Abstract
Diabetes is a recognized risk factor for cardiovascular diseases and heart failure. Diabetic cardiovascular dysfunction also underscores the development of diabetic retinopathy, nephropathy and neuropathy. Despite the broad availability of antidiabetic therapy, glycaemic control still remains a major challenge in the management of diabetic patients. Hyperglycaemia triggers formation of advanced glycosylation end products(AGEs), activates protein kinase C, enhances polyol pathway, glucose autoxidation, which coupled with elevated levels of free fatty acids, and leptin have been implicated in increased generation of superoxide anion by mitochondria, NADPH oxidases and xanthine oxidoreductase in diabetic vasculature and myocardium. Superoxide anion interacts with nitric oxide forming the potent toxin peroxynitrite via diffusion limited reaction, which in concert with other oxidants triggers activation of stress kinases, endoplasmic reticulum stress, mitochondrial and poly(ADP-ribose) polymerase 1-dependent cell death, dysregulates autophagy/mitophagy, inactivates key proteins involved in myocardial calcium handling/contractility and antioxidant defense, activates matrix metalloproteinases and redox-dependent pro-inflammatory transcription factors (e.g. nuclear factor kappaB) promoting inflammation, AGEs formation, eventually culminating in myocardial dysfunction, remodeling and heart failure. Understanding the complex interplay of oxidative/nitrosative stress with pro-inflammatory, metabolic and cell death pathways is critical to devise novel targeted therapies for diabetic cardiomyopathy, which will be overviewed in this brief synopsis.
Keywords: diabetic cardiomyopathy, protein oxidation, autophagy, oxidative stress
1. Introduction
Incidence of metabolic diseases, especially of obesity and type II diabetes is constantly growing worldwide. Diabetes is a well-recognized risk factor for cardiovascular diseases and heart failure [1]. The term of diabetic cardiomyopathy has been introduced by Rubler et al. back in 1972 [2]. Later the Framingham Heart Study has also demonstrated that the occurrence of heart failure in male and female diabetics is twice and five times more common, respectively, compared to age-matched control subjects [3]. Similar cross-sectional studies confirmed increased risk for heart failure development in diabetics, even when corrected for other confounding variables such as coronary artery disease, hypercholesterolemia, hypertension, and obesity [4-7]. Notably, the higher occurrence of biventricular dysfunction and cardiomyopathy in diabetics is also suggestive of the fact that diabetes is an independent risk factor or cause of cardiomyopathy [7-9].
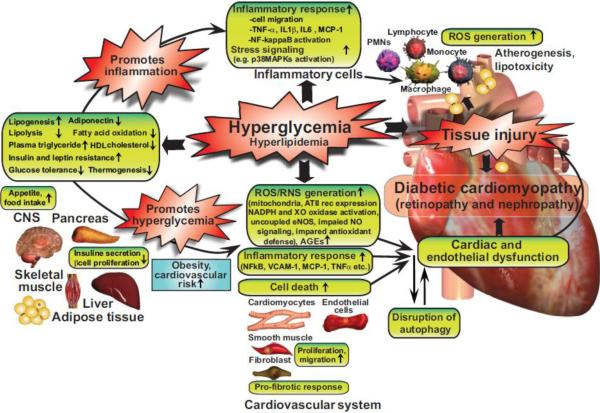
Although it is established that multiple factors may collectively contribute to the development and progression of diabetic cardiomyopathy (e.g. hyperglycaemia, insulin resistance, increased fatty acid metabolism, microcirculatory changes, sympathetic dysfunction, myocardial inflammation, remodelling and fibrosis), the exact molecular mechanisms that trigger and fuel these major pathological processes are not entirely clear (Figure 1).
Figure 1. Interplay of hyperglycemia and peripheral metabolism in cardiometabolic syndrome in mediating diabetic cardiovascular complications.
Hyperglycemia may indirectly (via its metabolic consequences) or directly enhance diabetes-associated inflammation and ROS generation, promoting tissue injury and the development of diabetic cardiovascular and other complications. AT II rec, angiotensin II receptor type 1; CNS, central nervous system; PMNs, polymorphonuclear leukocytes; XO, xanthine oxidase.
Accumulating recent evidence suggests that the complex interplay of oxidative, nitrosative and nitrative stress [10-12] with major proinflammatory, metabolic[13] and cell death pathways [14-17] play an essential role in the development of complex biochemical[18, 19], mechanical, and structural alterations associated with diabetic cardiomyopathy[8, 20, 21], which will be overviewed in this brief synopsis.
2. Oxidative, nitrosative and nitrative stress in diabetic cardiomyopathy
Oxidative and subsequent nitrosative damage of the myocardium and vasculature have been described as major primary mechanisms leading to pathologic alterations associated with diabetic cardiovascular complications [22-29]. Factors such as hyperglycaemia, glucose autoxidation, accumulation of advanced glycosylation end products (AGEs), enhanced receptor for advanced glycation end product (RAGE) and angiotensin II receptor type 1 (AT1R) signaling, elevated levels of free fatty acids and leptin, have been reported to contribute to increased production of reactive oxygen and nitrogen species production (ROS/RNS) in diabetic vessels and myocardium [22, 24], among others. In spite of the broad availability of antidiabetic therapy, glycaemic control still remains a major challenge in the management of diabetic patients [30]. Inadequate glycaemic control likely accounts for the high prevalence of diabetic complications, since high glucose or fluctuating glucose levels [31] can induce acute oxidative stress that might be a critical factor in the initiation of pathologic alterations eventually leading to development of diabetic cardiomyopathy [16, 24]. Oxidative protein modifications play a pivotal role in the development of diabetic myocardial dysfunction. Oxidation of proteins involved in contractility, excitation-contraction coupling, protein folding, antioxidant defence, metabolism (mainly fatty acid and glucose), and Ca2+ handling have been reported in diabetic hearts (see Table 1.). Oxidative modification may result in potentially harmful events including dissociation of catalytic subunits of enzymes, local or global unfolding, aggregation or fragmentation. Proteins can be oxidized directly by ROS, or by products of secondary oxidation reactions formed during lipid peroxidation (e.g., malondialdehyde or 4-hydroxynonenal). Moreover, oxidative protein modifications may also occur by reactive sugars in glycation or glycoxidation reactions [32]. Depending on the amount of modified proteins and the extent of oxidation, different degradation mechanisms are activated. Mildly oxidized proteins are broken down primarily by the proteasome, while heavily oxidized proteins (potentially forming aggregates) are degraded by the endosomal-lysosomal pathway thereafter [33]. Thus, an increase in oxidative stress might produce substrates that fuel autophagic protein degradation, at least in the first stages of oxidative injury [34]. In addition, certain proteins involved in autophagosome formation and maturation (Atg4, LC3-II) require oxidative posttranslational modifications [35, 36], thus, activity of these proteins are also strongly influenced by their redox status [35]. However, the accumulation of oxidized and nitrated proteins and lipids suggest abrogated autophagic processes in diabetic cardiomyopathy that may significantly contribute to myocardial dysfunction.
Table 1.
