Summary
Vasculitis of the medium and large arteries, most often presenting as giant cell arteritis (GCA), is an infrequent, but potentially fatal type of immune-mediated vascular disease. The site of the aberrant immune reaction, the mural layers of the artery, is strictly defined by vascular dendritic cells, endothelial cells, vascular smooth muscle cells and fibroblasts which engage in an interaction with T cells and macrophages to ultimately cause luminal stenosis or aneurysmal wall damage of the vessel. A multitude of effector cytokines, all known as critical mediators in host-protective immunity, has been identified in the vasculitic lesions. Two dominant cytokine clusters, one centering on the IL-6/IL-17 axis, the other on the IL-12/IFN-γ axis, have been connected with disease activity. These two clusters appear to serve different roles in the vasculitic process. The IL-6/IL-17 cluster is highly responsive to standard corticosteroid therapy, whereas the IL-12/IFN-γ cluster is resistant to steroid-mediated immunosuppression. The information exchange between vascular and immune cells and stabilization of the vasculitic process involves members of the NOTCH receptor and ligand family. Focusing on elements in the tissue context of GCA, instead of broadly suppressing host immunity, may allow for a more tailored therapeutic approach and spare patients the unwanted side-effects of aggressive immunosuppression.
Introduction
Human blood vessels range in diameter from 8 micrometers to 30,000 micrometers and span over 60,000 miles, making them one of the largest organ systems in the body. Like the immune system, blood vessels are distributed widely, ultimately reaching every, even remote, tissue site. Blood vessels are the major transit ways for immune cells, giving innate and adaptive immune cells rapid access to essentially all peripheral tissues as well as to the immune storage sites in lymphoid organs. Given the intimate relationship between the immune and vascular systems, it is surprising that immune-mediated vasculopathies are rare diseases.1-4 This statement does not hold for atherosclerotic disease, which remains the most frequent cause of death in the Western world. In that context it is intriguing that the pathogenic understanding of atherosclerosis has recently undergone a marked change. Previously recognized as a lipid storage disease, atherosclerosis is now emerging as an inflammatory syndrome in which innate and adaptive immune responses participate in every stage of the disease process.5, 6
Classical autoimmune inflammation of medium and large arteries (diameter >2000 micrometers) occurs infrequently. Large vessel vasculitides (LVV) affect the aorta and its major branches, and due to the vital role of such blood vessels are characterized by serious clinical complications. When attacked by misfunctional immunity, medium arteries respond with occlusion of the lumen and ischemic damage of dependent organs ensues. The aorta is more likely to develop signs of wall destruction instead of stenotic lesions; manifesting as aneurysm formation, rupture or dissection.7, 8 The pathological hallmark of LVV are chronic inflammatory lesions within the vessel wall, not outside the vessel wall, distinguishing LVV clearly from the small vessel vasculitides in which inflammation also occurs in the surrounding tissue.
Inflammatory infiltrates within the wall of the aorta and its major branches often display a distinct microarchitecture and are arranged as granulomatous lesions. Two syndromes account for most cases of LVV, giant cell arteritis (GCA) and Takayasu arteritis (TA).9 TA preferentially appears in the aorta and its primary branches. GCA lesions have a tendency to be localized in more peripheral, medium-sized arteries, affecting the 2nd-5th branches of the aorta. The manifestation pattern of both LVV makes it clear that vessel size and closely linked structural and functional attributes are key factors in the disease process.10 Which determinants within the wall of the major aortic branches (diameter of 5-30 mm) distinguish that tissue niche from the wall of an arteriole (diameter of 10-30 um) is currently not understood. Arterial diameter and wall thickness is directly correlated with body size.11, 12 In large human arteries the thickness of the wall exceeds the effective diffusion distance of oxygen and the medial smooth muscle cell layer, which has the highest metabolic demands, must be supplied from adventitial vessels.13 In contrast, in small animals the medial layer is thin enough to receive oxygen and nutrient supply solely via diffusion from the main lumen. Accordingly, it has been a major challenge to mimic LVV in model organisms that are much smaller than humans. On the other hand, access to the aorta of a human for tissue sampling occurs only under extremely restricted clinical conditions and these hurdles have hampered attempts to elucidate the pathogenesis of TA. The temporal artery, the preferred target of GCA, is easily accessible and is routinely biopsied for diagnostic purposes. Investigations of arterial immune infiltrates, coupled with studies of circulating immune cells, have supported the development of new pathogenic concepts directly relevant for humans. Considerable progress has been made in unraveling the misguided immune responses underlying LVV over the last decade and we focus this review on GCA as a richer source of new ideas and understandings. The emerging theme is that of multiple, fundamentally divergent immune pathways contributing to disease, making it unlikely that a single disease instigator causes LVV. Instead, recognition of the critical role of the artery as a player in immuno-stromal interactions is refocusing the pathogenic concepts, promoting the emergence of the new field of vasculo-immunology.14, 15
The cellular players of GCA
The immunopathology of GCA derives from dysregulated interaction between the vessel wall and the immune system (Figure 1).16 The vessel wall contributes endothelial cells (EC), vascular smooth muscle cells (VSMC), elastic membranes, matrix and fibroblasts. In arteries with a diameter of >2000 micrometers, the walls reach such a thickness that a supply network of vasa vasorum is required. Such arteries harbor dendritic cells, right at the interface of the adventitia and the media.17-19 Despite these vascular dendritic cells (vasDC), arterial walls are immuno-privileged, able to avoid spontaneous allorecognition.20 In vasculitic lesions, vasDC undergo a transformation, increase in number, distributed throughout the wall and are an absolute requirement to sustain the disease process.21 The immune system sends T cells, mostly CD4 T cells and macrophages to the vessel wall niche. They enter the wall through the vasa vasorum and penetrate through the tissue space in an adventitial-intimal direction. Typically, highly activated macrophages, so-called histiocytes, are arranged in granulomas, with surrounding T cells. B cells are absent from the infiltrates,22 and germinal center formation is not a feature of LVV. To break the immune privilege of normal human arteries wall-integrated DC need to be stimulated. Triggering of receptors recognizing danger signals, such as Toll-like receptors (TLR), is sufficient to break tissue tolerance and render arteries susceptible to immune attack.23, 24 It is currently not known whether such an event initiates LVV. Both, local as well as systemic, danger signals could break the immune privilege. As in all autoimmune syndromes of unknown etiology, infectious agents have been suspected to elicit the granulomatous reaction. A multitude of infectious organisms has been proposed, including a recent observation of a Burkholderia-like strain in some tissues.25, 26 Such observations require confirmation in independent studies. The relationship between infectious agents and vascular disease is certainly more complex, as evidenced by the recent report of diverse oral and gut bacteria (chryseomonas, veillonella and streptococcus) present in all tested atherosclerotic plaque samples.27 Equally likely is the local presence of autoantigens or stress-related proteins that can elicit autoreactivity.28
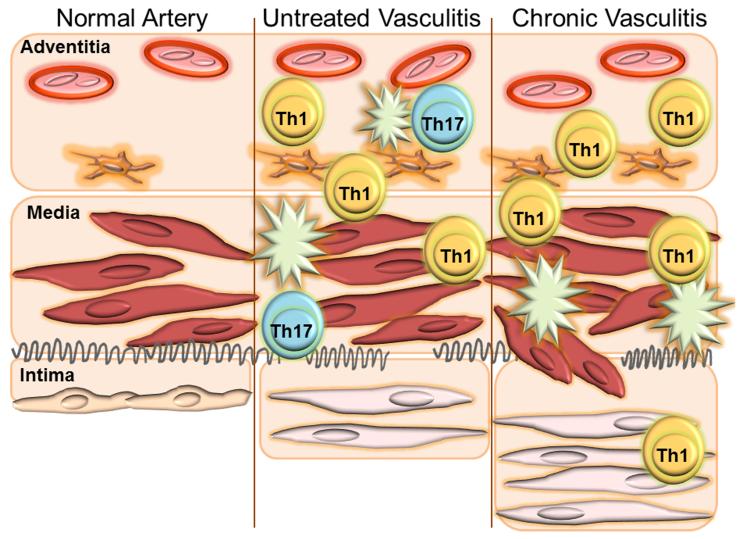
Figure 1.
