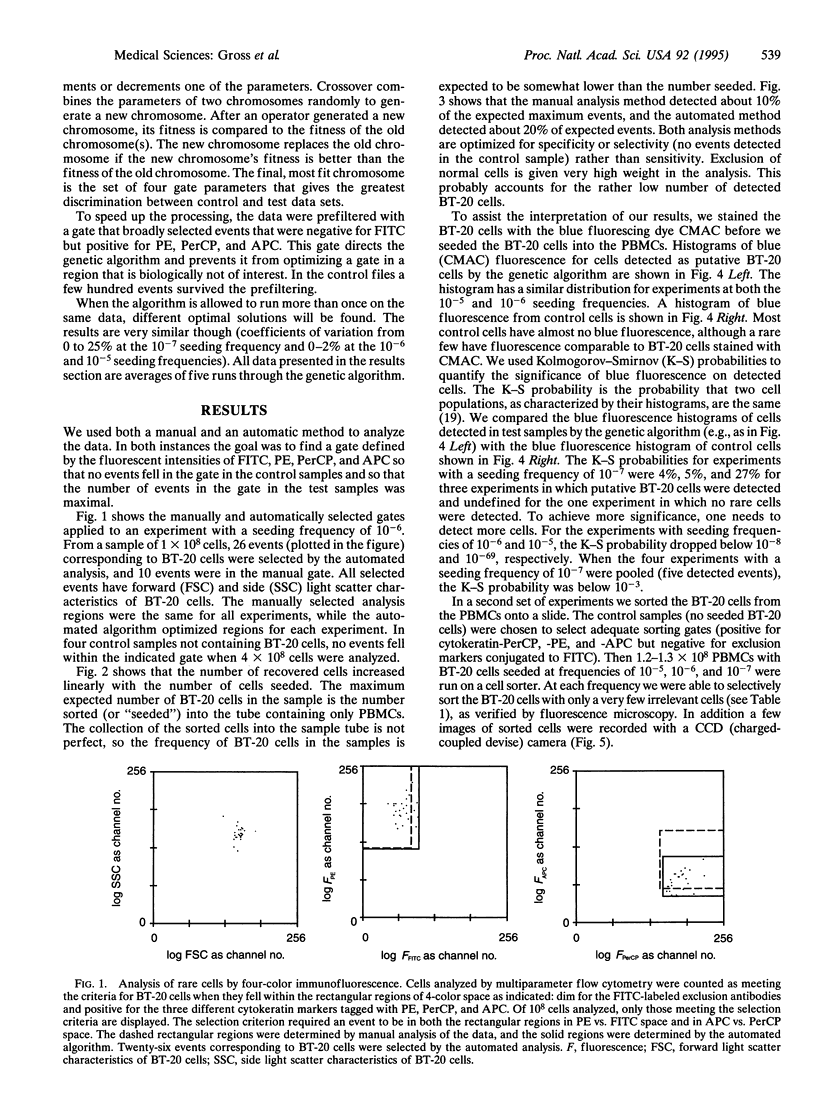
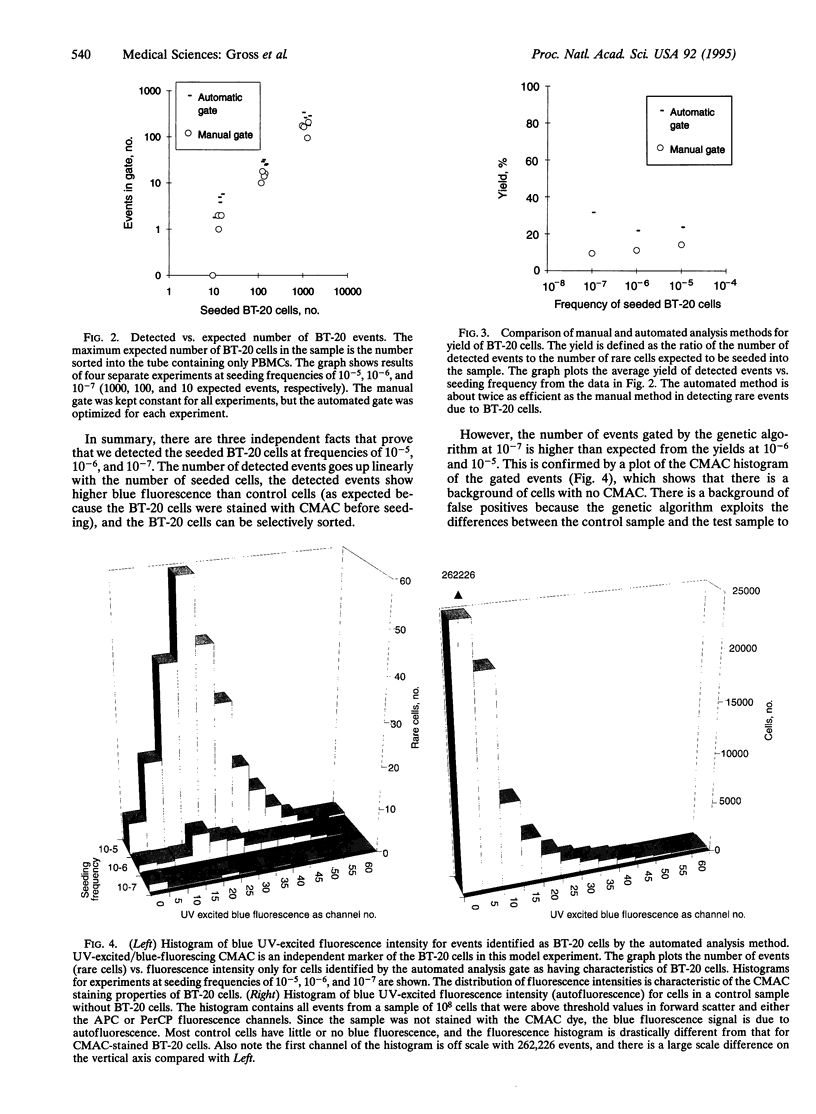
Abstract
A flow cytometric assay was developed to detect rare cancer cells in blood and bone marrow. Multiple markers; each identified by a separate color of immunofluorescence (yellow and two shades of red), are used to reliably identify the cancer cells. Blood or bone marrow cells, which are not of interest but interfere in detecting the cancer cells, are identified by a panel of immunofluorescence markers, each of which has the same color (green). Thus, the rare cancer cells of interest are yellow and two different shades of red but not green. The requirement that the rare cancer cell be simultaneously positive for three separate colors (the specific markers) and negative for a fourth color (the exclusion color) allowed detection of as few as one cancer cell in 10(7) nucleated blood cells (a frequency of 10(-7). To test this rare-event assay prior to clinical studies, a model study was performed in which the clinical sample was simulated by mixing small numbers of cells from the breast carcinoma line BT-20 with peripheral blood mononuclear cells. We detected statistically significant numbers of BT-20 cells at mixing frequencies of 10(-5), 10(-6), and 10(-7). In control samples, no target events (BT-20) were observed when more than 10(8) cells were analyzed. For additional confirmation that the BT-20 cells in the model study were correctly identified and counted, the BT-20 cells (and only BT-20 cells) were covalently stained with a fifth fluorescence dye, 7-amino-4-chloromethylcoumarin (CMAC). CMAC fluorescence data were not used in the assay for detecting BT-20 cells. Only after the analysis using data from the specific and exclusion colors had been completed were the events identified as BT-20 cells checked for CMAC fluorescence. The putative BT-20 events were always found to be positive for CMAC fluorescence, which further increases confidence in the assay. Manual data analysis and an automated computer program were compared. Results were comparable with the manual and automated methods, but the automated "genetic algorithm" always found more BT-20 events. Cell sorting of BT-20 cells from samples that contained BT-20 at frequencies of 10(-5), 10(-6), and 10(-7) provided further evidence that these rare cells could be reliably detected. The good performance of the assay with the model system will encourage further studies on clinical samples.