Oxidative and nitrative modifications of key myocardial proteins in diabetic cardiomyopathy
| Modified protein | Modification | Function | Reference |
|---|---|---|---|
| CaMK-II | oxidation | singalling | [45] |
| protein disulfide isomerase | oxidation | protein folding | [140] |
| complex I, III, and V | oxidation | ATP synthesis | [141] |
| mtHSP70, mitofilin | oxidation | mitochondrial protein import | [141] |
| alpha-enolase | oxidation/nitration | glycolysis | [142] |
| succinyl-CoA:3-oxoacid CoA transferase | nitration | ketone body metabolism | [143-145] |
| creatine kinase | nitration | muscle contraction | [144] |
| peroxiredoxin 3 | nitration | antioxidant defense | [144] |
| complex I (24 kDa subunit) | nitration | ATP synthesis | [144] |
| acyl coenzyme A thioester hydrolase | oxidation | fatty acid metabolism | [146] |
| acetyl-CoA acetyl transferase | oxidation | fatty acid metabolism | [146] |
| acyl CoA dehydrogenase | oxidation | fatty acid metabolism | [146] |
| 3-ketoacyl-CoA thiolase | oxidation | fatty acid metabolism | [146] |
| enoyl-CoA hydratase | oxidation | fatty acid metabolism | [146] |
| heart-type fatty acid-binding protein | oxidation | fatty acid metabolism | [146] |
| Glutathione Peroxidase-1 | oxidation | antioxidant defense | [146] |
| Glutathione-S-Transferase | oxidation | antioxidant defense | [146] |
| Superoxide Dismutase-2 | oxidation | antioxidant defense | [146] |
| Peroxiredoxin-1 | oxidation | antioxidant defense | [146] |
| Peroxiredoxin-2 | oxidation | antioxidant defense | [146] |
| Peroxiredoxin-3 | oxidation | antioxidant defense | [146] |
| Peroxiredoxin-6 | oxidation | antioxidant defense | [146] |
| Ryanodine receptors (RyR2) | oxidation | excitation-contraction | [147] |
| SERCA2a | oxidation | Ca2+ handling | [148] |
| Myosin heavy chain alpha and beta | oxidation | contraction | [149] |
| GAPDH | nitrosylation | glycolysis | [150] |
| Caspase-3 | nitrosylation | apoptosis | [150] |
| Connexin43 | nitration | cell-cell communication | [151] |
| ATP synthase subunits | nitration | ATP synthesis | [84] |
| thioredoxin-1 | nitration | antioxidant defense | [152, 153] |
Several cellular and subcellular sources were described that may account for enhanced ROS production in diabetic cardiovascular system and at other sites of diabetic complications (e.g. in the retina and kidneys) (Figure 2). This list includes nicotinamide adenine dinucleotide phosphate oxidases (NADPH oxidases) [37-39], xanthine oxidase/oxidoreductase (XO)[40, 41], enzymes of the arachidonic acid cascade (cyclooxygenase and lypoxygenase enzymes), microsomal enzymes, the uncoupled nitric oxide synthases (NOS) [10], and the mitochondrial respiratory chain[24]. Among these, increased mitochondrial ROS generation initially was considered to represent the primary source of high glucose-induced oxidative stress in several tissues and cell types, including cardiomyocytes [24, 37, 42] and endothelial cells [43, 44]. Luo et al. recently showed, that mitochondrial oxidants significantly contribute to the oxidation of the multifunctional enzyme, Ca2+/calmodulin-dependent protein kinase II (CaMKII) [45]. Diabetic mice treated with a mitochondria-targeted antioxidant, MitoTEMPO, showed reduced levels of oxidized-CaMKII, subsequently with preserved heart rates, and improved survival after myocardial infarction[45]. Mitoquinone, a mitochondrially targeted ubiquinone that can act as an antioxidant, also improves cardiac dysfunction [46] and activates autophagy in an Nrf2-dependent manner in a non-cardiac system [47], suggesting an interaction between mitochondrial redox balance and protein quality control mechanisms, such as autophagy.
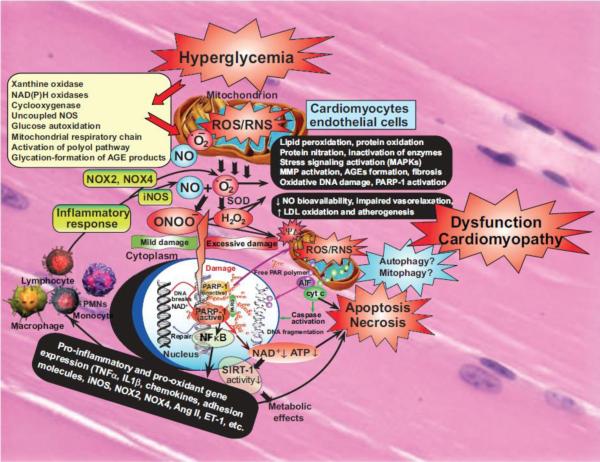
Figure 2. Interplay of oxidative and nitrosative/nitrative stress with cell death pathways in diabetic cardiomyopathy.
Hyperglycemia via activation of various pathways shown in the large yellow box increased superoxide anion (O2•−) production in cardiovascular cell types. NO and superoxide (O2•−) rapidly react to form peroxynitrite (ONOO−) which induces cell injury via enhanced lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration, and also activation of stress signaling, matrix metalloproteinases (MMPs) among others. Peroxynitrite also triggers the release of proapoptotic factors such as cytochrome c and apoptosis-inducing factor (AIF) from the mitochondria, which mediate caspase-dependent and -independent apoptotic cell demise pathways. Autophagy may be beneficial in diabetic cardiomyopathy in removal of injured cells, but additional support is required to prove its exact role. Peroxynitrite, in concert with other reactive oxidants, causes stand breaks in DNA, activating the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). Mild damage of DNA activates the DNA repair machinery, but once excessive oxidative/nitrosative stress-induced DNA damage occurs, like in diabetes, overactivated PARP initiates an energy-consuming cycle by transferring ADP-ribose units from nicotinamide adenine dinucleotide (NAD+) to nuclear proteins, resulting in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading to cellular dysfunction and demise. Poly(ADP-ribose) glycohydrolase (PARG) degrades poly(ADP-ribose) (PAR) polymers, generating free PAR polymer and ADP-ribose. Overactivated PARP also facilitates the activation of NFkB and expression of a variety of pro-inflammatory genes leading to increased inflammation and associated oxidative stress, thus facilitating the progression of cardiovascular dysfunction and heart failure. Via attenuation of the cellular NAD+ levels PARP activation may also promote metabolic dysfunction via decreased activity of SIRT-1 in various tissues. In addition to these adverse consequences the NO bioavailability and signaling is also impaired in diabetic hearts promoting impaired vasorelaxation and enhanced atherogenesis eventually facilitating increased cardiovascular inflammation, and lipid deposition in vessels and myocardium, functional ischemia and enhanced cardiac injury.