The walls of human arteries are multi-layered, with an endothelial barrier in the intima, sheets of vascular smooth muscle cells in the media and the vasa vasorum network in the adventitia. Endogenous vascular dendritic cells populate the adventitia (left) and are responsible for the recruitment of T cells and macrophages into the tissue niche. In early and untreated vasculitis, IFN-γ–producing Th1 cells and IL-17-secreting Th17 cells are abundant, surrounded by macrophages (middle). Corticosteroid therapy diminishes Th17 cells but cannot clear Th1 cells from the vascular lesions (right). Dysregulated VSMC migrate towards the lumen and lay down to form lumen-stenosing intimal hyperplasia.
The IL-6/IL-17 cytokine cluster in GCA
Two decades ago the elevation of circulating IL-6 was reported in patients with GCA and serum levels were correlated with disease activity.29 Later reports assigned IL-6 production to several cell populations in the vessel wall granulomas, except endothelial cells and multinucleated giant cells,30 and were able to correlate the intensity of the systemic inflammatory response to tissue expression of of IL-1β, TNFα and IL-6.31 Several clinical aspects of GCA have supported the concept that IL-6 is an important disease mediator. Patients have a combination of vascular and extravascular disease, almost always with a strong component of systemic inflammation. Amongst clinicians, GCA is considered a prototype of an aggressive systemic inflammation, often associated with constitutional symptoms (weight loss, fever, anorexia, failure-to-thrive). It is not unusual for this extravascular component to dominate the clinical presentation.32 The underlying molecular process is a vigorous acute phase response, which separates GCA from many other autoimmune syndromes. Biomarkers associated with the acute phase response are a strongly elevated erythrocyte sedimentation rate (ESR), often reaching 80-100 mm/hour, and C-reactive protein levels often extremely high. Acute phase proteins are produced by hepatocytes upon stimulation with IL-1– and IL-6–type cytokines.33 Accordingly, IL-6 was reported to be strongly elevated in GCA patients about 20 years ago.29, 30, 34 IL-6 is a pleiotropic cytokine, with a multiplicity of functions that specializes in the cross-talk between stromal cells and immune cells and ferries information between injured tissues and the immune system.35 ECs, VSMCs and fibroblasts can release IL-6, but it can also derive from lymphocytes and macrophages, allowing for positive feed-forward looping in the amplification of inflammatory reactions. Deriving from vascular cells, including ECs and VSMCs, IL-6 appears as an important connector between injured vascular walls and immune cells. Besides its systemic effect, activating hepatocytes, IL-6 also shapes the local cytokine environment and orchestrates the patterning of immune reactions. Of particular importance is the role of IL-6 as a polarizing cytokine, guiding the differentiation of T cells into selected functional lineages (Figure 2). Specifically, IL-6 is critically involved in promoting the differentiation of the Th17 lineage, a functional T cell population first described in 2005.36-39 Initially, it was believed that a combination of IL-1β, IL-6 and IL-23 was sufficient to direct the differentiation of a T cell into a Th17 cell. It is now clear that naïve T cells are negative for IL-1R and IL-23R, but these receptors are upregulated when the cells are exposed to TGF-β and IL-6 or IL-21.40, 41 This mechanism assigns a key role to IL-6 in directing T cell responses.
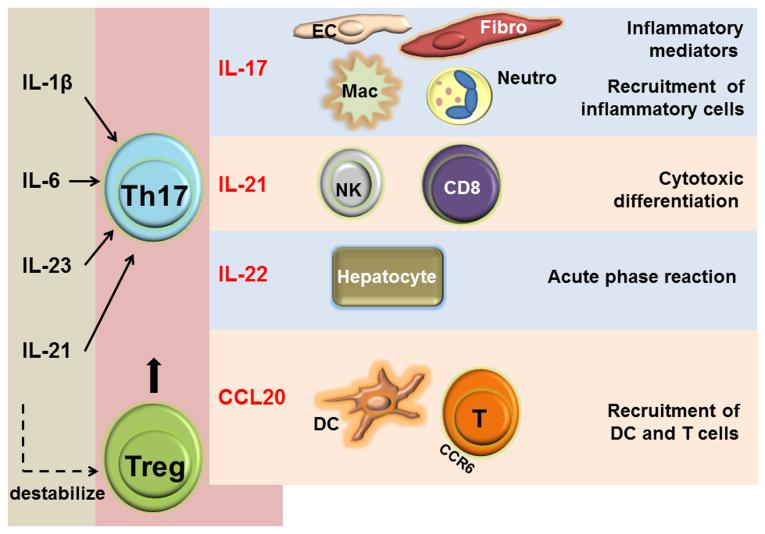
Figure 2.
IL-1β, IL-6, IL-23 and IL-21 shift T cell differentiation towards the Th17 lineage. Th17 cells produce a plethora of cytokines that regulate local and systemic inflammatory effects in GCA.
