardiology | M | 57 | 2006 | tracheal aspirate |
| 40 | Nini | Tripoli | ICU | M | 20 | 2012 | blood |
| 41 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 45 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 46 | Monla | Tripoli | NA | M | 37 | 2012 | chest drain |
| 47 | AWH | Beyrouth | NA | F | 65 | 2011 | sputum |
| 48 | RHH | Beyrouth | NA | M | 74 | 2011 | blood |
| 49 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 50 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 51 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 52 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 53 | Nini | Tripoli | Maternity | F | 25 | 2010 | urine |
| 56 | Nini | Tripoli | ICU | F | 79 | 2011 | bronchial aspirate |
| 58 | AWH | Beyrouth | NA | F | 39 | 2011 | sputum |
| 59 | RHH | Beyrouth | NA | F | 70 | 2011 | blood |
| 60 | RHH | Beyrouth | NA | NA | NA | 2011 | NA |
| 62 | Nini | Tripoli | Outpatient | F | 74 | 2012 | urine |
| 63 | Rahal | Akkar | Internal medicine | M | 46 | 2012 | wound |
| 65 | TGH | Tripoli | Internal medicine | M | 27 | 2012 | wound |
| 66 | TGH | Tripoli | ICU | M | 29 | 2012 | sputum |
| 67 | TGH | Tripoli | Internal medicine | F | 38 | 2012 | abdomen |
| 68 | TGH | Tripoli | Internal medicine | M | 29 | 2012 | sputum |
AWH: Ain Wazein Hospital; RHH: Rafic Hariri Hospital; TGH: Tripoli Governmental Hospital; ICU: Intensive care unit; NA: not available.
Carbapenem resistance mechanisms
Thirty-one strains showed carbapenem resistance phenotypes (Fig. 1). Among these strains, 28 harbored a bla OXA-23 gene, 2 a bla OXA-58 gene, and one a bla OXA-24 gene. No acquired bla ndm-1 or bla OXA-143 has been detected. ISAba1 was present in 37 strains. All carbapenem resistant strains except one (strain 53, bla OXA-24 positive) had this sequence in their genomes whereas 7 carbapenem susceptible strains only had this sequence. The research of ISAba1 presence before both bla OXA-51 and bla OXA-23 genes revealed its insertion upstream bla OXA-23 in bla OXA-23 producing strains but not upstream bla OXA-51 gene in both carbapenem resistant or susceptible strains. This insertion explains the high level of resistance to carbapenems (imipenem MIC>32, meropenem MIC>32, doripenem 12<MIC>32) for all bla OXA-23 producing A. baumannii strains. The bla OXA-24-producing strain (strain 53) had MIC: 8 mg/l, 16 mg/l, 16 mg/l for imipenem, meropenem and doripenem respectively, whereas the two bla OXA-58 - producing strains (strain 58 and 23) showed low level of carbapenem resistance: (4; 8 mg/l), (2; 4 mg/l), (2; 3 mg/l) for imipenem, meropenem and doripenem respectively.
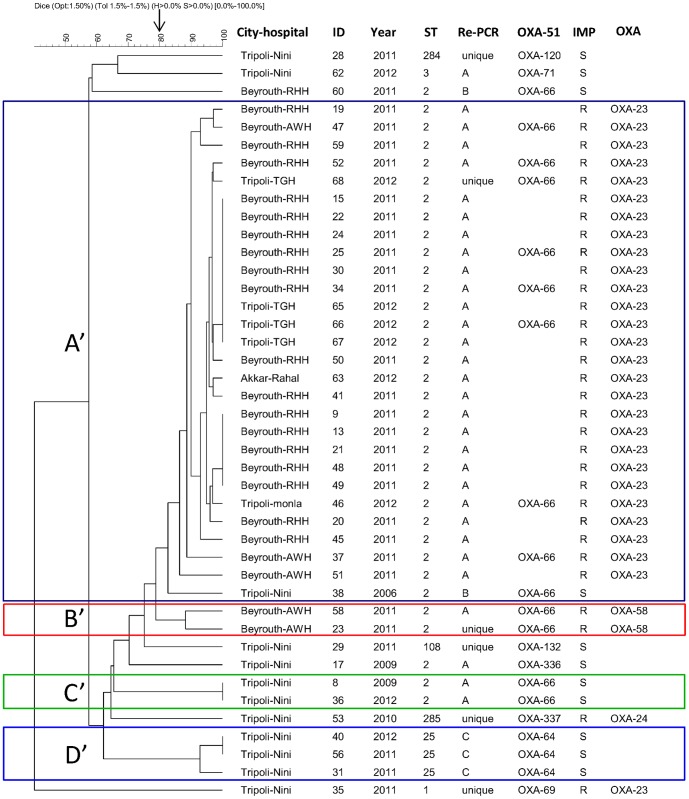
Figure 1. PFGE, MLST, DiversiLab and bla OXA-51-like comparison results for 42 A. baumannii strains.
