Abstract
Objective:
To evaluate adiponectin and leptin levels in older men and women with migraine.
Methods:
Fasting total and high molecular weight (HMW) adiponectin and leptin levels were evaluated in a case–cohort study of nondiabetic older migraine and nonmigraine control participants from the ongoing, longitudinal, general population, Atherosclerosis Risk in Communities Study at visit 1 (1987–1989). A standardized headache questionnaire was completed at visit 3 (1993–1995). Logistic regression models adjusted for age, sex, race, center, body mass index, and fasting glucose were used to evaluate the association of each adipocytokine with migraine.
Results:
Of the 981 participants, the mean age at baseline was 52.8 years (SE 0.3); 131 fulfilled migraine criteria. Crude, mean total adiponectin levels were greater in men and women with migraine (8.1 µg/mL, SE 0.5) as compared to those without migraine (7.0 µg/mL, SE 0.2) (p = 0.031). After adjustments, the odds of migraine were increased by 88% with each SD increase in total adiponectin in men (odds ratio [OR] 1.86; 95% confidence interval [CI] 1.15, 3.01; p = 0.011), but not in women (OR 1.05; 95% CI 0.80, 1.37; p = 0.728; p interaction = 0.029). Similar results were demonstrated for HMW adiponectin. Crude and adjusted leptin levels were not associated with migraine.
Conclusions:
Although crude, total adiponectin levels were higher in older men and women with migraine than controls, after adjustments, the prevalence of migraine was significantly associated with total adiponectin only in older men, suggesting the association may be confounded or absent in older women. Leptin was not associated with migraine in older men or women.
Migraine is a neurologic disorder associated with obesity,1 hyperlipidemia,2 and insulin resistance.3 Current theories suggest that the neurogenic inflammation4 of migraine involves neurotransmitters and proteins that participate in energy regulation and metabolism (e.g., serotonin).5,6 More recently, adipocytokines (cytokines predominantly secreted from fat cells, such as adiponectin and leptin) have been suggested to contribute as inflammatory mediators of migraine.7,8
While the high molecular weight (HMW) oligomer of adiponectin is largely proinflammatory, the low molecular weight (LMW) oligomer is anti-inflammatory. HMW adiponectin has been shown to activate nuclear factor κβ (NFκβ) and to induce interleukin-6 (IL-6).9,10 In contrast, LMW adiponectin has been shown to reduce IL-6 secretion.11
Although adiponectin levels are decreased in obesity states,12,13 recent studies reported increases of adiponectin levels in migraineurs. Specifically, these studies reported that baseline total adiponectin levels were elevated in reproductive-aged women with chronic migraine7 and that levels decline in episodic migraineurs in pain following successful treatment.8 In contrast to adiponectin, plasma levels of the adipocytokine leptin are increased in patients with obesity14; however, no changes in leptin levels have been reported in migraineurs after adjusting for body mass index (BMI).15,16
Recent epidemiologic research supports that obesity in migraineurs exhibits a differential effect by age and sex, with the greatest association in younger women (<50).17 Given previous data demonstrating age- and sex-specific differences in adiponectin and leptin in healthy individuals,18,19 our aim was to evaluate these levels in older migraineurs. We hypothesized that adiponectin, and not leptin, would be elevated in older migraineurs.
METHODS
Study design.
The Atherosclerosis Risk in Communities Study (ARIC) is a longitudinal community-based study conducted at 4 sites (Jackson, MS; Forsyth County, NC; Washington County, MD; suburban Minneapolis, MN). The initial recruitment and study participation has been previously described.20 In brief, ARIC includes a predominantly biracial population of 15,792 adults, who upon initial recruitment were between 45 and 64 years of age.20 The first visit occurred during 1987–1989, with visits 2 (1990–1992), 3 (1993–1995), and 4 (1996–1998) occurring approximately 3 years apart. Visit 5 occurred during 2011–2013 among surviving participants.
The current study used data from a prospective case–cohort within ARIC, which was originally designed to investigate the role of plasma biomarkers collected at visit 1 (1987–1989) in the development of diabetes.21 The study also used headache questionnaires collected approximately 6 years later at visit 3 (1993–1995). Of the 15,792 participants who attended visit 1, the case–cohort study excluded those with prevalent diabetes (n = 2,018), 95 with race other than black or white, the few black subjects from the Minnesota and Washington County sites, 853 not returning to any follow-up visit, 26 with no diabetes determination at follow-up, and 6 with restrictions on plasma use. Additionally, 12 were excluded for missing baseline anthropometrics, 2,514 participants in a previous ARIC case–control and case–cohort study using stored plasma, 212 for incomplete fasting, and 315 with missing information for key covariates.22 From this population, a random cohort (n = 591) and incident diabetes case sample (n = 599) were sampled by race strata. Additionally, participants who were missing adiponectin levels (n = 106) (none were missing leptin levels) and headache questionnaires (n = 103) were excluded, leaving a total of 981 participants (figure e-1 on the Neurology® Web site at www.neurology.org).