Th17 cells exert their multiple proinflammatory functions through the release of effector cytokines, including IL-17, IL-21, IL-22, CCL20, GM-CSF, IL-8 (CXCL8), and IL-26.42, 43 Multiple of these cytokines have been identified in the inflammatory lesions of GCA. The wide distribution of receptors for the Th17-related cytokines makes it possible for Th17 cells to participate in several layers of vascular wall-injurious immunity: IL-21–enhanced differentiation of cytotoxic cells; IL-22–mediated hepatocyte stimulation and acute phase amplification; IL-17–dependent stimulation of ECs, VSMCs and fibroblasts; IL-17–regulated recruitment of macrophages and neutrophils; CCL20-facilitated recruitment of dendritic cells and T cells (Figure 2).44-47
Frequencies of Th17 cells are up to tenfold increased in the blood of GCA patients.48-50 Th17 cells are part of the cellular infiltrates in the arteries. In a study comparing the composition of the vasculitic infiltrates before and after corticosteroid therapy, immunosuppression caused an intriguing shift in wall-infiltrating T cell populations. Prior to therapy, IL-17–producing T cells were mixed in with IFN-γ–producing Th1 cells to create the granulomas. Arteries from treated patients contained few Th17 cells, but were populated by Th1 cells.48 IL-21 has also been reported to appear within vasculitic infiltrates.50 IL-6 and IL-1 are certainly present and TGF-β has been detected in GCA-affected temporal artery biopsies.51, 52 In essence, a cluster of Th17-related cytokines participates in the vasculitic reaction.
Besides its role in inducing Th17 differentiation, IL-6 has also been implicated in regulating the induction of anti-inflammatory T regulatory (Treg) cells. Specifically, the developmental pathways leading the Th17 and Treg induction seem reciprocal with IL-6, IL-21 and IL-23 blocking the Treg-specifying transcription factor FoxP3 and instead enhancing the Th17-inducing RORγt transcription factor.53, 54 Accordingly, frequencies of Treg cells have been reported to be reduced in GCA patients.49, 50 Within the arterial wall infiltrates Treg cells seem to be distinctly low, although it does not seem to hold for FoxP3-expressing cells. Thus, IL-6 may have two major effects in LVV; boosting proinflammatory T cell immunity and paralyzing opposing anti-inflammatory T cells. A similar duality of IL-6 also applies to another immune-dependent vasculopathy, arterial allograft rejection.55, 56
Given that the frequencies of Th17 precursor cells, CD161+CD4+ T cells, have been reported to be similar in patients and controls,49 the defect in biasing T cell immunity towards Th17 differentiation appears to be extrinsic to the adaptive immune system. Whether the aging process and immunosenescence contribute to this deviation of T cell immunity is currently not known. But given the stringent age cutoff of GCA, appearing exclusively during the 6th-9th decade of life, age-related defects in the regulatory control of innate and adaptive immune responses almost certainly have a major pathogenic function.57
The IL-12/IFN-γ cytokine cluster in GCA
While available evidence suggests that the IL-6/IL-17 cytokine cluster plays an important role in early GCA, it may be less important during chronic disease.58 Support for this concept stems from a plethora of clinical observations, such as the persistence of vasculitis despite long-term and high-dose corticosteroid therapy, and from recent evaluations of GCA patients treated with the IL-6 pathway blocker tocilizumab.59 Unizony et al have reported that a patient treated with tocilizumab had persistent vasculitis of medium-sized and large vessels on autopsy59 and Xenitidis et al have encountered sustained inflammation of the aortic wall despite tocilizumab treatment in Takaysu arteritis .60 Clinical observations have already suggested that LVV may indeed not be a short-term, self-limiting condition but may persist over extended periods in a subset or most patients. Aortic aneurysm formation a decade after initial diagnosis and treatment and positivity of temporal artery biopsies in patients on corticosteroids is a suspicious finding, supporting a concept of persistent, chronic smoldering disease, contrasting the idea of GCA as a self-limiting syndrome. A recent study systematically evaluating temporal artery biopsies 3, 6, 9 and 12 months after treatment initiation has confirmed that the vasculitis does not go into remission, although IL-1, IL-6 and IL-17 production was effectively suppressed.48
Whereas untreated arteritic tissues contained a mixture of Th17 and Th1 cells, biopsies and blood samples of treated patients displayed a signature of a Th1 response, which was separable from the Th17 response (Figure 3). Plasma levels of IFN-γ, essentially non-detectable in healthy age-matched controls, are elevated in untreated GCA cases and remain elevated postcorticosteroid therapy. Corticosteroids rapidly control IL-1, IL-6 and IL-23 production, followed by suppression of IL-17 production, both in the blood and in the inflamed arteries.48 Despite of this effective immunosuppression though, the vasculitis persists and continues as a Th1-dependent disease. The cytokine signature associated with chronic disease includes IL-12 and IFN-γ and no longer relies on IL-6, IL-23 and IL-17.61 These recent data confirm previous work published in 1997 by Brack et al. That study explored therapeutic effects of corticosteroids in SCID-human artery chimeras engrafted with inflamed human temporal arteries. Even when extremely high doses of corticosteroids were used (dexamethasone 4 mg/kg, equivalent to >1900 mg prednisolone) the tissue T cell infiltrates persisted and tissue IFN-γ expression was barely affected.62 In contrast, the monocyte cytokines IL-1β and IL-6 were highly susceptible to steroid-mediated suppression. Thus, despite the limitations inherent to studies in human tissues, strong evidence has accumulated that vascular inflammation may persist despite effective IL-6 blockade.
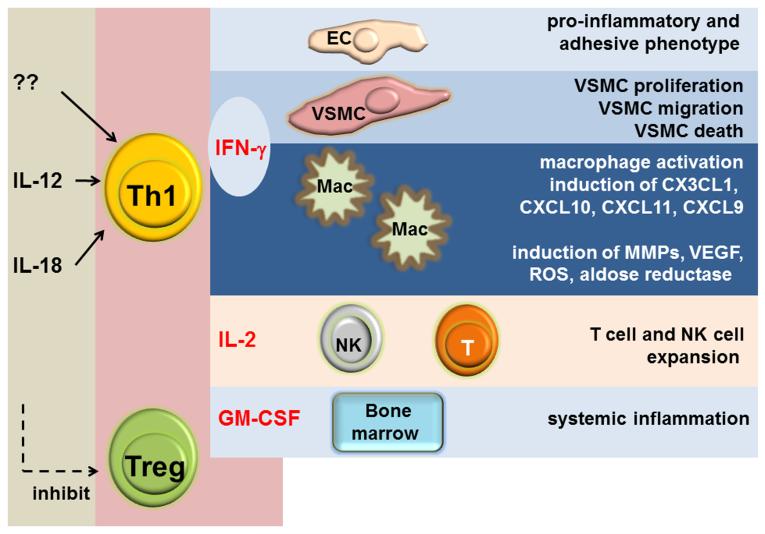
Figure 3.