Dendrogram was generated by cluster analysis of PFGE fingerprinting patterns. Arrow shows the adopted cut-off (80%) for definition of PFGE type. ID: Sample number, rep-PCR for rep-PCR type, IMP: imipenem susceptibility (S for susceptible and R for resistant), OXA: oxacillinase responsible for carbapenem resistance phenotype.
Epidemiological typing
PFGE
Using ≥80% similarity cut-off as a threshold, PFGE classified our strains in 11 types: 4 clusters (A' to D') and 7 unique profiles (Fig. 1). Cluster A' comprised 28 strains, 27 were bla OXA-23 producing strains and one an imipenem susceptible strain. This cluster contained strains from Beyrouth and North Lebanon. Cluster B' contained two bla OXA-58 producing strains that belonged to the same hospital. Cluster C' and D' contained two and three imipenem susceptible strains respectively.
MLST
MLST typing was performed on all strains. Seven ST(s) were identified (Fig. 1), two of which were novel and assigned as 284 and 285 by MLST Pasteur. The ST2 was the most predominant, present in 34 strains, followed by ST25, present in 3 strains; other ST(s) were present sporadically in our collection. The eBUSRT analysis of our ST(s) with all identified ST(s) in MLST database (28.03.2014) showed that ST 1, 2, 3, 25, 284 belonged to CC 1, 2, 3, 25 and 33 respectively, whereas ST285 was a singleton, and ST108 shared similarity with ST112 (we did not assign a name for this complex because no more of 2 ST(s) have been yet identified). Interestingly, our novel ST284 was a SLV of ST33 (the founder of CC33).
rep-PCR
Using ≥95% similarity cut-off as a threshold, rep-PCR identified 9 types (Fig. 1): 3 clusters (cluster A to C) and 6 unique profiles (ungrouped strains). Cluster A was the major cluster which contained 31 strains, whereas cluster B and C contained 2 and 3 strains respectively. Cluster A grouped only strains belonging to clone EU II except one strain (strain 62) belonging to EU clone III. This cluster comprised strains from different hospitals in Beyrouth (22 strains) and from North Lebanon (9 strains). Cluster B comprised two imipenem susceptible strains from Tripoli and Beyrouth, while cluster C contained all strains belonging to ST25 and coming from one hospital in Tripoli. Two unclustered strains belonged to ST2 while the remaining strains belonged to different ST(s).
bla OXA-51-like sequence-based typing
bla OXA-51 SBT has been performed with randomly selected strains from rep-PCR type A, and for all remaining strains. Notably, bla OXA-51 SBT correctly identified our strains. All strains belonging to ST2 (CC2) carried the bla OXA-66 gene, except one strain (strain 17) which was a colistin resistant one and which carried a single amino acid variant of OXA-66 described for the first time in this study (OXA-336, KF048907). The bla OXA-336 had a non-synonymous mutation from bla OXA-66 at nucleotide 518 (thymine became adenine) which led to the substitution of isoleucine by asparagine at amino acid 173. For the other strains, each ST had a unique OXA-51 type: ST1 carried OXA-69, ST3 OXA -71, ST25 OXA-64, ST108 OXA-132, ST284 OXA-120, and ST285 a new OXA (OXA-337, KF048908).