Ascertainment of headache status.
At visit 3, trained interviewers administered a headache questionnaire to all participants.23,24 Participants were queried as to whether they ever experienced headaches lasting 4 or more hours. Those reporting never experiencing headaches lasting ≥4 hours were categorized as nonmigraine controls, herein referred to as controls. Participants responding affirmatively were asked more detailed questions regarding location, quality, duration, and accompanying symptoms including nausea or vomiting, photophobia, phonophobia, and if they felt like lying down in a dark room during headaches. Additionally, participants were asked if they experienced a visual aura (spots, jagged lines, or heat waves) in association with their headaches.
Based on the responses to a headache questionnaire administered at visit 3, migraine was characterized for these analyses using modified International Classification of Headache Disorders (ICHD), 2nd edition, criteria. Participants were categorized as having migraine who fulfilled the following 4 criteria: (1) headache lasting ≥4 hours; (2) headache with at least 2 out of the following 3 features: (a) throbbing, pulsing, or pounding quality, (b) unilateral location, (c) desire to go to a dark room and lie down; (3) nausea or vomiting or both photophobia and phonophobia; and (4) ≥1 year history of headaches (at any point in their life). Those participants with a history of headache lasting ≥4 hours and satisfying 2 out of 3 of criteria of the second criteria were categorized as probable migraine (prob-Mig). Those not fulfilling migraine or prob-Mig criteria were categorized as controls. Additional analyses were conducted for the combined group of participants with migraine and prob-Mig (all-Mig). Finally, subgroup analyses were conducted for migraine groups based on the presence or absence of visual aura, with those responding affirmatively to experiencing a visual aura categorized as with aura and those responding negatively as without aura.
Covariates.
All covariates used in the regression models were assessed at visit 1.20 Covariates included age (years), sex, race/center (Maryland whites; Minnesota whites; North Carolina whites; North Carolina blacks; Mississippi blacks), BMI (kg/m2), and fasting blood glucose (mg/dL). A combined race/center variable was created to adjust for confounding between these 2 variables. BMI was calculated as weight in kilograms/height in meters.2
Laboratory methods.
Fasting plasma blood samples collected at visit 1 (1987–1989) were centrifuged, aliquoted, and stored at −70°C for approximately 20 years, until thawed and maintained at 4°C until measured (no longer than 24 hours later).22 Total and HMW adiponectin were assessed with sandwich ELISA (American Laboratory Products Company, Salem, NH) with a minimum assay sensitivity of 0.04 ng/mL. Pretreatment with proteinase K, which digests all isoforms except for HMW adiponectin, allowed for HMW adiponectin to be measured at the same time as total adiponectin. Reliability coefficients for total, HMW, and HMW:total adiponectin ratio were 0.93, 0.98, and 0.70, respectively.22 Leptin was measured in duplicate using a sandwich ELISA (Linco Research, St. Charles, MI) with a reliability coefficient of 0.94 and a minimum assay sensitivity of 0.5 ng/mL.25 Fasting glucose and cholesterol were measured using standard laboratory techniques.26
Statistical analysis.
All statistical analyses were weighted to account for the case–cohort study design using the svy command and pweights in Stata version 13 (StataCorp LP, College Station, TX). This method involves inverse weighting of the observations according to the sampling design, to permit statistical estimation and inference relevant to the entire cohort, which adjusts for the oversampling of incident diabetes. All standard errors were estimated using the Taylor series linearization method. p Values were calculated using linear regression and χ2 test statistics.
Means (SE) and proportions (SE) of baseline characteristics of study participants were reported overall and by migraine category. We examined the association of total, HMW, and the ratio of HMW to total adiponectin and leptin with migraine using logistic regression models. Odds ratios (ORs) from the logistic regression models were calculated per 1 SD increase in adipocytokine level, where the SD was based on the random cohort sample. Model 1 was adjusted for age, sex, race/center, and BMI. Given the association of migraine with impaired insulin sensitivity27 as well as the association of adiponectin with diabetes,22 model 2 was adjusted for variables in model 1 plus fasting glucose. We formally tested for multiplicative interaction by age, sex, and BMI and present stratified results when interaction was present with a p value <0.05. Our primary outcome was all-Mig; additionally, a sensitivity analysis using migraine as the outcome was conducted.
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of all participating institutions approved the study, and all participants provided written informed consent.