IL-12 is a major inducer of Th1 cells which release the highly potent cytokine IFN-γ into the microenvironment. IFN-γ controls macrophages activation and regulates disease-relevant functions of endothelial cells and vascular smooth muscle cells in vasculitis.
The IL-6–independent, IFN-γ–dependent arm of vasculitic immunity is reminiscent of graft arteriosclerosis (GA) (Table 1). GA occurs in transplanted hearts and is the dominant mechanism causing late cardiac allograft failure.63, 64 The disease process is characterized by concentric intimal hyperplasia which eventually leads to luminal stenosis and allograft ischemia. Outgrowth of the lumen-obstructing neotissue is driven by a T cell- and macrophage-dependent immune response which stimulates vascular smooth-muscle like cells to migrate, replicate and secrete extracellular matrix.65 This process closely mimics events in GCA, where the clinical complications (blindness, stroke, aortic arch syndrome) are a consequence of luminal occlusion.
Table 1.
Pathogenic pathways in Giant cell arteritis (GCA) and in Graft Arteriosclerosis (GA)
| Giant Cell Arteritis |
Graft Arteriosclerosis |
|
|---|---|---|
| Chronic smoldering course |
+ | + |
| Vascular remodelling | Luminal stenosis of medium arteries |
Luminal stenosis of conduit arteries |
| Secondary ischemic damage |
Optic nerve, CNS | Graft parenchyma |
| Key driver | IFN-γ-producing Th1 cells | IFN-γ-producing Th1 cells |
| Immuno-stromal interactions |
infiltrating immune and resident vascular cells |
infiltrating immune and resident vascular cells |
| Dysregulated VSMC function |
proliferation migration matrix production |
proliferation migration matrix production |
| Systemic inflammation | intense | subclinical |
| Responsive to corticosteroids |
partially | non-responsive |
Elegant work in the model system of GA has established IFN-γ as a key pathogenic factor (Table 1).64, 66 IFN-γ is a powerful cytokine, a prototype of an effector cytokine in anti-pathogen immune responses. The role of IFN-γ in conferring pathogen resistance is nonredundant.67, 68 Binding of the IFN-γ receptor elicits activation of the JAK-STAT signaling pathway, culminating in the activation of the STAT1 transcription factor. STAT1-dependent genes represent a complex program of immune effector genes, with hundreds of genes amplifying antigen presentation, lymphocyte and macrophages recruitment, proliferation and apoptosis or target cells.69 An important outcome of IFN-γ action is the activation of macrophages, differentiation of helper and regulatory T cells and the remodeling of stromal cells.70 Accordingly, IFN-γ integrates immune regulatory events with response patterns of the surrounding tissues, optimizing anti-bacterial and anti-viral immunity but also intensifying tissue damage. In the specialized tissue niche of the vessel wall, IFN-γ triggers migratory and proliferative signaling pathways in VSMC. In temporal artery lesions of patients with GCA, tissue levels of IFN-γ have been associated with the following pathologies: luminal compromise by hyperplastic intima,71 formation of a neoangiogenic network of capillaries that support the expanding intimal layer (Figure 3).72 In line with these observations, chronically inflamed arteries of treated patients contain dense infiltrates of IFN-γ producing Th1 cells. Tissue transcripts for IFN-γ and plasma concentrations of IFN-γ are essentially unaffected by corticosteroid treatment.48
While the source of tissue IFN-γ is clear, it derives from Th1 effector T cells, the spectrum of IFN-γ–induced processes in the arterial wall is steadily expanding.73 IFN-γ upregulates TLR3, rendering VSMC susceptible to activation by tissue-endogenous and exogenous danger signals.74 In an elegant study exploring interactions of IFN-γ with other T cell effector cytokines, such as IL-17, the two cytokines displayed a synergistic interaction with enhanced production of IL-6, CXCL8, and CXCL10.75 These data place IFN-γ at the top of the hierarchy of immune regulators in the mural infiltrates. IFN-γ seems to be particularly important in regulating the dialogue between tissue-infiltrating T cells and VSMC, as well as EC. Whereas some VSMC become migratory and move towards the central lumen, others disappear creating the typical loss of medial layer encountered in GCA-affected arteries. IFN-γ can induce apoptosis of VSMC and thus may well function as a triage signal that separates proliferating and dying VSMC.76 In its role as an immune amplifier, IFN-γ induces a set of chemokines, including IP-10 (CXCL10), Mig (CXCL9), RANTES (CCL5), ITAC (CXCL11) and CX3CL1. A detailed profiling of GCA lesions for such products has not been achieved. However, Th1 cells, in turn, are responsive to CXCR3 and CCR5-binding chemokines,77 establishing a potent reinforcing feedback loop sustaining in situ T cell activation, differentiation and recruitment which may be critically involved in the chronicity of medium and large vessel vasculitis.