Comparison of the four typing methods
The visual analysis of the collection (Fig. 1) showed an overall agreement between the different techniques, although some discrepancies have been noticed. The adjusted Wallace coefficient analysis (Table 2) revealed that all ST(s) obtained by MLST were predicted at 100% level by PFGE and bla OXA-51 SBT and at 81.5% by rep-PCR. Conversely, MLST was unable to predict PFGE and rep-PCR types, but able to well predict bla OXA-51 sequences (84.7%). As expected, PFGE types were not predicted by any other technique. In contrast, PFGE predicted at 100% level all ST(s) and bla OXA-51 sequences and at 69% rep-PCR types. Rep-PCR types were well predicted (69.0%) by PFGE, but not predicted neither by MLST nor by bla OXA-51 SBT. In contrast, rep-PCR predicted the ST(s) at 81.5% and bla OXA-51 at 67.2% but was unable to predict PFGE types. Finally for bla OXA-51 SBT, we have assumed that isolates belonging to ST2 and for which we didn't perform bla OXA-51 sequencing carried bla OXA-66 [17]. bla OXA-51 sequences were all predicted by PFGE, and at 84. 7% and 67.2% by MLST and rep-PCR respectively. Conversely bla OXA-51 SBT was able to predict all ST(s), but not PFGE and rep-PCR types.
Table 2. Concordance between the studied typing techniques using adjusted Wallace coefficient (95% confidence interval).
| PFGE (cut-off 80%) | rep-PCR (cut-off 95%) | MLST | blaOXA-51 SBT | |
| PFGE | 0.690 (0.313–1.000) | 1.000 (1.000–1.000) | 1.000 (1.000–1.000) | |
| rep-PCR | 0.462 (0.086–0.838) | 0.815 (0.473–1.000) | 0.672 (0.259–1.000) | |
| MLST | 0.422 (0.058–0.786) | 0.513 (0.107–0.919) | 0.847 (0.564–1.000) | |
| blaOXA-51 SBT | 0.498 (0.142–0.854) | 0.499 (0.085–0.914) | 1.000 (1.000–1.000) |
Discussion
This is the first study in Lebanon providing data about clonality and carbapenem resistance mechanisms of a set of isolates recovered from different hospitals in Beyrouth and North Lebanon. However, this study does not illustrate the overall A. baumannii molecular epidemiology in this country because it does not contain prospectively collected isolates from different hospitals in different Lebanese provinces.
Currently, worldwide carbapenem resistant strains are mostly associated with international clone 2 [2], with bla OXA-23 as the main carbapenem resistance mechanism [22]–[24]. Our results stick well to the global situation where the majority of carbapenem resistant strains belonged to ST2, but only two imipenem resistant strains in our collection belonged to ST1 and ST285 (Fig. 1). In several countries, bla OXA-58 [25] or bla OXA-24 [26] have been progressively replaced by bla OXA-23. In Beyrouth-Lebanon, the studied outbreaks between 2004–2007 [18]–[20] were caused by the three international clones (1 to 3) producing OXA-58 as a main carbapenem resistance mechanism. In the present study, we detected bla OXA-58 in two strains from a hospital in Beyrouth, but bla OXA-23 seemed to be an emerging carbapenemase in Beyrouth and North Lebanon as else. Emergence of bla OXA-23 in Lebanon is linked to the clonal spread of the PFGE type A'/ST2. It is noteworthy that we reported in this study the first detection of bla OXA-23 and bla OXA-24 in Lebanon. Interestingly, the PFGE type A'/ST2 (harboring bla OXA-66) dominated heavily in Beyrouth and North Lebanon suggesting an extensively inter-hospital dissemination and thus it could be considered as an epidemic cluster. Beside this epidemic clone, some hospitals had their unique PFGE clone (PFGE type B'/ST2, PFGE type C'/ST2 and PFGE type D'/ST25). In those cases, it is interesting to notice that some clones contained isolates separated by 4 years. These results suggest a successful spread of well-established clones and therefore the urgent need of effective infection control measures to eradicate such bugs.
Our A. baumannii strains were analyzed using four epidemiological typing techniques. PFGE was the most discriminating scheme allowing the recognition of 11 types, followed by DiversiLab with 9 types then bla OXA-51 with 8 types and finally MLST with 7 types.
To our knowledge, there is no sufficient use of adjusted Wallace coefficient in A. baumannii typing field, an area which has largely been expanded in the last decades. Many techniques have been proposed with an increasing trend to track this pathogen and assign it to its corresponding international clonal lineage. Therefore, there is an arising need to perform a quantitative comparison between available typing methods using this coefficient or other coefficients to assess their strengths and weaknesses, better understand and validate their fields of application.