RESULTS
Participants.
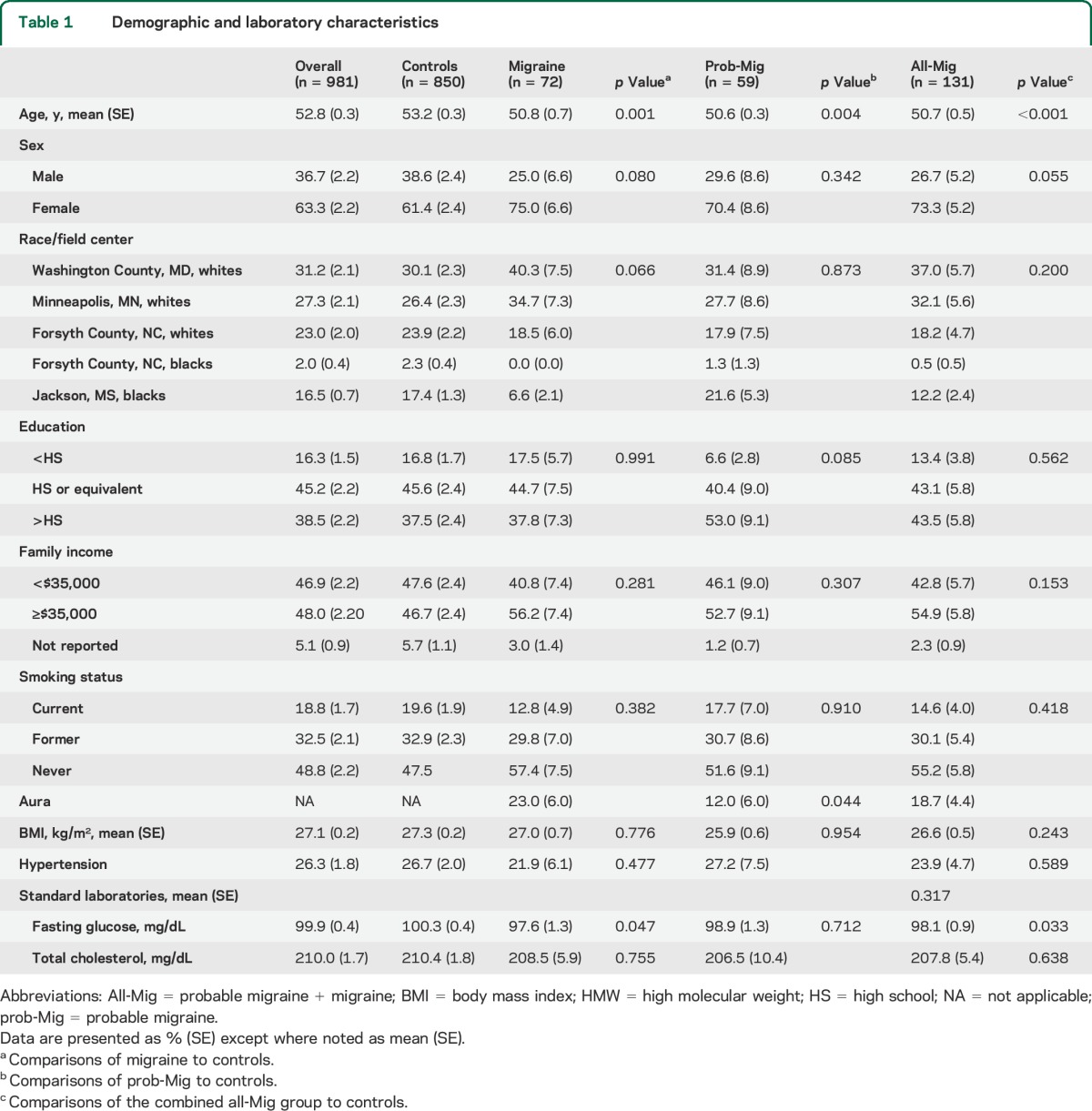
Demographic, socioeconomic, and laboratory characteristics of the 981 participants (360 men and 621 women) are presented in table 1. The mean age of participants was 52.8 years (SE 0.3) (range 45–64). A total of 13.4% (131/981) of participants met criteria for all-Mig (72 migraine, 59 prob-Mig). Participants with all-Mig were more likely to be female (p = 0.055) and younger (p < 0.001) and have lower fasting glucose (p = 0.033) than controls. A total of 18.7% of participants (SE 4.4) with all-Mig had a history of aura (table 1).
Table 1.
Demographic and laboratory characteristics
Adipocytokines.
Adiponectin.