There is also evidence for increased production of ROS from non-mitochondrial sources. NADPH oxidases (NOX) are unique enzymes that may be responsible for large amounts of superoxide and hydrogen peroxide (H2O2) production under various pathological conditions. Activity or expression of various NOX isoenzymes (that are involved in superoxide or H2O2 generation, has been found to be significantly higher in the heart with metabolic derangements such as diabetes [37-39, 48, 49] and hypercholesterolemia [50]. The increased activity and expression of NOX4 in the diabetic heart [49] is therapeutically targetable, and NOX4 inhibitors are indeed in preclinical development for various cardiovascular indications [51]. Diabetes induced increase in NADPH activity is further accentuated by the increased production of NADPH by glucose-6-phosphate dehydrogenase, as it was described in the heart and aorta of Zucker diabetic fatty rats [52]. Elevated concentration of hydrogen peroxide has been shown to modulate autophagy by several mechanisms. For example, H2O2, activates the LKB1/AMPK pathway which leads to inhibition of the mTORC1 complex and induced autophagy [53]. NOX4-mediated production of hydrogen peroxide also induces autophagy in human umbilical vein endothelial cells [54] and in cardiomyocytes [55] after induction of endoplasmic reticulum stress. Although, it is plausible that excessive amounts of ROS might incapacitate certain players of autophagy. Vascular NADPH oxidase is also an important downstream target of angiotensin II, which has been proposed to play a pivotal pathological role in the development and progression of diabetic cardiovascular [56-59] and other diabetic complications. This is supported by convincing evidence obtained from preclinical rodent models of STZ (streptozotocin) -induced type 1 diabetes, as well as from human myocardial biopsy specimens, suggesting that the renin-angiotensin system is up-regulated in diabetes and angiotensin II locally through AT1R, which is overexpressed in diabetic hearts or in cardiomyocytes exposed to high glucose, importantly contributes to the development of diabetic cardiomyopathy [38, 57-60]. The beneficial effects of AT1R blockade in diabetic hearts involve, but are not limited to, the attenuation of myocardial NADPH oxidase-dependent (such as p47phox) ROS generation, inflammation, cell death, fibrosis, and contractile dysfunction [57-60]. AT1 receptor blockade also prevents glucose-induced cardiac dysfunction directly in ventricular myocytes via attenuating the AT1-NADPH oxidase signaling [61]. Consistently with the important pathological role of NADPH oxidases-derived ROS generation in diabetic hearts, apocynin (a putative non-specific NADPH oxidase inhibitor) not only attenuated the enhanced superoxide generation in diabetic hearts, but also improved the diabetes-associated cardiac dysfunction in type I diabetic mice[39].
Increased activation of another ROS generating enzyme, the XO, has also been shown in different organs [62] of diabetic rats or mice, including in the heart [41]. Conversely, inhibition of XO by allopurinol significantly attenuated most pathological features of diabetic cardiomyopathy (e.g. myocardial ROS/RNS generation, iNOS expression, cell death, and fibrosis) and improved both systolic and diastolic dysfunctions in type I diabetic mouse[41] or rat [63] hearts. Importantly, there is also current evidence, coupled with numerous previous studies[40], for the beneficial cardiac effects of allopurinol in humans [64]. Rekhraj at al. recently described, that in patient with ischemic heart disease, high dose off allopurinol was capable to reduce left ventricular muscle mass and improve the symptoms of the disease[65].
A substantial amount of experimental evidence suggests that there is reduced nitric oxide (NO) availability in diabetic tissues. Different mechanisms have been proposed to be responsible for the diabetes-induced dysfunction of NO production, bioavailability and/or signaling. A generally accepted mechanism is the alterations in nitric oxide synthases (NOS), particularly in its endothelial isoform (eNOS), function [10, 66]. The composition of the eNOS complex is critical for the relative formation of NO or superoxide. The mechanisms by which eNOS dysfunction develops remain elusive, however, a decrease in the dimer to monomer eNOS ratio within the myocardium of diabetic animals has been reported [67]. Monomerization i.e. uncoupling of NOS results in increased oxidative stress and decreased NO bioavailability (since uncoupled NOS generates superoxide anion instead of NO), which has been implicated in the pathophysiology of numerous cardiovascular diseases. Accordingly, eNOS uncoupling has also been reported in diabetic hearts, while administration of the tetrahydrobiopterin precursor, sepiapterin inhibited uncoupling of NOS and improved LV function [68]. Similarly, ascorbic acid or N-acetyl-cystein is also capable to increase BH4/BH2 ratio and prevent NOS uncoupling in the diabetic heart [69]. In addition to uncoupling the major cardiac NOS isoforms, iNOS and eNOS expression has been shown to be increased (particularly iNOS) in the diabetic hearts [37, 38, 70, 71]. The increase in NOS expression in the diabetic heart is associated with an increase in lipid peroxidation and 3-nitrotyrosine formation (marker of peroxynitrite generation and nitrative stress), which might be related to the uncoupled and monomer state of the enzyme, which most likely produces superoxide instead of NO, as well as triggers the formation of peroxynitrite. Interestingly, peroxynitrite itself also causes NOS un-coupling by selectively targeting and disrupting caveolae-NOS interaction [72].
The rapid diffusion-limited reaction of superoxide with NO indeed forms peroxynitrite, a very strong cytotoxic oxidant, which attacks and damages various biomolecules via multiple mechanisms [10]. In agreement with these, inhibition of NOS activity (by L-NAME or L-NMMA) improves myocardial performance in diabetic hearts, suggesting that the increased production of superoxide and peroxynitrite rather than NO is a major contributor of suppressed contractile performance in diabetes [39, 66, 73, 74]. NO and peroxynitrite has a very complex relation to autophagy. Nitric oxide donors have been shown to decreased autophagy in HeLa cells, which has been confirmed by the overexpression of eNOS [75]. Although, LPS-induced up-regulation of NOS izoenzymes were followed by an accelerated autophagy in HL-1 cells and in mice [76]. Since LPS induces the production of not only NO, but peroxynitrite, these seemingly opposing results can be explained. It is further evidenced by other studies, where peroxynitrite exerted inhibitory effects on autophagy. For example, peroxynitrite induced autophagy in endothelial cells and increased LC3-II protein [77]. These data well demonstrate that sub-physiological NO levels and nitrosative/nitrative stress which are characteristic to diabetes, might facilitate autophagic processes in the heart, however, direct observations are yet to be made. Peroxynitrite formation and consequent protein nitration (nitrative stress) have been suggested to play a central role in the development of cardiovascular dysfunction associated with diabetes, as well as in the development of diabetic retinopathy, nephropathy and neuropathy. Peroxynitrite in hearts not only promotes apoptotic or necrotic cell death in cardiomyocytes and endothelial cells via activation of stress signaling pathways (e.g. mitogen activated protein kinases (MAPKs) and poly ADP ribose polymerase 1 (PARP-1)-dependent pathways), but also induces nitration and consequent inactivation of key proteins involved in intracellular calcium cycling, energy homeostasis, antioxidant defense, and myocardial contractility (see Table 2.) [10]. Peroxynitrite, in concert with other oxidant may also activate matrix metalloproteinases (MMPs) in the failing hearts to promote pathological remodeling [23, 37, 78] and may impair the NO-soluble guanylate cyclase signaling pathway rendering NO to unable to activate its primary protective signaling machinery [79, 80]. Furthermore, peroxynitrite decomposition catalysts have been shown to restore the normal contractile phenotype of high glucose treated cardiomyocytes and attenuate cardiac dysfunction in diabetic hearts [74]. The importance of peroxynitrite has also been shown in diabetic patients. Increased serum and/or vascular 3-nitrotyrosine levels were positively correlated with increased blood pressure, and or endothelial dysfunction in diabetic patients [29, 81]. The persistently increased myocardial oxidative and nitrative stress in diabetic hearts eventually also leads to dysfunction of important antioxidant defense mechanisms [10] (e.g. inactivation of important antioxidant enzymes such as superoxide dismutases and catalase, depletion of endogenous antioxidants (e.g. metallothionein, glutathione) [37, 82-84]) and dysregulation of important redox-dependent transcription factors (e.g. NFE2L2 nuclear factor, erythroid 2-like 2 (Nrf2)) [85, 86], among others. Increased oxidative and nitrative stress in diabetes may also promote oxidation and/or nitration of various insulin receptors in peripheral tissues, which may contribute to the development of insulin resistance [87].