GCA lesions have all the features of a Th1 lesion (Figure 3). IFN-γ–producing T cells are surrounded by highly activated macrophages, committed to either the M1 or the M2 polarization lineage.78, 79 Overexpression of IL-32, an IFN-γ-inducible cytokine has been described in inflamed temporal arteries.80 Whether Th1 cells are recruited as committed cells or whether they acquire this functional phenotype within the tissue niche is currently unknown. The functional activity of Th1 cells is regulated by IL-12 and this polarizing cytokine has been identified in the plasma of GCA, with plasma levels indistinguishable in untreated and treated patients.48, 58 The cellular source of IL-12 has not been unequivocally identified, but wall-embedded DC certainly can serve as a local reservoir of IL-12. Some information is available on triggers that can induce local release of IL-12, to direct T cells towards the Th1 differentiation pathway. Ligands for TLR4 and TLR5 have been demonstrated in experimental systems to activate arterial wall-integrated DC and initiate and promote Th1 cell accumulation.24 Vascular DC are equipped with a broad portfolio of pattern recognition receptors, including TLR2, 3, 4, 5, 6, and 8.18 Whether all of these receptors have a potential role in kick-starting immune activation in the otherwise protected artery wall, needs to be examined. Theoretically, different DC, expressing distinct repertoires of danger receptors, could activate different T cell response patterns. The separability of Th1 and Th17 immunity in untreated and treated GCA patients strongly supports the concept that independent axes exist that regulate vasculitic immunity.58
Clinical observations have also supported the concept of two components of immunopathology in GCA; systemic inflammation mostly associated with IL-6-driven immunity and vaso-occlusion sustained by a less inflammatory but more tissue-remodeling process. Supportive evidence includes the close association of intimal hyperplasia on biopsy with neuro-ophthalmologic complications,81 a reduced frequency of constitutional symptoms in patients with vision loss 82, 83 and the stable incidence of visual manifestations despite aggressive management with corticosteroids over the last four decades.84
As in all inflammatory disease, a multitude of cytokines that do not belong to the IL-12/IFN-γ cluster and the IL-6/IL-17 cluster can be expected to contribute to GCA. More mechanistic studies, applying pharmacologic and genetic knockdown technologies in experimental systems and in clinical trials are required to decipher the role of individual cytokines. Notably, TNF-α blockade was explored in a well-designed clinical study involving 44 patients. While the trail was too small to draw definite conclusions, the investigators concluded that using infliximab as maintenance therapy was of no benefit and could be harmful.85 A review of clinical trials in GCA has emphasized the urgent need for improved study design and inclusion of larger patient groups to enhance the yield of useful information.86 Going beyond classical cytokine blockade holds great promise as it may provide clues on distinct cellular and molecular pathways in GCA pathogenesis. One example is the recent observation on the efficiency of leflunamide in difficult-to-treat polymyalgia rheumatic and GCA.87
Immuno-stromal interactions in GCA
Classifying GCA as an autoimmune disease emphasizes the multi-faceted and convincing evidence that host-beneficial immunity is misdirected to lead to granuloma formation in the mural layers of medium and large arteries. That paradigm misses to conceptualize the tissue tropism of the disease and underestimates the stromal milieu as a pathogenic force. By histomorphology the secondary and tertiary branches of the aorta may all appear to be similar, but recent molecular studies have provided a framework to define the selective immunological identity of distinct vascular beds. GCA outside of a vascular wall structure is extremely rare and may not be the same disease as classical granulomatous arteritis. Within the vasculature, GCA displays a clear preference for certain sites and avoids others. At-risk arteries are the temporal artery, the vertebral artery, the distal subclavian artery, the axillary artery and the aorta. On the other hand, intracerebral arteries, mesenteric arteries and lower extremity arteries are at much lower risk. All of these arteries have wall-embedded vasDC, all should be able to attract and stimulate T cells and macrophages. Functional testing in a humanized mouse model has shown that engrafted human arteries are not recognized by allogeneic T cells, unless the immune privilege of the arterial wall has been broken.20, 88 Injection of lipopolysaccharides which trigger TRL4 on vasDC is sufficient to render the vessel wall susceptible to immunological attack. The ability of human arteries to sense lipopolysaccharides, a bacterial product, emphasizes the potential of the vasculature to fulfill immunological functions. More importantly, each artery participates in this function in a selective way. The pattern of Toll-like receptors expressed on the surface of vasDC changes from vessel to vessel, providing a molecular mechanism how different arteries respond to immune stimuli and interact with the adaptive immune system. Carotid, subclavian, mesenteric and femoral arteries derived from the same donor each attract allogeneic T cells but elicited a different response pattern (own unpublished data). Current studies explore whether the aging process alters vasDC function to generate age-related susceptibility, as encountered in GCA.89-91
Given the cellular diversity of a blood vessel wall, it is unlikely that vasDC are the only cells making contact with the immune system. Rather, it is becoming increasingly clear that the tissue stroma through its cellular and matrix components defines “address-codes” that determine recruitment, retention and survival of infiltrating immune cells.92, 93 The wall of a temporal artery is normally free of T cells and macrophages. To establish granulomatous lesions, such cells need to be attracted and then retained in the tissue site. Understanding how long wall-residing T cells survive and whether they recirculate between the disease lesion and secondary lymphoid organs would be valuable in conceptualizing the dynamics of vasculitic reactions, their durability and the molecular pathways that are promising in the therapy of vasculitis. Disease chronicity requires presence of vasculitic T cells and macrophages over years. Whether this requires continuous influx and in situ differentiation would be important to know. A recent study has begun to shed light on possible communication pathways that connect T cells, VSMCs and ECs (Figure 4). Inflamed temporal arteries from patients with GCA contain a strong gene expression signal for NOTCH receptors and ligands.94 The NOTCH signaling pathway is well known for its pivotal role in the development of vertebrate organ systems, including development of vascular structures.95 Also, >50% of T cell acute lymphoblastic leukemias have activating point mutations in the NOTCH1 receptor, implicating NOTCH in T cell proliferation and survival.96 In healthy arteries, multiple NOTCH receptors, NOTCH ligands and their downstream signaling machineries regulate VSMC differentiation, plasticity and phenotype switching and facilitate VSMC-EC communication.97 In patient-derived T cells NOTCH1 receptor expression is 20-fold increased, enabling these T cells to interact with ligand-expressing DC, VSMC and EC.94 Therapeutic blockade of NOTCH-NOTCH ligand interactions effectively suppresses experimentally-induced vasculitis, emphasizing the disease relevance of this cellular communication pathway. Disruption of NOTCH signaling blocks T cell retention and effector differentiation and downregulates proinflammatory networks in vessel wall lesions during early and established disease. Notably, VSMC and EC can express both, NOTCH receptors and NOTCH ligands.98 Vice versa, CD4 T cells can carry NOTCH1 receptors but also Jagged2 ligands.99 Thus, VSMC can serve as signal-sending and as signal-receiving cells. The results of the NOTCH blockade experiments emphasize that such communication fluxes are relevant in vasculitis. Treatment of humanized mouse chimeras engrafted with human arteries revealed that disrupting NOTCH signaling effectively suppressed both T cell and macrophage functions.94 Density of tissue-infiltrating T cells declined in the absence of NOTCH signaling, suggesting a role of the VSMC environment in providing survival signals for T cells. Chimeras treated with a NOTCH signal blocking enzyme blocker produced only low levels of IL-17, whereas tissue IFN-γ levels were more resistant to this form of immunosuppression. One possible explanation is that VSMC selectively communicate with different T cell lineages and thus are a major shaping force in orchestrating vasculitis.100
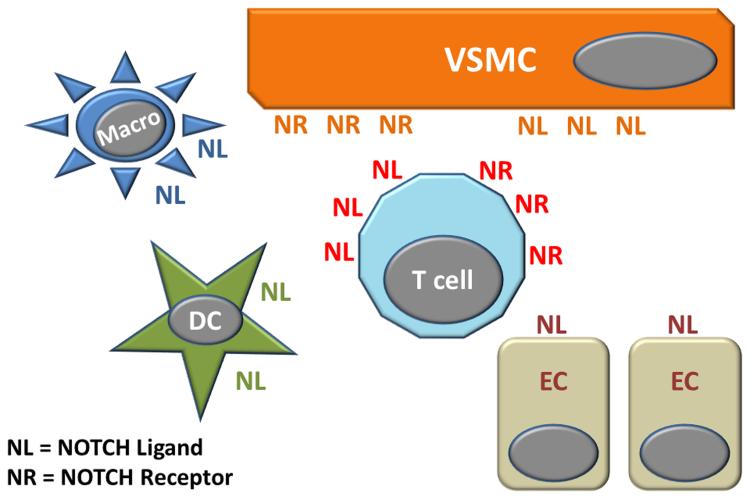
Figure 4.