As previously described in the literature,18,28 total and HMW adiponectin levels in our sample were higher in women (total 8.15, SE 0.23 µg/mL, HMW 3.80, SE 0.15 µg/mL) than men (total 5.41, SE 0.21 µg/mL, p < 0.001; HMW 2.1, SE 0.11 µg/mL, p < 0.001; table e-1). As compared to controls, crude total adiponectin levels were increased in those with all-Mig as well as those with migraine alone but were not significantly different in those with prob-Mig (table 2 and figure 1). HMW adiponectin levels were nominally higher in those with all-Mig and migraine alone, as compared to controls, but did not reach statistical significance (table 2, figure 1). The HMW:total adiponectin ratio was not different across groups (table 2). Mean crude total, HMW, and HMW:total adiponectin levels were not different between all-Mig participants with and without aura (table e-2).
Table 2.
Crude adipocytokines levels in migraine and nonmigraine control participants in the Atherosclerosis Risk in Communities Study
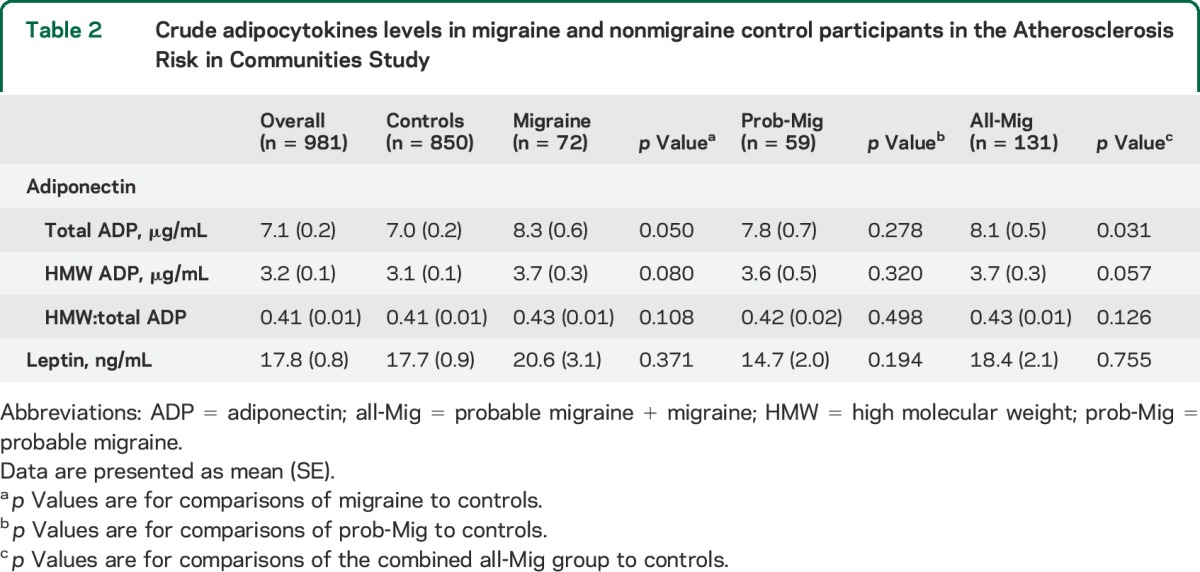
Figure 1. Crude mean total and high molecular weight adiponectin levels in migraine and control participants in the Atherosclerosis Risk in Communities Study.
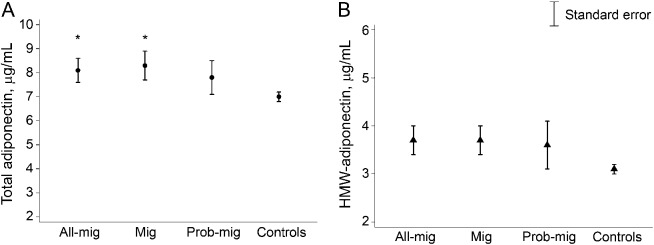
(A) Crude mean total and (B) high molecular weight (HMW) adiponectin levels. *p < 0.05 Compared to controls. All-Mig = both probable migraine and migraine; Mig = migraine; prob-Mig = probable migraine.