PFGE has been considered as a gold standard for outbreak investigations due to its higher discriminatory power which impaired its use for large investigations and population structure studies. As we have shown, PFGE is a very good method to predict bla OXA-51, MLST and rep-PCR types, but its higher resolution prevents other methods to successfully predict its types. Indeed, PFGE is a time demanding and labor intensive method with many intra and inter laboratory reproducibility problems [27]. ApaI, the classical restriction enzyme used for almost A. baumannii PFGE protocols generates complex DNA patterns with more than 40 fragments [28]. Chang et al., 2013 [28] suggested the use of two other infrequent cutting restriction enzymes (AscI and AsiSI) generating clear patterns with only 10–20 fragments per pattern.
MLST is a highly informative technique which puts isolates in a global context [24] and can directly assign them to their clonal complex. Thus, it is regarded as the method of choice for long term and phylogenetic studies. Although it is a reproducible and portable method, MLST is expensive and time demanding. As expected, MLST was unable to predict PFGE and rep-PCR types because of a discriminatory power lesser than found in PFGE and rep-PCR [12], [24]. Besides, MLST could predict very well (84.7%) all bla OXA-51 sequences except the colistin resistant strain that belonged to ST2 and had an OXA-336, a single amino acid variant of OXA-66 enzyme.
DiversiLab is a commercial rep-PCR typing system which benefits from several advantages: rapidity with an ability to investigate large number of isolates, standardization, reproducibility with conserved clustering between laboratories [29], and allowing in house libraries building. For A. baumannii, rep-PCR had revealed a comparable discriminatory power to PFGE [15]. It has been suggested to study A. baumannii population structure with rep-PCR as it can identify eight international clones within 492 isolates from a worldwide collection [7] and generate concordant results with MLST and bla OXA-51 SBT [16], [30]. Our rep-PCR results showed an overall agreement with PFGE, whenever some exceptions (Fig. 1) have been noticed which explained the low adjust Wallace coefficient (PFGE predicted 69% of rep-PCR types). Compared to PFGE clustering, rep-PCR grouped differently some strains belonging to the same PFGE type or assembled some strains belonging to different PFGE types in the same rep-PCR types, as reported with some other authors [31], [32]. One discrepant result was the strain belonging to ST3 which regrouped with rep-PCR type A, whereas it had a unique PFGE type. When we visually checked the graphs of samples and overlayed functions, we noticed the presence of another peak which was the source of this confusion between strains belonging ST2/rep-PCR type A and strain ST3/rep-PCR type A. Hence, this noticed the importance of careful interpretation of rep-PCR results [31].
The bla OXA-51 gene is an intrinsic chromosomal beta-lactamase gene naturally found in A. baumannii and up to 95 enzyme variants have been identified to date [33]. Sequencing of the entire gene was proposed as a bla OXA-51 SBT [12]. This SBT could correctly identify the eight international clones characterized by rep-PCR [16]. Also, it correlated well with Bartual's MLST [12]. Compared with Pasteur's MLST, SBT could correctly assign the nine clonal complexes in such manner each CC had specific bla OXA-51 alleles [17]. We have found similar results, where each CC or ST had a specific bla OXA-51 variant confirmed by adjusted Wallace coefficient (bla OXA-51 SBT predict 100% all ST). Indeed, the colistin strain (strain 17) carried OXA-336 which differed only by a single amino acid from OXA-66, indicating that bla OXA-51 SBT could correctly identify it as belonging to CC2. Compared with PFGE clustering; bla OXA-51 SBT had a lower discriminatory power. bla OXA-66 characteristically linked to ST2 has been found in 3 PFGE types (A', B' and C') and one unique profile. The bla OXA-64 variant carried by ST25 had characteristically been linked to PFGE type D'. Finally, other bla OXA-51-like variants had unique PFGE profiles.
Conclusion
This report describes the first detection of bla OXA-23 and bla OXA-24 in Lebanon. Although, our collection is unable to give the real picture of molecular epidemiology in Lebanon, it shed lights on circulating clones and on the mechanisms of carbapenem resistance. Other multi center studies are obviously required to better understand the epidemiology of this bug in the country.
Overall, a good concordance with the four typing methods was shown. PFGE and MLST are reference methods in local and long term epidemiological studies respectively, although both methods are time and cost consuming. bla OXA-51 SBT seems to be an excellent choice for initial epidemiological screening of isolates. rep-PCR is a rapid tool to access outbreaks at local scale but careful interpretation of results must be done.