CD4 T cells from patients with GCA spontaneously express the NOTCH1 receptor, enabling them to engage dendritic cells and macrophages, but more importantly, to exchange information with endothelial cells and vascular smooth muscle cells. Both, CD4 T cells and VSMC express NOTCH receptors and NOTCH ligands, thus functioning as signal-receiving and signal-sending cells in immuno-stromal communications.
An integral part of the vessel wall microenvironment is the extracellular matrix, generally produced by stromal cells. While little is known about the composition of matrix in different human arteries, an emerging field in immunology addresses how stromal cells regulate recruitment, retention, and clearance of immune-competent cells in a tissue niche and how such stromal cells impose regulatory control over host protective and host injurious immune responses. The carbohydrate-binding protein Galectin-1A, produced by stromal cells and deposited into the extracellular matrix, has been implicated in a potent immunoregulatory circuit. Galectin-1A has been described to initiate IL-27–mediated immunosuppressive functions in human DC,101 giving rise to the novel concept that stromal cell populations are signal-sending cells that deviate DC differentiation. As demonstrated in chimera experiments, human medium sized arteries are primarily immunoprivileged, despite being occupied by potent vasDC. An environment-imposed skewing of DC towards a regulatory phenotype would provide an adaptable mechanism to protect vital arteries from immune attack. Stromal cells in lymphoid organs modulate DC and T cell function through a variety of mechanisms, including production of IL-10, TGF-β and nitric oxide.102 Given the aggressiveness of DC and T cell function in GCA-affected arteries, failure of such mechanisms would need to be proposed. More sophisticated experimental systems are required to explore whether vasculitic lesions in GCA result from the inability of the stroma to inhibit localized immunity. The potency of microenvironment-dependent pathways in controlling tissue inflammation is now recognized for the kidney and the lung,103, 104 emphasizing that affected organ structures are not just innocent bystanders but active participants in dysfunctional immune responses. Two other matrix proteins involved in immune modulation are fibronectin and syndecan.105 Syndecan, a heparan sulfate proteoglycan, displays protective functions in abdominal aortic aneurysm formation and acts through regulating T cell and macrophage accumulation.106
An important constituent of the blood vessel wall are endothelial cells which are the first to interact with immune cells infiltrating into the wall layers. Endothelial cells participate in immune responses in a number of inflammatory conditions and are certainly involved when pathogenic immunity unfolds in the vessel wall. Evidence for active recognition of endothelial cells in GCA derives from elegant recent studies demonstrating that patients build anti-endothelial-cell antibodies, including such reacting against vinculin, lamin A/C, voltage-dependent anion-selective channel protein 2, and Annexin V.107 Mouthon and colleagues are actively pursuing the concept that such antibodies have tissue-damaging functions. Evidence for activation of endothelial cells in GCA-affected arteries has come from studies describing the expression of endothelin-1 and endothelin receptors.108
In essence, stromal cells in the vascular wall, together with the matrix proteins they produce, can profoundly influence inflammatory events.109 Under physiologic conditions, the arterial wall is relatively unattainable for the immune system. A breakdown of such protective measures allows recruitment of innate and adaptive immune cells that then form organized microarchitectures in this tissue microenvironment. The intensity and direction of the inflammatory response depends on the immune system, possible recognition of antigen in the tissue site, but, to a large extent, on the immuno-stromal interactions between resident vascular cells and immune effectors.
Conclusions
The walls of medium and large arteries are multilayered structures built by EC, VSMC, fibroblasts, elastic membranes and matrix proteins. Dedicated microvessels, the vasa vasorum, provide access for inflammatory cells. Interconnected dendritic cells, placed in the vicinity of the vasa vasorum, guard this life-essential structure and play a critical role in initiating and sustaining vessel wall inflammation. The immunopathogenesis of GCA is defined by a cytokine cascade in which the initiators are ill-defined. Much progress has been made in identifying the effectors, essentially a mixture of cytokines released by differentiated CD4 T cells, macrophages and resident vascular cells. Two major cytokine clusters have been associated with arterial wall injury but are also identifiable in the peripheral blood, raising the intriguing possibility that inflammatory cells recirculate from the vasculitic lesions and become accessible in the periphery. The IL-6/IL-17 cytokine cluster appears to be playing a redundant role in vasculitis and is highly sensitive to corticosteroid-mediated suppression. In contrast, the IL-12/IFN-γ cluster has been linked to persistent vasculitis and is resistant to standard therapy in the clinical setting, supporting shared pathogenic mechanisms in GCA and allograft atherosclerosis. Interdependence of the two cytokine clusters is insufficiently understood, but mechanistic similarities with allograft atherosclerosis in which vascular remodeling and luminal stenosis occurs in the absence of intense systemic inflammation suggests relative independence of the IL-12/IFN-γ cluster. Growing information on the molecular interactions that define the tissue microenvironment of GCA shed light on the immuno-stromal interactions between vascular cells and leukocytes which determine recruitment, retention and survival of inflammatory cells and thus control chronicity of vasculitis. Expanding pathogenic concepts in GCA beyond a simplified view of aberrant antigen recognition in a tissue site considerably widens the spectrum of therapeutic possibilities (Table 2).
Table 2.
Pathogenesis-based therapeutic interventions in GCA
| Currently used | Unexplored |
|---|---|
| Suppression of the IL-6-IL 17 cytokine cluster (corticosteroids) |
Suppression of the IL-12-IFN-γ cytokine cluster |
| Untargeted T cell suppression | Inhibition of immuno-stromal interactions |
| Suppression of dendritic cell stimulation/function |
|
| Suppression of endothelial function | |
| Suppression of VSMC function |
Review Criteria.