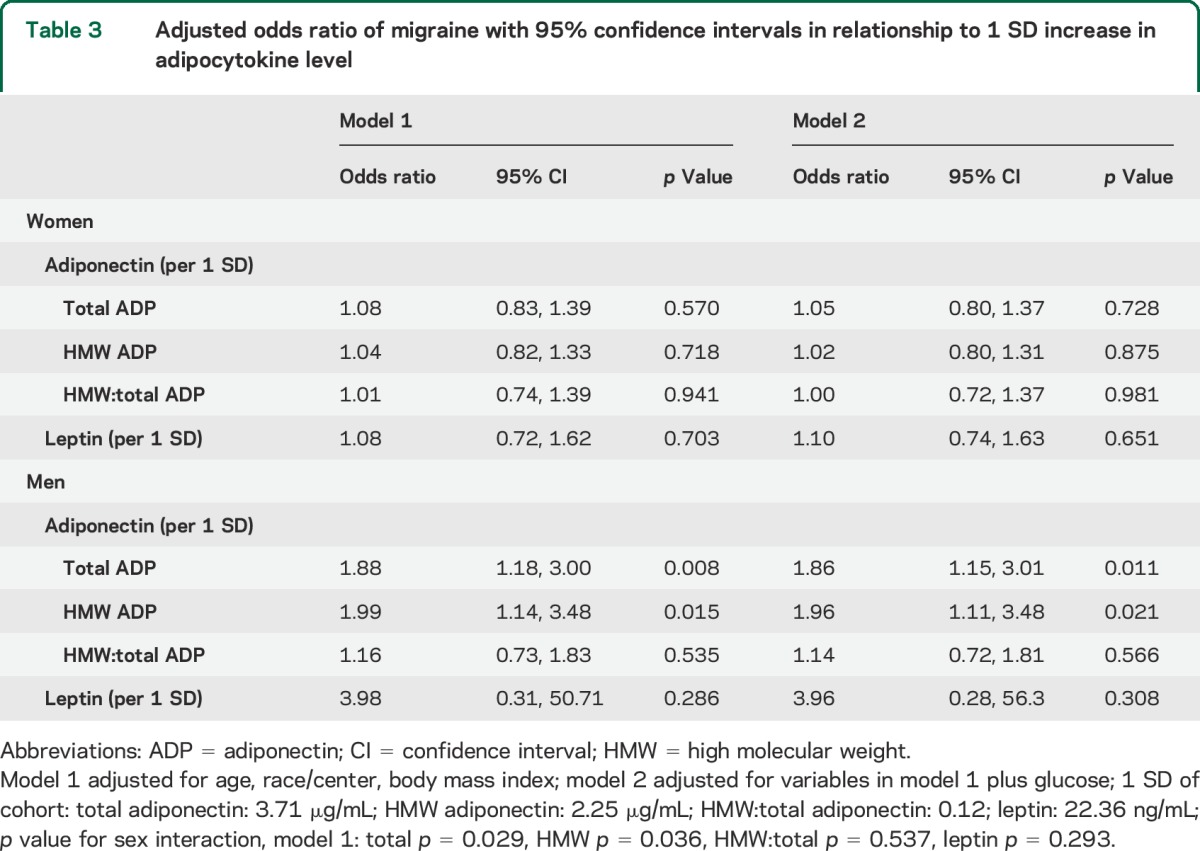
Although there was no difference in crude, sex-stratified adiponectin levels across migraine groups compared to controls (table e-1), after adjustments, the odds of all-Mig were increased by 86% in men (OR 1.86, 95% confidence interval [CI] 1.15–3.01) for each 1-SD increase in total adiponectin (table 3). Similarly, after adjustment, the odds of all-Mig was almost twofold greater in men for each SD increase in HMW adiponectin (OR 1.96; 95% CI 1.11, 3.48). The HMW:total ratio was not associated with increased odds of all-Mig in men. In contrast, the odds of all-Mig and migraine did not increase with increasing total, HMW, or HMW:total adiponectin levels in women (table 3, p value, sex interaction: total p = 0.029, HMW p = 0.036, HMW:total p = 0.537). Due to the small number of male participants with aura (n = 6), reliable interpretation of adiponectin levels stratified by sex with and without aura was not possible.
Table 3.
Adjusted odds ratio of migraine with 95% confidence intervals in relationship to 1 SD increase in adipocytokine level
Leptin.
As previously described in the literature,28,29 crude leptin levels were lower in men than in women, independent of migraine status (mean leptin: women 24.6, SE 1.2 ng/mL; men 6.0, SE 0.4 ng/mL, p < 0.001). However, crude and adjusted leptin levels were not significantly different in any of the migraine groups compared to controls (tables 2 and 3), and there was no sex-specific interaction for leptin (p = 0.293). Additionally, leptin levels did not vary in all-Mig participants by aura status (table e-2).
DISCUSSION
We conducted an analysis in the first large general population study to investigate the association between migraine and adipocytokines and present 3 main findings. First, crude total adiponectin levels were higher in those with migraine as compared to those without migraine. Second, there was an interaction by sex such that increasing total adiponectin was associated with increased odds of migraine in older men, but not women. Finally, leptin did not significantly differ in migraineurs as compared to nonmigraine controls. Supporting our findings that adiponectin differs by migraine status is a prior clinical trial of 37 reproductive-aged women that demonstrated higher total adiponectin levels in women with chronic migraine7; however, no studies have interpreted the sex-specific effect.