Table 2.
Main therapeutic approaches and targets in diabetic cardiomyopathy
| Therapeutic target | Approach | Reference | Potential effect on autophagy |
|---|---|---|---|
| Oxidative, nitrosative, nitrative stress | |||
| NADPH oxidases | Enzyme inhibition | ||
| apocynin, (VAS2870 or GKT137831) | [39, 154] | Potential inhibitors of autophagy [55, 155] | |
| AT-1 receptor antagonist | [61] | Potential inhibitors of autophagy [156] | |
| Xanthine oxidase | Enzyme inhibition: | ||
| allopurinol | [40, 41, 63, 64] | No information | |
| eNOS | Uncoupling inhibitors | ||
| L-Arginine | [157] | Potentially inhibits autophagy by NO [75, 158] | |
| tetrahydrobiopterin | [68] | Potentially inhibits autophagy by NO [159] | |
| IGF-1 | [160] | Potential inhibitor of autophagy [161] | |
| iNOS | Enzyme inhibition: | ||
| L-NIL | [162, 163] | No information in the literature. | |
| Peroxynitrite | Decomposition catalysts: | ||
| MnTMPyP | [28] | Potential inhibitors of autophagy [77] | |
| FP15 | [164] | ||
| Mitochondria oxidative stress | MitoTEMPO | [45] | No information in the literature. |
| NRF2 | Activators: | ||
| Sulforafan | [165] | Potential autophagy inducer [166] | |
| DH404 | [167] | ||
| ROCK | Enzyme inhibition: | ||
| Fasudil | [168] | Potential autophagy inducer [169] | |
| Cell death pathways – Autophagy/Mitophagy, Apoptosis, PARP-dependent cell death | |||
| p38 MAPK | Enzyme inhibition: | ||
| SB 239063 | 170] | Potential inhibitors of autophagy [76] | |
| PARP | Enzyme inhibition: | ||
| PJ34 | [118] | ||
| 3-aminobenzamide | [171] | Potential inhibitors of autophagy [172] | |
| AMPK activation | Metformin | [104] | Potential autophagy inducer [99] |
| GLP-1 | GLP-1 agonists , DPP-4 inhibitors | [173-177] | Potential autophagy inducers [178] |
| Inflammation | |||
| Cytokine expression (TNFα, IL1β, TGFβ) | p38 MAPK inhibition | [122] | Potential inhibitors of autophagy [76] |
| AT-1 receptor antagonist | [57] | Potential inhibitors of autophagy [156] | |
| Endocannabinoid system | CB1 antagonist | [38] | Potential inhibitors of autophagy [139] |
Hyperglycemia and/or hyperglycemia-induced ROS has been reported to increase accumulation of products of nonenzymatic glycation/oxidation of proteins/lipids (AGE) and enhanced the expression of their receptor (RAGE) in cardiovascular tissues, which thought to play a key role in the development and progression of cardiovascular complications of diabetes [88, 89]. Accumulation of AGEs and increased expression of RAGE have been associated with diabetes-induced dysfunction and structural alterations in hearts of type 1 diabetic rodents [88, 89]. Furthermore, in diabetic heart failure patients with reduced LV ejection fraction, the accumulation of AGEs and fibrosis appear to contribute to the increased diastolic stiffness, but not in patients with normal LV ejection fraction, where the increased cardiomyocyte resting tension is responsible for this phenomenon[90].
3. Autophagy and cell death mechanisms in diabetic cardiomyopathy
It has extensively been documented that increased oxidative and nitrative stress in diabetic hearts may trigger activation of stress signalling pathways facilitating apoptosis in cardiomyocytes and endothelial cells [14]. However, recent evidence also suggests that in addition to apoptosis, other processes such as autophagy, mitophagy and PARP-dependent cell death pathways may also play an important role in controlling the cell demise during the course of cardiovascular disease[91] and diabetic cardiomyopathy [17], which will be discussed in the following part.
Autophagy is a cellular housekeeping process that is essential for removal of damaged or unwanted organelles, protein and lipid aggregates. It is a dynamic process which is tightly regulated by the availability of nutrients and the metabolic balance of the cell, and functional autophagy is indispensable for cellular survival in low-energy conditions [92]. Since in diabetes, where oxidative stress, protein- and lipid oxidation are elevated and cellular energy balance is disturbed, functional autophagy might have even higher importance in the maintenance of cardiac cellular integrity. In the heart, constitutive autophagy is a homeostatic mechanism for maintaining cardiac structure and function [93], while disruption of autophagy leads to heart failure [94]. Interestingly, in healthy animals, autophagy seems to be higher in males than in females suggesting that the male heart has a major constitutive autophagy [95]. This gender difference may elucidate the observation of the Framingham Heart Study showing markedly increased occurrence of diabetic cardiomyopathy in females [3, 96]. Autophagy is suppressed at high glucose concentrations, such as present in diabetes, which might be associated with the development of diabetic cardiomyopathy [97]. In addition, the diabetic heart is characterized by excessive fatty acid uptake and oxidation, resulting in accumulation of toxic lipid intermediates (long-chain acyl-CoA, diacylglycerol, ceramides), known as lipotoxicity. Cardiac lipotoxicity contributes to insulin resistance, impairment of mitochondrial function, CM hypertrophy, and apoptosis, which may ultimately lead to left ventricular remodelling of the heart [98].
Elevated oxidative stress, apoptosis, inflammation, and ER stress by increased production of monocyte chemoatractic protein-1 (MCP-1) is suspected to contribute to the disruption of cardiac autophagy in diabetes [97, 99-101] Several upstream signalling pathways of autophagy are altered in diabetes or metabolic syndrome as well [102]. The mammalian target of rapamycin (mTOR) pathway, a negative regulator of autophagy is blunted in obese, hyperlipidemic rats fed a high cholesterol-high fructose diet showing most characteristics of type II diabetes [103]. Also, activity of AMP-activated protein kinase (AMPK) is reduced in high glucose conditions, leading to disturbed autophagy[104]. Although the majority of reports agree on the attenuation of the autophagy in diabetes, not all studies are equivocal (possibly due to the different experimental models used). For example, in a fructose-fed mouse model, induction of autophagy has been described[105], and milk fat-based chow blunted cardiac autophagy and facilitated cardiomyopathy more efficiently than a lard-based chow [106]. Further complicating the picture is the discrepancy that oxidative stress is elevated in diabetes, which is supposed to induce autophagy, however, the majority of studies evidence quite the opposite. This phenomenon and the lack of any direct evidence from diabetic patients warrant further investigations on the status of cardiac autophagy in disease models relevant to human pathological conditions.