Materials and Methods
Bacterial strains
A total of 42 non redundant clinical strains of A. baumannii isolated from various clinical samples were collected between 2006 and 2012 from the following hospitals: Rafic Harrii Beyrouth governmental hospital, Tripoli governmental hospital (TGH), Nini hospital, Rahal hospital, Monla hospital and Ain Wazein Hospital (AWH). All the bacterial strains were de-identified and a number was attributed prior to access and analysis. No consent was needed since strains used in this study were those isolated during routine analysis in the different laboratories. The clinical sources of the different strains are noted in Table 1.
Identification
Isolates were routinely cultured on Blood agar at 37°C, and stored at -80°C until further study. Identification to A. calcoaceticus-baumannii complex was initially performed using MALDI-TOF Vitek MS (bioMérieux, Marcy-l'Etoile, France) and confirmation of identification at species level was done by real time PCR of bla OXA-51 gene [34] and partial RNA polymerase b-subunit (rpoB) gene sequencing [35].
Susceptibility testing and investigation of carbapenem resistance mechanisms
Antibiotic Susceptibility testing was determined by the disc diffusion method according to the guidelines of the French Society of Microbiology (www.sfm-microbiologie.org/). Resistance to carbapenem and colistin were confirmed by determining imipenem, meropenem, doripenem and colistin minimum inhibitory concentration (MICs) by Etest strips (bioMérieux, Marcy-l'Étoile, France). Carbapenem resistant isolates were investigated for the presence of carbapenem resistance genes bla OXA-23, bla OXA-24, bla OXA-58, bla OXA-143, bla ndm-1 by PCR (Table 3). Presence of the insertion sequence ISAba1 was also screened. The association ISAba1-bla OXA-23 and ISAba1-bla OXA-51 was tested using a combination of primers ISAba1F with reverse primers targeting bla OXA-23 or bla OXA-51 respectively (Table 3).
Table 3. Oligonucleotide primers and TaqMan* fluorescent probes used in this study.
| Gene | Primer | Primer Sequences | Amplicon size (bp) | References |
| blaOXA51-like | OXA51like-F | 5'-AACATTAAAGCACTCTTACTTATAAC | 171 | [22] |
| OXA51like-R | 5′-TTGTTGGATAACTAAAACACCCGT | |||
| OXA51like-Dye | FAM-CTCACCTTATATAGTGTCTGCTAA-BHQ1 | |||
| blaOXA23-like | OXA23-F1 | 5′-TGCTCTAAGCCGCGCAAATA | 130 | [39] |
| OXA23-R1 | 5′-TGACCTTTTCTCGCCCTTCC | |||
| OXA23-probe | FAM-GCCCTGATCGGATTGGAGAACCA-BHQ1 | |||
| blaOXA24-like | OXA24-F | 5′-CAAATGAGATTTTCAAATGGGATGG | 123 | [39] |
| OXA24-R | 5′-TCCGTCTTGCAAGCTCTTGAT | |||
| OXA24-probe | FAM-GGTGAGGCAATGGCATTGTCAGCA-BHQ1 | |||
| blaOXA58-like | OXA58-F | 5′-CGCAGAGGGGAGAATCGTCT | 102 | [39] |
| OXA58-R | 5′-TTGCCCATCTGCCTTTTCAA | |||
| OXA58-probe | FAM-GGGGAATGGCTGTAGACCCGC- BHQ1 | |||
| blaOXA143-like | OXA-143-F | 5′-TGGCACTTTCAGCAGTTCCT | 149 | [40] |
| OXA-143-R | 5′-TAATCTTGAGGGGGCCAACC | |||
| blaNDM | NDM-F | 5′-GGTGCATGCCCGGTGAAATC | 661 | [41] |
| NDM-R | 5′-ATGCTGGCCTTGGGGAACG | |||
| ISAba1 | ISAba1 | 5′-CATTGGCATTAAACTGAGGAGAAA | 451 | [42] |
| ISAba2 | 5′-TTGGAAATGGGGAAAACGAA | |||
| blaOXA51-like | OXA-69A | 5′-CTAATAATTGATCTACTCAAG | 975 | [12] |
| OXA-69B | 5′-CCAGTGGATGGATGGATAGATTATC | |||
| rpoB | Ac696F | 5′-TAYCGYAAAGAYTTGAAAGAAG | 350 | [23] |
| Ac1093R | 5′-CMACACCYTTGTTMCCRTGA |
*Eurofins MWG Operon, Courtaboeuf, France.