There are several potential explanations for the sex interaction with adiponectin and migraine. First, it is possible that the sex differences in the expression of circulating total and multimeric adiponectin contribute partially to the increased risk of migraine in older men but not women. Younger women of reproductive age have been shown to have higher total adiponectin and HMW adiponectin, but comparable LMW adiponectin levels, in comparison with younger men.18,28,30 The lower adiponectin levels in younger men may be, at least in part, due to the effects of testosterone.31 Castrated mice have high levels of plasma adiponectin and testosterone treatment reduced plasma adiponectin.32 Further, in rat adipocytes, testosterone selectively impeded HMW adiponectin but not middle molecular weight (MMW) or LMW adiponectin.33 As such, it may be that the multimers MMW or LMW are more important for migraine, and these levels are differentially expressed between sexes.
It is also possible that by looking only at older migraineurs, we are missing a differential sex effect by age, in which adiponectin levels are increased in young but not older women with migraine. Adiponectin levels were shown to increase with age, particularly in men.19,34 Whereas men over 65–70 years of age have significantly higher plasma adiponectin levels than young men, women may have less of an increase in total adiponectin with age. Thus with age (and declining testosterone), men have a greater increase in total adiponectin and specifically more HMW adiponectin than older women, potentially placing men with higher adiponectin at greater risk of migraine in the age group studied.
We were not able to evaluate the LMW and MMW adiponectin oligomers. It is possible that the extent of the increase in the ratio of the proinflammatory (HMW) to anti-inflammatory (LMW) oligomers contributes to migraine risk. Thus, as older men have a larger increase in total and HMW adiponectin than women, they may also have larger increases in the HMW:LMW ratio and thus greater vulnerability to inflammation and migraine as compared to older women. As LMW adiponectin was not evaluated, we were not able to test this hypothesis.
It is also possible that total and HMW adiponectin may be only increased ictally in women with active migraine pain, and not interictally when migraineurs are pain-free.7 Previous studies have shown that total and HMW adiponectin were elevated in chronic migraineurs with active pain as compared to both episodic migraineurs when interictal and controls without pain.7 In addition, a second study demonstrated that total, HMW adiponectin, and HMW:LMW ratio were increased during acute moderate to severe pain in episodic migraineurs as compared to levels after successful treatment with mild or no pain.8 As we were unable to assess the level of migraine pain at the time of the blood draws, it is possible that pain severity is an unmeasured confounder in sex interaction in migraineurs resulting in a missed association of adiponectin and migraine in older women.
Leptin has been studied in relationship to migraine in prior clinic-based studies. One study measured leptin levels in 84 women16 and found that although crude leptin levels were lower in migraineurs than controls, this difference was attenuated after adjusting for fat mass.15 Our results show no association with migraine and leptin, and taken together this suggests that while leptin may participate in energy homeostasis and modulation of inflammatory process (e.g., modulation of NFκβ), it may have less or no direct role in the modulation of inflammation and pain related to migraine.
Our study has several strengths. It permitted assessment of the association between migraine and adiponectin levels in a large biracial population-based sample, not impacted by the limited generalizability inherent in clinical samples. Participants in a community-based sample of older individuals allowed investigation into associations with migraine, independent of BMI and other rigorously measured confounders. Our ascertainment of migraine is in close alignment with standardized criteria.
There are several limitations of our study. First, migraine assessments and blood draws were done approximately 6 years apart, thus we were not able to determine the presence or absence of active migraine pain at the time of the blood draw. As a result, while we can say that in older men the risk of migraine is increased with increasing total adiponectin, because pain is unmeasured, it is not clear that there is an absence of an association in older women between total adiponectin and migraine. The nonconcurrent cross-sectional design permits the possibility that some participants classified as nonmigraine controls developed migraine between visits 1 and 3, and thus the adiponectin values would have been drawn prior to development of migraine. It is likely that this only occurred in a small number of participants, as migraine has the highest incidence in the second decade of life, with a very low incidence after age 50 years.35,36 Another consideration is that adiponectin levels increase with age,17 and are likely lower at visit 1 than would be projected 6 years later at visit 3; however, this should occur nondifferentially across all participants included in the current analysis (i.e., irrespective of presence or absence of migraine).
Second, adiponectin is a complex hormone with respect to its role in inflammation, with both proinflammatory and anti-inflammatory properties, depending on the oligomers and ratio of the HMW:LMW units of adiponectin. As LMW adiponectin was not evaluated, a potentially important piece of the inflammatory role adiponectin plays in older migraineurs may be missing from the current study. It is possible that the LMW or HMW:LMW ratio may be more sensitive of a marker of migraine in both women and men.