Aiming to restore pathological cardiac consequences of diabetes modulation of autophagy has been attempted by different tools. Chronic activation of AMPK (therefore autophagy) by metformin or overexpression of ATG7 reduced cardiomyocyte apoptosis, cardiac fibrosis, and improved myocardial functions in streptozotocin (STZ)-induced diabetic mice [104]. Overexpression of hem oxygenase-1 in STZ-treated mice also induced autophagy, reduced oxidative stress and inflammation and prevented cardiac dysfunction [97]. In a recent report, inhibition of mTOR signalling by the overexpression of PRAS40 prevented the development of cardiomyopathy in high-fat diet-fed mice [107]. Furthermore, it was also demonstrated that disturbed autophagy undermine the benefit of exercise in protection against high-fat-diet-induced glucose intolerance [108]. However, a few papers disagree with this conclusion. Suppression of autophagy prevented apoptosis in neonatal and adult cardiomyocytes in extremely high glucose conditions resembling to uncontrolled diabetes [109]. Furthermore, overexpression of beclin-1, an inducer of autophagy, worsened cardiac function and enhanced apoptosis in STZ-treated mice, while cardiac damage by STZ-induced diabetes was blunted in beclin-1+/− mice [110]. In summary, although the usefulness of induction of autophagy in diabetes is still a controversial issue, the majority of reports implicate that the restoration of cellular energy household, induction of antioxidant systems and reduction of the detrimental protein synthesis by the modulation of autophagy-related pathways might serve as therapeutic targets in the treatment or prevention of diabetes and its cardiac complications, once we better understand these processes.
ROS and RNS under pathological conditions may also lead to oxidative DNA injury and overactivation of the nuclear enzyme poly (ADP-ribose) polymerase 1 (PARP-1), the predominant isoform of the PARP enzyme family, which normally participates in the regulation of DNA repair, cell death, metabolism, and inflammatory responses [111]. Following binding to damaged DNA, PARP-1 forms homodimers and catalyzes the cleavage of its substrate NAD+ into nicotinamide and ADP-ribose resulting in formation of longbranches of ADP-ribose polymers on target proteins such as histones and PARP-1 itself, which results in cellular energetic depletion, mitochondrial dysfunction, and ultimately necrosis. Numerous transcription factors involved in controlling inflammation such as NFkB, and various signaling molecules have also been shown to become poly(ADP-ribosylated) by PARP-1 [112]. Thus, overactivation of PARP-1 due to ROS/RNS formation not only promotes cell death by ATP and NAD+ depletion, but also stimulates proinflammatory mediator production. PARP inhibitors exerted marked tissue protective and antiinflammatory effects in preclinical models of ischemia-reperfusion injury, endothelial and cardiac dysfunction, circulatory shock, heart failure and diabetic complications [16, 112]. More excitingly several recent studies have also suggested that PARP-1 and PARP-2 (a minor isoform of the PARP enzyme family) are involved in regulation of mitochondrial function/biogenesis, and adipogenesis in various organ systems [113, 114], including in the liver, via modulation of NAD+ levels and consequently sirtuin 1 activity [111, 115]. Specifically, PARP inhibitors in rodent models of type I diabetes were very effective in improving endothelial [116, 117] and cardiac function [118], and the PARP activation from skin biopsies in microvessels positively correlated with the degree of endothelial dysfunction in prediabetic and diabetic human subjects [29]. PARP inhibition also prevented the hyperglycemia-induced pathological activation of PKC isoforms, hexosaminase pathway flux, and AGE formation in vitro, suggesting its key role in regulating pathological processes promoting the development of all major diabetic complications [16] .
4. Inflammation in diabetic cardiomyopathy
Tschope et al. found using a STZ-induced rat model of type I diabetes that the diabetic cardiomyopathy was characterized by significant increases in myocardial intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 (ICAM-1 and VCAM-1) expressions, as well as of beta2-leukocyte-integrins+ (CD18+, CD11a+, CD11b+) and cytokine tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β))-expressing infiltrating immune cells. And these pro-inflammatory changes were positively correlated with oxidative stress and decline of left ventricular function [119]. Interestingly, transgenic activation of the kallikrein-kinin system inhibited intramyocardial inflammation, endothelial dysfunction and oxidative stress in diabetic hearts [119]. They found that the AT-1 receptor antagonists irbesartan attenuated cardiac failure by decreasing cardiac inflammation (IL1β, TNFα, and TGFβ levels) and normalizing MMP activity, leading to attenuated cardiac fibrosis in STZ-induced diabetic cardiomyopathy [57]. Using mouse or rat models of type I diabetes they also demonstrated that neutralization of TNFα [120] or genetic deletion of kinin receptor B [121] attenuated the development of experimental diabetic cardiomyopathy associated with a reduction of intramyocardial inflammation and cardiac fibrosis. They also showed that inhibition of p38 mitogen-activated protein kinase attenuated left ventricular dysfunction by decreasing the level of myocardial pro-inflammatory cardiac cytokines in a mouse model of diabetes mellitus[122], and more recently they suggested that the STZ-induced diabetic cardiomyopathy is a robust model for investigating cardiac immune response and LV remodeling processes under diabetic conditions [123]. Several studies from other groups have also found similar pro-inflammatory changes in the myocardium of diabetic rodent hearts [37, 38, 41]. Recently, using type I and II models of diabetes in mice several studies have also shown activation of the major pro-inflammatory transcription factor nuclear factor kappa B in diabetic hearts[37, 124] or in human cardiomyocytes or coronary artery endothelial cells exposed to high glucose concentrations which triggered enhanced ROS/RNS generation[37]. Both studies demonstrated that the anti-inflammatory compounds used for the treatments by attenuating inflammation also decreased oxidative/nitrative stress, myocardial cell death, remodeling, and improved diabetes-induced contractile dysfunction in rodent hearts [37, 124]. On the other hand, it has been also reported that the clinically effective drugs (statins [125], RAAS inhibitors [57], metformin [126], and thiazolidinediones [127]) used in the treatment of metabolic syndrome attenuate the inflammatory response in diabetes.
Cytokines can attenuate myocyte contractility and viability by various mechanisms. For example by formation of peroxynitrite, which was shown to be a major contributor to cytokine-induced myocardial contractile failure[128]. Peroxynitrite is also a major trigger of apoptosis in cardiomyocytes exposed to various insults in vitro or in vivo [129, 130]. Other proposed mechanisms may involve direct action on sarcoplasmic reticulum function and on the regulation on the SR calcium ATPase expression and activity by oxidation/nitration [10, 131]. It is also known, that inflammatory cytokines may regulate the extracellular matrix composition and dynamics in the heart, which is a known important determinant of myocardial function [132]. In agreement with this, inhibition of TNFα signalling with an antibody for 6 weeks attenuated myocardial inflammation and fibrosis in experimental diabetes [120].
The role of myocardial cytokine signaling in the regulation of autophagy and protein quality control is unknown. In other cell types, autophagosomes are formed in response to a number of environmental stimuli, including both host- and pathogen-derived molecules, toll-like receptor ligands or various cytokines[133, 134]. In particular, the cytokines IFN-γ, TNF-α, IL-1, IL-2, IL-6 and TGF-β have been shown to induce autophagy, while IGF-1, IL-4, IL-10 and IL-13 have inhibitory effect. In parallel, autophagy itself can regulate expression of several cytokines, including IL-1, IL-18, TNF-α, and IFN-γ (for an extensive review see [134]).