Epidemiological typing
Pulsed field gel electrophoresis (PFGE)
PFGE using ApaI as a restriction enzyme was done as described previously [36]. DNA fragments were separated in CHEF-DRIII system (Biorad, Marne LA Coquette, France) at 6V/cm and 14°C for 21 hours with pulse times ranging from 3 s to 20 s. Computer-assisted analysis was performed by using fingerprinting II (Biorad, Marne LA Coquette, France) with the unweighted pair-group method with artithmetic averages (UPGMA) and Dice similarity coefficient for banding pattern comparison. A PFGE type was defined by a cluster of isolates showing ≥80% similarity
MLST
MLST was performed according to the Pasteur scheme (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html). The internal fragments of seven housekeeping genes (fusA, pyrG, rpoB, rplB, cpn60, gltA and recA) were amplified, then purified and sequenced by an ABI 3130XL DNA sequencer (Applied Biosystems, Foster City, United States). The sequences were compared to the available sequences present in the MLST website. When a new allele or ST was identified, it was submitted and codified by Institut Pasteur MLST Database. The eBURST [37] was used to compare ST to the existed ST(s) and to assign isolates to their clonal complexes. A clonal complex (CC) is defined as a set of similar ST(s) having 6 identical loci among 7, so a CC is formed by the founder ST and its single locus variants (SLV) [10].
rep-PCR
Rep-PCR was performed using the automated system DiversiLab, version 3.4 (bioMérieux, Marcy-l'Étoile, France) following the manufacturer recommendations. Briefly, bacteria were cultured on sheep blood agar (Oxoid). DNA was extracted using the MoBio Ultra Clean microbial DNA extraction Kit, and adjusted to 50 ng/µl. After extraction, DNA was amplified using DiversiLab Acinetobacter kit, and the amplified DNA was separated and detected by Agilent 2100 Bioanalyser (Agilent Technologies). The resulted fingerprints were analyzed using the DiversiLab software with the modified Kullback-Leibler (KL) as a statistical method and ≥95% as a threshold to define a cluster of closely related isolates or a rep-PCR type.
bla OXA-51 sequence-based typing (SBT)
This typing method consists to sequence the full length (825 bp) of a single locus bla OXA-51 gene. The bla OXA-51 was amplified by external primers OXA-69A/OXA-69B as described [12]. PCR products were purified and sequenced by ABI 3130xl DNA sequencer (Applied Biosystems). The resulted sequences were compared to all variants present in BLAST. When a novel variant was detected, it was submitted to GenBank and assigned by the Lahey database for beta-lactamase classification (http://www.lahey.org/studies/webt.asp).
Concordance between techniques
The online tool (http://www.comparingpartitions.info/) was used to calculate the adjusted Wallace coefficient. This coefficient is an objective and quantitative measure of clustering agreement between the studied techniques which indicates the probability of a pair of isolates assigned at the same type by one technique is also reassigned at the same by the other technique [32], [38].
Nucleotide sequence accession numbers and novel sequence types
Two new nucleotide sequences of bla OXA-51 were submitted to GenBank under accession number KF048907 and KF048908 and assigned respectively by Lahey center as bla OXA-336 and bla OXA-337. Two new sequence types were identified and coded by MLST Pasteur as ST284 (3-5-2-1-7-1-4) and ST285 (1-52-2-2-9-4-2). The latter had a new fusA allele.