Finally, migraine ascertainment is subject to recall bias, and we were not able to exclude remitted migraineurs or nonmigraine headaches (e.g., tension-type headache) from our control group, which may have biased our findings toward a null effect.37 While it is possible that some migraineurs may have been part of the nonmigraine control group, the migraine groups more accurately reflect those with actual migraine, therefore any associations observed are conservative estimates. This potential misclassification could lead to attenuation of the results described, especially in women.38 Unlike a clinic-based population, expanding the modified ICHD criteria to the ARIC cohort study has an unknown sensitivity for detecting migraine, and has not been validated with physician adjudication. Although aura was not a main outcome, it was classified based on one question, which can lead to misclassification of aura status. Most importantly, participants were only asked about visual symptoms, and aura can take many other forms.
Despite these limitations, the present study demonstrates that in older individuals, total adiponectin is increased in migraineurs and that the risk of migraine demonstrates a sex-specific effect such that higher total adiponectin levels are associated with a greater prevalence of migraine in older men, but not older women. Additionally, leptin is not associated with migraine in older men or women. Further research examining the association between migraine and adiponectin, and particularly LMW adiponectin and the HMW:LMW ratio in older men and women inside and outside acute pain states, is warranted.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of ARIC for their contributions.
GLOSSARY
- all-Mig
combined group of participants with migraine and probable migraine
- ARIC
Atherosclerosis Risk in Communities Study
- BMI
body mass index
- CI
confidence interval
- HMW
high molecular weight
- ICHD
International Classification of Headache Disorders
- IL-6
interleukin-6
- LMW
low molecular weight
- MMW
middle molecular weight
- NFκβ
nuclear factor κβ
- OR
odds ratio
- prob-Mig
probable migraine
Footnotes
Editorial, page 2198
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jennifer Dearborn: primary authorship of manuscript, data interpretation, approval of final manuscript. Andrea L.C. Schneider: primary statistical analyst, manuscript revision, approval of final manuscript. Rebecca F. Gottesman: statistical assistance, data interpretation, manuscript revision, approval of final manuscript. Tobias Kurth: study design, data interpretation, manuscript revision, approval of final manuscript. James S. Pankow: acquisition of data, data interpretation, manuscript revision, approval of final manuscript. David Couper: statistical assistance, data interpretation, manuscript revision, approval of final manuscript. Kathryn M. Rose: data interpretation, manuscript revision, approval of final manuscript. Michelle Williams: study design, data interpretation, manuscript revision, approval of final manuscript. B. Lee Peterlin: study conception and design, data interpretation, manuscript revision, approval of final manuscript.
STUDY FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) and National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK56918.
DISCLOSURE
J. Dearborn reports no disclosures relevant to the manuscript. A. Schneider is supported by NIH/NHLBI training grant T32HL007024. R. Gottesman is supported by R01 (NIA G040282). T. Kurth has received investigator-initiated research funding from the French National Research Agency and the US NIH within the last 2 years and has received honoraria from BMJ and Cephalalgia for editorial services. J. Pankow, D. Couper, K. Rose, and M. Williams report no disclosures relevant to the manuscript. B. Peterlin received investigator-initiated research support from GSK and Luitpold Pharmaceuticals for studies unrelated to the current manuscript, funding by NIH/NINDS grant K23-NS078345, and a Landenberger Foundation grant for a study unrelated to the current manuscript, and serves as an associate editor for Headache. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Chai NC, Scher AI, Moghekar A, Bond DS, Peterlin BL. Obesity and headache: part I: a systematic review of the epidemiology of obesity and headache. Headache 2014;54:219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology 2005;64:614–620. [DOI] [PubMed] [Google Scholar]
- 3.Rainero I, Limone P, Ferrero M, et al. Insulin sensitivity is impaired in patients with migraine. Cephalalgia 2005;25:593–597. [DOI] [PubMed] [Google Scholar]
- 4.Ward TN. Migraine diagnosis and pathophysiology. Continuum 2012;18:753–763. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea G, Leon A. Pathogenesis of migraine: from neurotransmitters to neuromodulators and beyond. Neurol Sci 2010;31(suppl 1):S1–S7. [DOI] [PubMed] [Google Scholar]