Recent preclinical and clinical studies have also highlighted the role of endocannabinoids (novel lipid mediators) and their G protein coupled cannabinoid receptors (CB1 and CB2 receptors) in the regulation of food intake, energy balance, and metabolism (for extensive review see: [135]). The endocannabinoid system also plays a prominent role in the pathology of diabetes and obesity [136, 137]. We recently explored the role of endocannabinoids and CB1 receptors in a type I model of diabetic cardiomyopathy. The diabetic cardiomyopathy was characterized by myocardial inflammation, increased expression of CB1, oxidative/nitrative stress, β-MHC isoenzyme switch, AT1R up-regulation, myocardial RAGE and AGE expression/accumulation, MAPK activation, cell death, and cardiac dysfunction (both systolic and diastolic). Pharmacological inhibition or genetic deletion of CB1 receptors was associated with attenuated diabetes-induced myocardial inflammation, decreased MAPK activation, oxidative/nitrative stress, β-MHC isoenzyme switch, AT1R up-regulation, myocardial RAGE and AGE expression/accumulation, MAPK activation, and largely diminished cell death and better preservation of cardiac function [38]. Recent studies indicate that cannabinoids (especially CB1 agonists) may be important inductors of autophagy in preimplantation mouse embryos [138] and in human epithelial colorectal adenocarcinoma cells (CaCo2) [139]. Although, the evidences outlined here clearly show that inflammation is one of the key therapeutically important components associated with diabetic cardiomyopathy, to see if pharmacologic modulation of cytokine as well as cannabinoid signalling and thereby autophagy has therapeutic potential, further experimental studies are required.
5. Conclusions
Despite our accumulating knowledge based on preclinical reports, the treatment of diabetic cardiomyopathy is still largely symptomatic once it fully develops, which inevitably progresses to heart failure (see Figure 3 for the progression of diabetic cardiomyopathy to heart failure). Therefore, an early diagnosis of this condition and rigorous glycaemic control is very important to try to slow down the progression of the disease as much as possible. From preclinical and human studies it has become clear that oxidative/nitrative stress and inflammation are central components in triggering and driving the pathological processes associated with diabetic cardiomyopathy and other cardiovascular complications. However, recognizing the largely disappointing results of clinical trials with global antioxidants in diabetes, it is essential to keep in mind the necessity of more thorough understanding the complex interplay of oxidative/nitrosative stress with pro-inflammatory, metabolic and cell death signaling pathways, the specificity of these processes, and their temporal-spatial relationship.
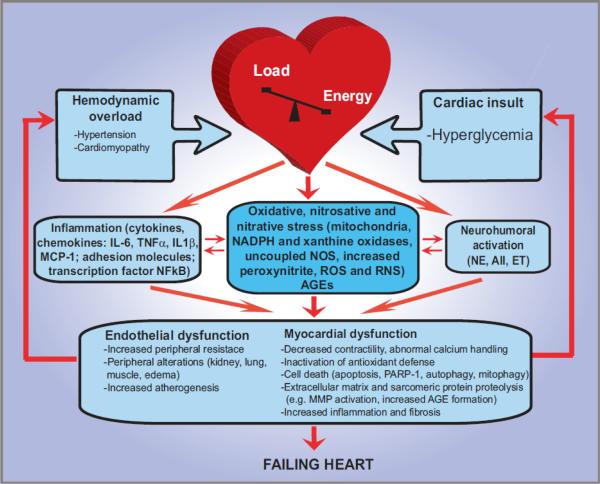
Figure 3. Progression of diabetic cardiomyopathy: role of oxidative/nitrosative stress, inflammation, cell death and remodeling.
The mechanisms leading to diabetic cardiomyopathy and failure are complex. Eventually the pathological alterations will result in mismatch between the load applied to the heart and the energy needed for contraction, leading to mechanoenergic uncoupling. After initial insults (episodes of hyperglycemia), secondary mediators such as angiotensin II (AII), norepinephrine (NE), endothelin (ET), proinflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α) and interleukin 6 and IL1β (IL-6 and IL1β), in concert with oxidative stress and peroxynitrite, activate downstream effectors (e.g., PARP-1 or MMPs)], act directly on the myocardium or indirectly via changes in hemodynamic loading conditions to cause endothelial and myocardial dysfunction, cardiac and vascular remodeling with hypertrophy, fibrosis, cardiac dilation, and myocardial necrosis, leading eventually to heart failure. The adverse remodeling and increased peripheral resistance further aggravate heart failure. MMPs, matrix metalloproteinases; PARP-1, poly(ADP-ribose) polymerase.
HIGHLIGHTS.
Clinical treatment of diabetic cardiomyopathy is still largely symptomatic
Oxidative stress and inflammation fuel development of diabetic cardiomyopathy
Cell death pathways, including autophagy, are dysregulated in diabetic hearts
Cell death has complex interplay with oxidative stress and inflammation
These pathways are promising therapeutic targets in the diabetic heart
Acknowledgements
This study was supported by Intramural Research Program of NIH/NIAAA (to PP), the New Horizons Grant of the European Foundation for the Study of Diabetes, and Hungarian Scientific Research Fund (OTKA K109737) (to PF, ZG, and ZVV). ZG holds a „János Bolyai Fellowship” from the Hungarian Academy of Sciences. PF is a Szentágothai Fellow of the National Program of Excellence (TAMOP 4.2.4.A/2-11-1-2012-0001).
Authors are indebted to Dr. George Kunos, the Scientific Director of NIAAA for continuous support.
Abbreviations
- AGEs
advanced glycosylation end products
- AMPK
AMP-activated protein kinase
- AT1R receptor
angiotensine II receptor type 1
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CB1/2 receptor
cannabinoid 1 and 2 receptors
- eNOS
endothelial nitric oxide synthase
- ER stress
endoplasmatic reticulum stress
- ICAM-1
intercellular adhesion molecule 1, also known as CD54
- iNOS
inducible nitric oxide synthase
- MAPKs
mitogen activated protein kinases
- MCP-1
monocyte chemoatractic protein-1
- MMPs
matrix metalloproteinases
- mTOR
mammalian target of rapamycin
- NADPH oxidase/NOX
nicotinamide adenine dinucleotide phosphate-oxidase
- NFkB
nuclear factor kappaB
- NO
nitric oxide
- NOS
nitric oxide synthases
- Nrf2
NFE2L2 nuclear factor, erythroid 2-like 2
- PARP-1
poly(ADP)ribose polymerase 1
- PARP-1
poly(ADP-ribose) polymerase 1
- RAGE
receptor for advanced glycation end product
- ROS/RNS
reactive oxygen and nitrogen species
- SERCA2a
sarco/endoplasmic reticulum Ca2+-ATPase
- STZ
streptozotocin
- VCAM-1
vascular cell adhesion molecule 1, also known as CD106
- XO
xanthine oxidase/oxidoreductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, E.S.C.C.f.P. Guidelines. Zamorano JL, S. Achenbach, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 2.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 4.Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115:867–868. doi: 10.1378/chest.115.3.867. [DOI] [PubMed] [Google Scholar]
- 5.Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791–2795. doi: 10.2337/diacare.26.10.2791. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Deng W, Zhou L, Fang P, He D, Zhang W, Liu K, Hu R. Prevalence, incidence and risk factors of chronic heart failure in the type 2 diabetic population: systematic review. Curr Diabetes Rev. 2009;5:171–184. doi: 10.2174/157339909788920938. [DOI] [PubMed] [Google Scholar]
- 7.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–318. doi: 10.1016/j.ijcard.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Fein FS. Diabetic cardiomyopathy. Diabetes Care. 1990;13:1169–1179. doi: 10.2337/diacare.13.11.1169. [DOI] [PubMed] [Google Scholar]
- 9.Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 10.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, Palesty A, McFadden DW, Parinandi NL, Maulik N. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386:233–249. doi: 10.1007/s11010-013-1861-x. [DOI] [PubMed] [Google Scholar]
- 13.Isfort M, Stevens SC, Schaffer S, Jong CJ, Wold LE. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3:219–228. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- 15.Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res. 2007;39:672–676. doi: 10.1055/s-2007-985823. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi S, Liang Q. Autophagy and Mitophagy in Diabetic Cardiomyopathy. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbadis.2014.05.020. in press. [DOI] [PubMed] [Google Scholar]