Ethic statement
Not applicable
Acknowledgments
We thank the team of the curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles and isolates at http://www.pasteur.fr/mlst. We thank also Catherine Ramont, Mariam Yehya and Taha Abdo for excellent technical assistance. A part of results of carbapenemase detection was presented at the 9th International Symposium on the Biology of Acinetobacter, Cologne 2013, poster presentation P5–7.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Kempf M, Rolain J-M (2012) Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents 39:105–114 doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2. Karah N, Sundsfjord A, Towner K, Samuelsen Ø (2012) Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Updat 15:237–247 doi: 10.1016/j.drup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3. Zarrilli R, Pournaras S, Giannouli M, Tsakris A (2013) Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19 doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4. Dijkshoorn L, Aucken H, Gerner-Smidt P, Janssen P, Kaufmann M, et al. (1996) Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol 34:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Dessel H, Dijkshoorn L, Van Der Reijden T, Bakker N, Paauw A, et al. (2004) Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol 155:105–112. [DOI] [PubMed] [Google Scholar]
- 6. Seifert H, Dolzani L, Bressan R, van der Reijden T, van Strijen B, et al. (2005) Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J Clin Microbiol 43:4328–4335 doi: 10.1128/JCM.43.9.4328-4335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins PG, Dammhayn C, Hackel M, Seifert H (2010) Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238 doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 8. Nemec A, Dijkshoorn L, van der Reijden TJK (2004) Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J Med Microbiol 53:147. [DOI] [PubMed] [Google Scholar]
- 9. Bartual SG, Seifert H, Hippler C, Luzon MAD, Wisplinghoff H, et al. (2005) Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:4382–4390 doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S (2010) The Population Structure of Acinetobacter baumannii: Expanding Multiresistant Clones from an Ancestral Susceptible Genetic Pool. PLoS ONE 5:e10034 doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turton J, Gabriel S, Valderrey C, Kaufmann M, Pitt T (2007) Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin Microbiol Infect 13:807–815. [DOI] [PubMed] [Google Scholar]
- 12. Hamouda A, Evans BA, Towner KJ, Amyes SGB (2010) Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of blaOXA-51-like genes. J Clin Microbiol 48:2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pourcel C, Minandri F, Hauck Y, D'Arezzo S, Imperi F, et al. (2011) Identification of variable-number tandem-repeat (VNTR) sequences in Acinetobacter baumannii and interlaboratory validation of an optimized multiple-locus VNTR analysis typing scheme. J Clin Microbiol 49:539–548 doi: 10.1128/JCM.02003-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosso F, Carvalho KR, Quinteira S, Ramos A, Carvalho-Assef APD, et al. (2011) OXA-23-Producing Acinetobacter Baumannii: A New Hotspot of Diversity in Rio De Janeiro? J Antimicrob Chemother 66:62–65 doi: 10.1093/jac/dkq406. [DOI] [PubMed] [Google Scholar]
- 15. Grisold A, Zarfel G, Strenger V, Feierl G, Leitner E, et al. (2010) Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J Infect 60:44–51. [DOI] [PubMed] [Google Scholar]
- 16. Zander E, Nemec A, Seifert H, Higgins PG (2012) Association between β-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J Clin Microbiol 50:1900–1904 doi: 10.1128/JCM.06462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pournaras S, Gogou V, Giannouli M, Dimitroulia E, Dafopoulou K, et al. (2014) Single locus sequence-based typing of blaOXA-51-like gene for rapid classification of Acinetobacter baumannii clinical isolates to international clones. J Clin Microbiol. doi: doi: 10.1128/JCM.03565-13.. [DOI] [PMC free article] [PubMed]
- 18. Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, et al. (2008) A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob Agents Chemother 52:4115–4120 doi: 10.1128/AAC.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giannouli M, Tomasone F, Agodi A, Vahaboglu H, Daoud Z, et al. (2009) Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii strains in intensive care units of multiple Mediterranean hospitals. J Antimicrob Chemother 63:828–830 doi: 10.1093/jac/dkp032. [DOI] [PubMed] [Google Scholar]
- 20. Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R (2011) Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin Microbiol Infect Dis 17:197–201 doi: 10.1111/j.1469-0691.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 21. Rafei R, Dabboussi F, Hamze M, Eveillard M, Lemarié C, et al. (2014) First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int J Infect Dis 21:21–23 doi: 10.1016/j.ijid.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 22. Adams-Haduch JM, Onuoha EO, Bogdanovich T, Tian G-B, Marschall J, et al. (2011) Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol 49:3849–3854 doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schleicher X, Higgins PG, Wisplinghoff H, Körber-Irrgang B, Kresken M, et al. (2013) Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005–2009). Clin Microbiol Infect 19:737–742 doi: 10.1111/1469-0691.12026. [DOI] [PubMed] [Google Scholar]