- 6.Peroutka SJ. Calcitonin gene-related peptide targeted immunotherapy for migraine: progress and challenges in treating headache. BioDrugs 2014;28:237–244. [DOI] [PubMed] [Google Scholar]
- 7.Peterlin BL, Alexander G, Tabby D, Reichenberger E. Oligomerization state-dependent elevations of adiponectin in chronic daily headache. Neurology 2008;70:1905–1911.18474846 [Google Scholar]
- 8.Peterlin BL, Tietjen GE, Gower BA, et al. Ictal adiponectin levels in episodic migraineurs: a randomized pilot trial. Headache 2013;53:474–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao TS, Tomas E, Murrey HE, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 2003;278:50810–50817. [DOI] [PubMed] [Google Scholar]
- 10.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–783. [DOI] [PubMed] [Google Scholar]
- 11.Peterlin BL, Bigal ME, Tepper SJ, Urakaze M, Sheftell FD, Rapoport AM. Migraine and adiponectin: is there a connection? Cephalalgia 2007;27:435–446. [DOI] [PubMed] [Google Scholar]
- 12.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- 13.Kovacova Z, Tencerova M, Roussel B, et al. The impact of obesity on secretion of adiponectin multimeric isoforms differs in visceral and subcutaneous adipose tissue. Int J Obes 2012;36:1360–1365. [DOI] [PubMed] [Google Scholar]
- 14.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 15.Guldiken B, Guldiken S, Demir M, Turgut N, Tugrul A. Low leptin levels in migraine: a case control study. Headache 2008;48:1103–1107. [DOI] [PubMed] [Google Scholar]
- 16.Bernecker C, Pailer S, Kieslinger P, et al. GLP-2 and leptin are associated with hyperinsulinemia in non-obese female migraineurs. Cephalalgia 2010;30:1366–1374. [DOI] [PubMed] [Google Scholar]
- 17.Peterlin BLRA, Williams MA, Rosenberg JR, et al. Episodic migraine and obesity and the Influence of age, race, and sex. Neurology 2013;81:1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bidulescu A, Liu J, Hickson DA, et al. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovasc Disord 2013;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol 2005;153:91–98. [DOI] [PubMed] [Google Scholar]
- 20.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2004;53:2473–2478. [DOI] [PubMed] [Google Scholar]
- 22.Zhu N, Pankow JS, Ballantyne CM, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC Study. J Clin Endocrinol Metab 2010;95:5097–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson AP, Rose KM, Sanford CP, et al. Lifetime prevalence of migraine and other headaches lasting 4 or more hours: the Atherosclerosis Risk in Communities (ARIC) Study. Headache 2004;44:20–28. [DOI] [PubMed] [Google Scholar]
- 24.Hamedani AG, Rose KM, Peterlin BL, et al. Migraine and white matter hyperintensities: the ARIC MRI study. Neurology 2013;81:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt MI, Duncan BB, Vigo A, et al. Leptin and incident type 2 diabetes: risk or protection? Diabetologia 2006;49:2086–2096. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999;353:1649–1652. [DOI] [PubMed] [Google Scholar]
- 27.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46:459–469. [DOI] [PubMed] [Google Scholar]
- 28.Boyne MS, Bennett NR, Cooper RS, et al. Sex-differences in adiponectin levels and body fat distribution: longitudinal observations in Afro-Jamaicans. Diabetes Res Clin Pract 2010;90:e33–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad MF, Damani S, Gingerich RL, et al. Sexual Dimorphism in plasma leptin Concentration. J Clin Endocrinol Metab 1997;82:579–584. [DOI] [PubMed] [Google Scholar]
- 30.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol 2005;153:409–417. [DOI] [PubMed] [Google Scholar]
- 31.Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of Androgens on circulating adiponectin in male and female rodents. PLoS One 2012;7:e47315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishizawa H, Shimomura I, Kishida K, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002;51:2734–2741. [DOI] [PubMed] [Google Scholar]
- 33.Xu A, Chan KW, Hoo RLC, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by Inhibiting its secretion from adipocytes. J Biol Chem 2005;280:18073–18080. [DOI] [PubMed] [Google Scholar]
- 34.Obata Y, Yamada Y, Takahi Y, et al. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol 2013;79:204–210. [DOI] [PubMed] [Google Scholar]
- 35.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin 2009;27:321–334. [DOI] [PubMed] [Google Scholar]
- 36.Rozen TD, Swanson JW, Stang PE, McDonnell SK, Rocca WA. Increasing incidence of medically recognized migraine headache in a United States population. Neurology 1999;53:1468–1473. [DOI] [PubMed] [Google Scholar]
- 37.Peterlin BL, Scher AI. Migraine and the social selection vs causation hypotheses: a question larger than either/or? Neurology 2013;81:942–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart WF, Roy J, Lipton RB. Migraine prevalence, socioeconomic status, and social causation. Neurology 2013;81:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