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Shpall E. J., Leslie D. S., Nustad K., Ugelstad J., Peters W. P., Bast R. C., Jr Elimination of malignant clonogenic breast cancer cells from human bone marrow. Cancer Res. 1989 Aug 15;49(16):4659–4664. [PubMed] [Google Scholar]
- Cote R. J., Rosen P. P., Lesser M. L., Old L. J., Osborne M. P. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991 Oct;9(10):1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- Deisseroth A. B., Zu Z., Claxton D., Hanania E. G., Fu S., Ellerson D., Goldberg L., Thomas M., Janicek K., Anderson W. F. Genetic marking shows that Ph+ cells present in autologous transplants of chronic myelogenous leukemia (CML) contribute to relapse after autologous bone marrow in CML. Blood. 1994 May 15;83(10):3068–3076. [PubMed] [Google Scholar]
- Ellis G., Ferguson M., Yamanaka E., Livingston R. B., Gown A. M. Monoclonal antibodies for detection of occult carcinoma cells in bone marrow of breast cancer patients. Cancer. 1989 Jun 15;63(12):2509–2514. doi: 10.1002/1097-0142(19890615)63:12<2509::aid-cncr2820631225>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Frey T., Houck D. W., Shenker B. J., Hoffman R. A. Bivariate flow karyotyping with air-cooled lasers. Cytometry. 1994 Jun 1;16(2):169–174. doi: 10.1002/cyto.990160211. [DOI] [PubMed] [Google Scholar]
- Glorieux P., Bouffet E., Philip I., Biron P., Holzapfel L., Floret D., Bouvier R., Vitrey D., Pinkerton R., Brunat-Mentigny M. Metastatic interstitial pneumonitis after autologous bone marrow transplantation. A consequence of reinjection of malignant cells? Cancer. 1986 Nov 1;58(9):2136–2139. doi: 10.1002/1097-0142(19861101)58:9<2136::aid-cncr2820580929>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Verwer B., Houck D., Recktenwald D. Detection of rare cells at a frequency of one per million by flow cytometry. Cytometry. 1993;14(5):519–526. doi: 10.1002/cyto.990140511. [DOI] [PubMed] [Google Scholar]
- Kemshead J. T. Immunomagnetic manipulation of hematopoietic cells: a review of current technology. J Hematother. 1992 Spring;1(1):35–44. doi: 10.1089/scd.1.1992.1.35. [DOI] [PubMed] [Google Scholar]