We searched PubMed for the search terms “Giant Cell Arteritis, Large Vessel Vasculitis, Takayasu Arteritis, Takayasu’s Arteritis, Polymyalgia rheumatic, Temporal arteritis, Arteritis and Vasculitis”. Publications from the past 10 years were analyzed for pathogenic studies. If appropriate to support scientific concepts, older publications were included. Case reports were not included, unless providing unique insights into pathogenic pathways. The date of the last search was June 22, 2013.
Key Points.
GCA, the most frequent form of LVV, occurs in a strictly defined tissue context and requires corruption of the immune-privileged tissue niche of the arterial wall.
Receptors and ligands from the NOTCH family facilitate information exchange between vascular stromal cells and immune cells and are critically involved in vasculitis.
The therapeutic potential of targeting the stromal compartment in vasculitis is currently unexplored.
The granulomatous inflammation of GCA is characterized by a cytokine cascade, in which the initiating signals are poorly defined but the effectors are multifold and match those encountered in protective immune responses.
A cytokine cluster involving the IL-6/IL-17 axis is highly active in early and untreated disease, is rapidly suppressed by corticosteroids and is nonredundant for vasculitis.
A cytokine cluster centering on the IL-12/IFN-γ axis is more resistant to immunosuppression and reveals pathogenic similarities between allograft arteriosclerosis and GCA.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01 EY011916, P01 HL058000, U19 AI057266 and U19 AI090019) and the Hedy Govenar Discovery Fund.
REFERENCES
- 1.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clin Exp Rheumatol. 2008;26:S94–104. [PubMed] [Google Scholar]
- 3.Richards BL, March L, Gabriel SE. Epidemiology of large-vessel vasculidities. Best Pract Res Clin Rheumatol. 2010;24:871–883. doi: 10.1016/j.berh.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Weiss PF. Pediatric vasculitis. Pediatr Clin North Am. 2012;59:407–423. doi: 10.1016/j.pcl.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackie SL, Hensor EM, Morgan AW, Pease CT. Should I send my patient with previous giant cell arteritis for imaging of the thoracic aorta? A systematic literature review and meta-analysis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202145. [DOI] [PubMed] [Google Scholar]
- 8.Kermani TA, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luqmani R. Large vessel vasculitides: update for the cardiologist. Curr Opin Cardiol. 2012;27:578–584. doi: 10.1097/HCO.0b013e32835895ea. [DOI] [PubMed] [Google Scholar]
- 10.Jennette JC, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 11.Shiran H, Haddad F, Miller DC, Liang D. Comparison of aortic root diameter to left ventricular outflow diameter versus body surface area in patients with marfan syndrome. Am J Cardiol. 2012;110:1518–1522. doi: 10.1016/j.amjcard.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 12.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 13.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels. 1979;16:225–238. doi: 10.1159/000158209. [DOI] [PubMed] [Google Scholar]
- 14.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 15.McGettrick HM, Butler LM, Buckley CD, Rainger GE, Nash GB. Tissue stroma as a regulator of leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91:385–400. doi: 10.1189/jlb.0911458. [DOI] [PubMed] [Google Scholar]
- 16.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 17.Weyand CM, et al. Vascular dendritic cells in giant cell arteritis. Ann N Y Acad Sci. 2005;1062:195–208. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- 18.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenmark KR, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma-Krupa W, et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupa WM, et al. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–1823. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Taboada V, Brack A, Hunder GG, Goronzy JJ, Weyand CM. The inflammatory infiltrate in giant cell arteritis selects against B lymphocytes. J Rheumatol. 1996;23:1011–1014. [PubMed] [Google Scholar]
- 23.Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol. 2005;115:38–46. doi: 10.1016/j.clim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Deng J, et al. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenig CL, et al. Identification of a Burkholderia-like strain from temporal arteries of subjects with giant cell arteritis. Arthritis Rheum. 2012;64:S373. [Google Scholar]
- 26.Rodriguez-Pla A, Stone JH. Vasculitis and systemic infections. Curr Opin Rheumatol. 2006;18:39–47. doi: 10.1097/01.bor.0000197999.58073.2e. [DOI] [PubMed] [Google Scholar]
- 27.Koren O, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejaco C, et al. NKG2D stimulated T-cell autoreactivity in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-201660. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta B, Panayi GS. Interleukin-6 in serum of patients with polymyalgia rheumatica and giant cell arteritis. Br J Rheumatol. 1990;29:456–458. doi: 10.1093/rheumatology/29.6.456. [DOI] [PubMed] [Google Scholar]
- 30.Roche NE, et al. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum. 1993;36:1286–1294. doi: 10.1002/art.1780360913. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Rodriguez J, et al. Tissue production of pro-inflammatory cytokines (IL-1beta, TNFalpha and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology (Oxford) 2004;43:294–301. doi: 10.1093/rheumatology/keh058. [DOI] [PubMed] [Google Scholar]
- 32.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 33.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000;43:1041–1048. doi: 10.1002/1529-0131(200005)43:5<1041::AID-ANR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 36.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 41.Lee WW, et al. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torchinsky MB, Blander JM. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci. 2010;67:1407–1421. doi: 10.1007/s00018-009-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly RP, et al. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010;21:393–401. doi: 10.1016/j.cytogfr.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland AP, et al. IL-21 Promotes CD8+ CTL Activity via the Transcription Factor T-bet. J Immunol. 2013 doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 46.Camporeale A, Poli V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci. 2012;17:2306–2326. doi: 10.2741/4054. [DOI] [PubMed] [Google Scholar]
- 47.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 48.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samson M, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012;64:3788–3798. doi: 10.1002/art.34647. [DOI] [PubMed] [Google Scholar]
- 50.Terrier B, et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64:2001–2011. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 51.Wagner AD, Goronzy JJ, Weyand CM. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest. 1994;94:1134–1140. doi: 10.1172/JCI117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weyand CM, Wagner AD, Bjornsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 54.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52–77. doi: 10.1111/imr.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fogal B, et al. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. J Immunol. 2011;187:6268–6280. doi: 10.4049/jimmunol.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Issa F, Chandrasekharan D, Wood KJ. Regulatory T cells as modulators of chronic allograft dysfunction. Curr Opin Immunol. 2011;23:648–654. doi: 10.1016/j.coi.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13:231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weyand CM, Younge BR, Goronzy JJ. IFN-gamma and IL-17: the two faces of T-cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23:43–49. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unizony S, et al. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken) 2012;64:1720–1729. doi: 10.1002/acr.21750. [DOI] [PubMed] [Google Scholar]