- 18.Brownlee M, Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- 19.Sarkozy M, Zvara A, Gyemant N, Fekete V, Kocsis GF, Pipis J, Szucs G, Csonka C, Puskas LG, Ferdinandy P, Csont T. Metabolic syndrome influences cardiac gene expression pattern at the transcript level in male ZDF rats. Cardiovasc Diabetol. 2013;12:16. doi: 10.1186/1475-2840-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 22.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma H, Li SY, Xu P, Babcock SA, Dolence EK, Brownlee M, Li J, Ren J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:1751–1764. doi: 10.1111/j.1582-4934.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Cai L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 2006;41:851–861. doi: 10.1016/j.freeradbiomed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1:181–193. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 29.Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 30.Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(Suppl 3):13–20. doi: 10.1185/03007995.2011.621416. [DOI] [PubMed] [Google Scholar]
- 31.Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A. Reactive oxygen species mediate a cellular 'memory' of high glucose stress signalling. Diabetologia. 2007;50:1523–1531. doi: 10.1007/s00125-007-0684-2. [DOI] [PubMed] [Google Scholar]
- 32.Fu S, Fu MX, Baynes JW, Thorpe SR, Dean RT. Presence of dopa and amino acid hydroperoxides in proteins modified with advanced glycation end products (AGEs): amino acid oxidation products as a possible source of oxidative stress induced by AGE proteins. Biochem J. 1998;330(Pt 1):233–239. doi: 10.1042/bj3300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunlop RA, Brunk UT, Rodgers KJ. Oxidized proteins: mechanisms of removal and consequences of accumulation. IUBMB Life. 2009;61:522–527. doi: 10.1002/iub.189. [DOI] [PubMed] [Google Scholar]
- 34.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 35.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 37.Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Hasko G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roe ND, Thomas DP, Ren J. Inhibition of NADPH oxidase alleviates experimental diabetes-induced myocardial contractile dysfunction. Diabetes Obes Metab. 2011;13:465–473. doi: 10.1111/j.1463-1326.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 40.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajesh M, Mukhopadhyay P, Batkai S, Mukhopadhyay B, Patel V, Hasko G, Szabo C, Mabley JG, Liaudet L, Pacher P. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:2330–2341. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos DL, Palmeira CM, Seica R, Dias J, Mesquita J, Moreno AJ, Santos MS. Diabetes and mitochondrial oxidative stress: a study using heart mitochondria from the diabetic Goto-Kakizaki rat. Mol Cell Biochem. 2003;246:163–170. [PubMed] [Google Scholar]
- 43.Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia. 2008;51:1930–1932. doi: 10.1007/s00125-008-1100-2. [DOI] [PubMed] [Google Scholar]
- 44.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1095–1102. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 49.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, Abboud HE. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597–604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varga ZV, Kupai K, Szucs G, Gaspar R, Paloczi J, Farago N, Zvara A, Puskas LG, Razga Z, Tiszlavicz L, Bencsik P, Gorbe A, Csonka C, Ferdinandy P, Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wolin MS, Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scherz-Shouval R, Shvets E, Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3:371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 54.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res. 2013;113:1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou G, Li X, Hein DW, Xiang X, Marshall JP, Prabhu SD, Cai L. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–666. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56:641–646. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- 58.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 59.Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- 60.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 61.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 62.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 63.Gao X, Xu Y, Xu B, Liu Y, Cai J, Liu HM, Lei S, Zhong YQ, Irwin MG, Xia Z. Allopurinol attenuates left ventricular dysfunction in rats with early stages of streptozotocin-induced diabetes. Diabetes Metab Res Rev. 2012;28:409–417. doi: 10.1002/dmrr.2295. [DOI] [PubMed] [Google Scholar]
- 64.Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang CC, Morris AD, George J, Struthers AD. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol. 2013;62:2284–2293. doi: 10.1016/j.jacc.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 65.Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG, Lang CC, George J, Struthers AD. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61:926–932. doi: 10.1016/j.jacc.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 66.Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo H, Otani H, Jo F, Shimazu T, Okazaki T, Yoshioka K, Fujita M, Kosaki A, Iwasaka T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol. 2011;38:485–493. doi: 10.1111/j.1440-1681.2011.05535.x. [DOI] [PubMed] [Google Scholar]
- 69.Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Iwasaka T. Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radic Res. 2011;45:1173–1183. doi: 10.3109/10715762.2011.605361. [DOI] [PubMed] [Google Scholar]
- 70.Farhangkhoee H, Khan ZA, Mukherjee S, Cukiernik M, Barbin YP, Karmazyn M, Chakrabarti S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J Mol Cell Cardiol. 2003;35:1439–1448. doi: 10.1016/j.yjmcc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Stockklauser-Farber K, Ballhausen T, Laufer A, Rosen P. Influence of diabetes on cardiac nitric oxide synthase expression and activity. Biochim Biophys Acta. 2000;1535:10–20. doi: 10.1016/s0925-4439(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 72.Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63:1381–1393. doi: 10.2337/db13-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JM, Paulson DJ, Romano FD. Inhibition of nitric oxide synthase by L-NAME improves ventricular performance in streptozotocin-diabetic rats. J Mol Cell Cardiol. 1997;29:2393–2402. doi: 10.1006/jmcc.1997.0474. [DOI] [PubMed] [Google Scholar]
- 74.Esberg LB, Ren J. Role of nitric oxide, tetrahydrobiopterin and peroxynitrite in glucose toxicity-associated contractile dysfunction in ventricular myocytes. Diabetologia. 2003;46:1419–1427. doi: 10.1007/s00125-003-1183-8. [DOI] [PubMed] [Google Scholar]
- 75.Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo S, Underwood BR, Kroemer G, O'Kane CJ, Rubinsztein DC. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han F, Chen YX, Lu YM, Huang JY, Zhang GS, Tao RR, Ji YL, Liao MH, Fukunaga K, Qin ZH. Regulation of the ischemia-induced autophagy-lysosome processes by nitrosative stress in endothelial cells. Journal of pineal research. 2011;51:124–135. doi: 10.1111/j.1600-079X.2011.00869.x. [DOI] [PubMed] [Google Scholar]
- 78.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoeldtke RD, Bryner KD, McNeill DR, Hobbs GR, Baylis C. Peroxynitrite versus nitric oxide in early diabetes. Am J Hypertens. 2003;16:761–766. doi: 10.1016/s0895-7061(03)00976-2. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009;58:1391–1402. doi: 10.2337/db08-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 84.Cong W, Zhao T, Zhu Z, Huang B, Ma W, Wang Y, Tan Y, Chakrabarti S, Li X, Jin L, Cai L. Metallothionein prevents cardiac pathological changes in diabetes by modulating nitration and inactivation of cardiac ATP synthase. J Nutr Biochem. 2014;25:463–474. doi: 10.1016/j.jnutbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 85.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Li B, Liu S, Miao L, Cai L. Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. 2012;2012:216512. doi: 10.1155/2012/216512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stadler K. Peroxynitrite-driven mechanisms in diabetes and insulin resistance - the latest advances. Curr Med Chem. 2011;18:280–290. doi: 10.2174/092986711794088317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–792. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 89.Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53:463–473. doi: 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- 90.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 91.Mei Y, Thompson M, Cohen R, Tong YX. Autophagy and oxidative stress in cardiovascular diseases. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbadis.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong Y, Undyala VV, Gottlieb RA, Mentzer RM, Jr., Przyklenk K. Autophagy: definition, molecular machinery, and potential role in myocardial ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2010;15:220–230. doi: 10.1177/1074248410370327. [DOI] [PubMed] [Google Scholar]