- LASFARGUES E. Y., OZZELLO L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958 Dec;21(6):1131–1147. [PubMed] [Google Scholar]
- Mansi J. L., Berger U., Easton D., McDonnell T., Redding W. H., Gazet J. C., McKinna A., Powles T. J., Coombes R. C. Micrometastases in bone marrow in patients with primary breast cancer: evaluation as an early predictor of bone metastases. Br Med J (Clin Res Ed) 1987 Oct 31;295(6606):1093–1096. doi: 10.1136/bmj.295.6606.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi J. L., Easton D., Berger U., Gazet J. C., Ford H. T., Dearnaley D., Coombes R. C. Bone marrow micrometastases in primary breast cancer: prognostic significance after 6 years' follow-up. Eur J Cancer. 1991;27(12):1552–1555. doi: 10.1016/0277-5379(91)90413-8. [DOI] [PubMed] [Google Scholar]
- Moss T. J., Xu Z. J., Mansour V. H., Hardwick A., Kulcinski D., Ishizawa L., Law P., Gee A. Quantitation of tumor cell removal from bone marrow: a preclinical model. J Hematother. 1992 Spring;1(1):65–73. doi: 10.1089/scd.1.1992.1.65. [DOI] [PubMed] [Google Scholar]
- Nademanee A., Schmidt G. M., O'Donnell M. R., Snyder D. S., Parker P. A., Stein A., Smith E., Lipsett J. A., Sniecinski I., Margolin K. High-dose chemoradiotherapy followed by autologous bone marrow transplantation as consolidation therapy during first complete remission in adult patients with poor-risk aggressive lymphoma: a pilot study. Blood. 1992 Sep 1;80(5):1130–1134. [PubMed] [Google Scholar]
- Peters W. P., Shpall E. J., Jones R. B., Olsen G. A., Bast R. C., Gockerman J. P., Moore J. O. High-dose combination alkylating agents with bone marrow support as initial treatment for metastatic breast cancer. J Clin Oncol. 1988 Sep;6(9):1368–1376. doi: 10.1200/JCO.1988.6.9.1368. [DOI] [PubMed] [Google Scholar]
- Redding W. H., Coombes R. C., Monaghan P., Clink H. M., Imrie S. F., Dearnaley D. P., Ormerod M. G., Sloane J. P., Gazet J. C., Powles T. J. Detection of micrometastases in patients with primary breast cancer. Lancet. 1983 Dec 3;2(8362):1271–1274. doi: 10.1016/s0140-6736(83)91150-9. [DOI] [PubMed] [Google Scholar]
- Young I. T. Proof without prejudice: use of the Kolmogorov-Smirnov test for the analysis of histograms from flow systems and other sources. J Histochem Cytochem. 1977 Jul;25(7):935–941. doi: 10.1177/25.7.894009. [DOI] [PubMed] [Google Scholar]