- 60.Xenitidis T, Horger M, Zeh G, Kanz L, Henes JC. Sustained inflammation of the aortic wall despite tocilizumab treatment in two cases of Takayasu arteritis. Rheumatology (Oxford) 2013 doi: 10.1093/rheumatology/ket107. [DOI] [PubMed] [Google Scholar]
- 61.Weyand CM, Liao YJ, Goronzy JJ. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J Neuroophthalmol. 2012;32:259–265. doi: 10.1097/WNO.0b013e318268aa9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brack A, et al. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997;99:2842–2850. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min W, Pober JS. AIP1 in graft arteriosclerosis. Trends Cardiovasc Med. 2011;21:229–233. doi: 10.1016/j.tcm.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 66.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 67.MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang SY, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 69.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Curr Top Microbiol Immunol. 2007;316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]
- 70.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–633. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 72.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L, et al. AIP1 prevents graft arteriosclerosis by inhibiting interferon-gamma-dependent smooth muscle cell proliferation and intimal expansion. Circ Res. 2011;109:418–427. doi: 10.1161/CIRCRESAHA.111.248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad U, et al. IFN-gamma primes intact human coronary arteries and cultured coronary smooth muscle cells to double-stranded RNA- and self-RNA-induced inflammatory responses by upregulating TLR3 and melanoma differentiation-associated gene 5. J Immunol. 2010;185:1283–1294. doi: 10.4049/jimmunol.0902283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eid RE, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai Y, et al. Interferon-gamma induces X-linked inhibitor of apoptosis-associated factor-1 and Noxa expression and potentiates human vascular smooth muscle cell apoptosis by STAT3 activation. J Biol Chem. 2008;283:6832–6842. doi: 10.1074/jbc.M706021200. [DOI] [PubMed] [Google Scholar]
- 77.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 78.Wagner AD, Bjornsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 79.Ciccia F, et al. IL-33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis. 2013;72:258–264. doi: 10.1136/annrheumdis-2012-201309. [DOI] [PubMed] [Google Scholar]
- 80.Ciccia F, et al. Expression of interleukin-32 in the inflamed arteries of patients with giant cell arteritis. Arthritis Rheum. 2011;63:2097–2104. doi: 10.1002/art.30374. [DOI] [PubMed] [Google Scholar]
- 81.Makkuni D, et al. Is intimal hyperplasia a marker of neuro-ophthalmic complications of giant cell arteritis? Rheumatology (Oxford) 2008;47:488–490. doi: 10.1093/rheumatology/ken012. [DOI] [PubMed] [Google Scholar]
- 82.Salvarani C, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum. 2005;53:293–297. doi: 10.1002/art.21075. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Gay MA, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore) 2000;79:283–292. doi: 10.1097/00005792-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Singh AG, et al. Visual manifestations in giant cell arteritis: trend over five decades. Arthritis Rheum. 2012;64:S993. [Google Scholar]
- 85.Hoffman GS, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 86.Kotter I, Henes JC, Wagner AD, Loock J, Gross WL. Does glucocorticosteroid-resistant large-vessel vasculitis (giant cell arteritis and Takayasu arteritis) exist and how can remission be achieved? A critical review of the literature. Clin Exp Rheumatol. 2012;30:S114–129. [PubMed] [Google Scholar]
- 87.Adizie T, Christidis D, Dharmapaliah C, Borg F, Dasgupta B. Efficacy and tolerability of leflunomide in difficult-to-treat polymyalgia rheumatica and giant cell arteritis: a case series. Int J Clin Pract. 2012;66:906–909. doi: 10.1111/j.1742-1241.2012.02981.x. [DOI] [PubMed] [Google Scholar]
- 88.Han JW, et al. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–553. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 89.Agrawal A, Sridharan A, Prakash S, Agrawal H. Dendritic cells and aging: consequences for autoimmunity. Expert Rev Clin Immunol. 2012;8:73–80. doi: 10.1586/eci.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaw AC, et al. Dysregulation of human Toll-like receptor function in aging. Ageing Res Rev. 2011;10:346–353. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barone F, Nayar S, Buckley CD. The role of non-hematopoietic stromal cells in the persistence of inflammation. Front Immunol. 2012;3:416. doi: 10.3389/fimmu.2012.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 94.Piggott K, et al. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123:309–318. doi: 10.1161/CIRCULATIONAHA.110.936203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 96.Tzoneva G, Ferrando AA. Recent advances on NOTCH signaling in T-ALL. Curr Top Microbiol Immunol. 2012;360:163–182. doi: 10.1007/82_2012_232. [DOI] [PubMed] [Google Scholar]
- 97.Boucher J, Gridley T, Liaw L. Molecular pathways of notch signaling in vascular smooth muscle cells. Front Physiol. 2012;3:81. doi: 10.3389/fphys.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang K, Proweller A. Vascular smooth muscle Notch signals regulate endothelial cell sensitivity to angiogenic stimulation. J Biol Chem. 2011;286:13741–13753. doi: 10.1074/jbc.M110.181842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koyanagi A, Sekine C, Yagita H. Expression of Notch receptors and ligands on immature and mature T cells. Biochem Biophys Res Commun. 2012;418:799–805. doi: 10.1016/j.bbrc.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 100.Van de Walle I, et al. Specific Notch receptor-ligand interactions control human TCR-alphabeta/gammadelta development by inducing differential Notch signal strength. J Exp Med. 2013 doi: 10.1084/jem.20121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 102.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 103.Huang Y, et al. Kidney-derived stromal cells modulate dendritic and T cell responses. J Am Soc Nephrol. 2009;20:831–841. doi: 10.1681/ASN.2008030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38:2751–2761. doi: 10.1002/eji.200838542. [DOI] [PubMed] [Google Scholar]
- 105.Zhang M, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 106.Xiao J, et al. Syndecan-1 displays a protective role in aortic aneurysm formation by modulating T cell-mediated responses. Arterioscler Thromb Vasc Biol. 2012;32:386–396. doi: 10.1161/ATVBAHA.111.242198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 108.Dimitrijevic I, Andersson C, Rissler P, Edvinsson L. Increased tissue endothelin-1 and endothelin-B receptor expression in temporal arteries from patients with giant cell arteritis. Ophthalmology. 2010;117:628–636. doi: 10.1016/j.ophtha.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 109.Regent A, et al. Identification of target antigens of anti-endothelial cell and anti-vascular smooth muscle cell antibodies in patients with giant cell arteritis: a proteomic approach. Arthritis Res Ther. 2011;13:R107. doi: 10.1186/ar3388. [DOI] [PMC free article] [PubMed] [Google Scholar]