- 24. Runnegar N, Sidjabat H, Goh HMS, Nimmo GR, Schembri MA, et al. (2010) Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a single institution over a 10-year period. J Clin Microbiol 48:4051–4056 doi: 10.1128/JCM.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minandri F, D'Arezzo S, Antunes LCS, Pourcel C, Principe L, et al. (2012) Evidence of diversity among epidemiologically related carbapenemase-producing Acinetobacter baumannii strains belonging to international clonal lineage II. J Clin Microbiol 50:590–597 doi: 10.1128/JCM.05555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grosso F, Quinteira S, Peixe L (2011) Understanding the dynamics of imipenem-resistant Acinetobacter baumannii lineages within Portugal. Clin Microbiol Infect Dis 17:1275–1279 doi: 10.1111/j.1469-0691.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 27. Li W, Raoult D, Fournier P-E (2009) Bacterial strain typing in the genomic era. FEMS Microbiol Rev 33:892–916 doi: 10.1111/j.1574-6976.2009.00182.x. [DOI] [PubMed] [Google Scholar]
- 28. Chang K-M, Huang W-C, Chiou C-S, Shen G-H, Huang C-C, et al. (2013) Suitable restriction enzyme for standardization of pulsed-field gel electrophoresis protocol and interlaboratory comparison of Acinetobacter baumannii. J Microbiol Immunol Infect 46:195–201 doi: 10.1016/j.jmii.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 29. Higgins PG, Hujer AM, Hujer KM, Bonomo RA, Seifert H (2012) Interlaboratory reproducibility of DiversiLab rep-PCR typing and clustering of Acinetobacter baumannii isolates. J Med Microbiol 61:137–141 doi: 10.1099/jmm.0.036046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins PG, Janßen K, Fresen MM, Wisplinghoff H, Seifert H (2012) Molecular Epidemiology of Acinetobacter baumannii Bloodstream Isolates Obtained in the United States from 1995 to 2004 Using rep-PCR and Multilocus Sequence Typing. J Clin Microbiol 50:3493–3500 doi: 10.1128/JCM.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuetz AN, Huard RC, Eshoo MW, Massire C, Della-Latta P, et al. (2012) Identification of a novel Acinetobacter baumannii clone in a US hospital outbreak by multilocus polymerase chain reaction/electrospray-ionization mass spectrometry. Diagn Microbiol Infect Dis 72:14–19 doi: 10.1016/j.diagmicrobio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 32. Maâtallah M, Bakhrouf A, Habeeb MA, Turlej-Rogacka A, Iversen A, et al. (2013) Four Genotyping Schemes for Phylogenetic Analysis of Pseudomonas aeruginosa: Comparison of Their Congruence with Multi-Locus Sequence Typing. PLoS ONE 8:e82069 doi: 10.1371/journal.pone.0082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans BA, Amyes SGB (2014) OXA β-Lactamases. Clin Microbiol Rev 27:241–263 doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kempf M, Rolain J-M, Diatta G, Azza S, Samb B, et al. (2012) Carbapenem resistance and Acinetobacter baumannii in Senegal: the paradigm of a common phenomenon in natural reservoirs. PloS One 7:e39495 doi: 10.1371/journal.pone.0039495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Gundi VAKB, Dijkshoorn L, Burignat S, Raoult D, La Scola B (2009) Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology 155:2333–2341 doi: 10.1099/mic.0.026054-0. [DOI] [PubMed] [Google Scholar]
- 36.Kempf M, Rolain J-M, Azza S, Diene S, Joly-Guillou M-L, et al. (2012) Investigation of Acinetobacter baumannii resistance to carbapenems in Marseille hospitals, south of France: a transition from an epidemic to an endemic situation. Acta Pathol Microbiol Immunol Scand. doi: doi: 10.1111/j.1600-0463.2012.02935.x.. [DOI] [PubMed]
- 37. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG (2004) eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Severiano A, Pinto FR, Ramirez M, Carrico JA (2011) Adjusted Wallace Coefficient as a Measure of Congruence between Typing Methods. J Clin Microbiol 49:3997–4000 doi: 10.1128/JCM.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mesli E, Berrazeg M, Drissi M, Bekkhoucha SN, Rolain J-M (2013) Prevalence of carbapenemase-encoding genes including New Delhi metallo-β-lactamase in Acinetobacter species, Algeria. Int J Infect Dis 17:e739–e743 doi: 10.1016/j.ijid.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 40. Higgins PG, Lehmann M, Seifert H (2010) Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 35:305 doi: 10.1016/j.ijantimicag.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 41. Bonnin RA, Naas T, Poirel L, Nordmann P (2012) Phenotypic, Biochemical, and Molecular Techniques for Detection of Metallo-?-Lactamase NDM in Acinetobacter baumannii . J Clin Microbiol 50:1419–1421 doi: 10.1128/JCM.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruiz M, Marti S, Fernandez-Cuenca F, Pascual A, Vila J (2007) Prevalence of IS(Aba1) in epidemiologically unrelated Acinetobacter baumannii clinical isolates. Clin Microbiol Infect Dis 13:1192–1198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.