- 93.Martinet W, Knaapen MW, Kockx MM, De Meyer GR. Autophagy in cardiovascular disease. Trends Mol Med. 2007;13:482–491. doi: 10.1016/j.molmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 95.Campesi I, Straface E, Occhioni S, Montella A, Franconi F. Protein oxidation seems to be linked to constitutive autophagy: a sex study. Life Sci. 2013;93:145–152. doi: 10.1016/j.lfs.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Ren J, Ceylan-Isik AF. Diabetic cardiomyopathy: do women differ from men? Endocrine. 2004;25:73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G, Wang L, Peng Y, Dong X, Huang H, Si L, Zhang X, Li J, Wang W, Zhou L, Gao X. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One. 2013;8:e75927. doi: 10.1371/journal.pone.0075927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–2076. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie Z, He C, Zou MH. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy. 2011;7:1254–1255. doi: 10.4161/auto.7.10.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87:665–674. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giricz Z, Mentzer RM, Jr., Gottlieb RA. Autophagy, myocardial protection, and the metabolic syndrome. J Cardiovasc Pharmacol. 2012;60:125–132. doi: 10.1097/FJC.0b013e318256ce10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng JY, Huang JP, Lu LS, Hung LM. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am J Physiol Heart Circ Physiol. 2007;293:H978–987. doi: 10.1152/ajpheart.01002.2006. [DOI] [PubMed] [Google Scholar]
- 104.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol. 2011;50:1035–1043. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, Herzig S, Glembotski CC, Sussman MA. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med. 2014;6:57–65. doi: 10.1002/emmm.201303183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He X, Ma Q. Redox regulation by nuclear factor erythroid 2-related factor 2: gatekeeping for the basal and diabetes-induced expression of thioredoxin-interacting protein. Mol Pharmacol. 2012;82:887–897. doi: 10.1124/mol.112.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kobayashi S, Xu X, Chen K, Liang Q. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy. 2012;8:577–592. doi: 10.4161/auto.18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, Gulick J, Yue Z, Robbins J, Epstein PN, Liang Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem. 2013;288:18077–18092. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bai P, Canto C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 112.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bai P, Canto C, Brunyanszki A, Huber A, Szanto M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H, Gergely P, Menissier-de Murcia J, Schreiber V, Sauve AA, Auwerx J. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukhopadhyay P, Rajesh M, Cao Z, Horvath B, Park O, Wang H, Erdelyi K, Holovac E, Wang Y, Liaudet L, Hamdaoui N, Lafdil F, Hasko G, Szabo C, Boulares AH, Gao B, Pacher P. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology. 2014;59:1998–2009. doi: 10.1002/hep.26763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 117.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 118.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 119.Tschope C, Walther T, Escher F, Spillmann F, Du J, Altmann C, Schimke I, Bader M, Sanchez-Ferrer CF, Schultheiss HP, Noutsias M. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. Faseb J. 2005;19:2057–2059. doi: 10.1096/fj.05-4095fje. [DOI] [PubMed] [Google Scholar]
- 120.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschope C. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 121.Westermann D, Walther T, Savvatis K, Escher F, Sobirey M, Riad A, Bader M, Schultheiss HP, Tschope C. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–1381. doi: 10.2337/db08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 123.Becher PM, Lindner D, Frohlich M, Savvatis K, Westermann D, Tschope C. Assessment of cardiac inflammation and remodeling during the development of streptozotocin-induced diabetic cardiomyopathy in vivo: a time course analysis. Int J Mol Med. 2013;32:158–164. doi: 10.3892/ijmm.2013.1368. [DOI] [PubMed] [Google Scholar]
- 124.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Y, Ohmori K, Mizukawa M, Yoshida J, Zeng Y, Zhang L, Shinomiya K, Kosaka H, Kohno M. Differential impact of atorvastatin vs pravastatin on progressive insulin resistance and left ventricular diastolic dysfunction in a rat model of type II diabetes. Circ J. 2007;71:144–152. doi: 10.1253/circj.71.144. [DOI] [PubMed] [Google Scholar]
- 126.Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, da Silva NF, Sabico SL, Kumar S, McTernan PG, Harte AL. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol. 2009;8:20. doi: 10.1186/1475-2840-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elrashidy RA, Asker ME, Mohamed HE. Beneficial effects of pioglitazone against cardiovascular injury are enhanced by combination with aliskiren in a rat model of diabetic nephropathy. J Pharm Pharmacol. 2012;64:862–871. doi: 10.1111/j.2042-7158.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 128.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 129.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 132.Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. Embo J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harris J. Autophagy and cytokines. Cytokine. 2011;56:140–144. doi: 10.1016/j.cyto.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 135.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, Ju C, Aouadi M, Czech MP, Kunos G. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh HA, Kwon S, Choi S, Shin H, Yoon KH, Kim WJ, Lim HJ. Uncovering a role for endocannabinoid signaling in autophagy in preimplantation mouse embryos. Molecular human reproduction. 2013;19:93–101. doi: 10.1093/molehr/gas049. [DOI] [PubMed] [Google Scholar]
- 139.Koay LC, Rigby RJ, Wright KL. Cannabinoid-induced autophagy regulates suppressor of cytokine signaling (SOCS)-3 in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2014 doi: 10.1152/ajpgi.00317.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toldo S, Boccellino M, Rinaldi B, Seropian IM, Mezzaroma E, Severino A, Quagliuolo L, Van Tassell BW, Marfella R, Paolisso G, Rossi F, Natarajan R, Voelkel N, Abbate A, Crea F, Baldi A. Altered oxido-reductive state in the diabetic heart: loss of cardioprotection due to protein disulfide isomerase. Mol Med. 2011;17:1012–1021. doi: 10.2119/molmed.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol. 2011;300:R186–200. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu N, Zhang Y, Li H, Gao Z. Oxidative and nitrative modifications of alpha-enolase in cardiac proteins from diabetic rats. Free Radic Biol Med. 2010;48:873–881. doi: 10.1016/j.freeradbiomed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 143.Turko IV, Marcondes S, Murad F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferase. Am J Physiol Heart Circ Physiol. 2001;281:H2289–2294. doi: 10.1152/ajpheart.2001.281.6.H2289. [DOI] [PubMed] [Google Scholar]
- 144.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 145.Cong W, Ma W, Zhao T, Zhu Z, Wang Y, Tan Y, Li X, Jin L, Cai L. Metallothionein prevents diabetes-induced cardiac pathological changes, likely via the inhibition of succinyl-CoA:3-ketoacid coenzyme A transferase-1 nitration at Trp(374) Am J Physiol Endocrinol Metab. 2013;304:E826–835. doi: 10.1152/ajpendo.00570.2012. [DOI] [PubMed] [Google Scholar]
- 146.Dennis KE, Hill S, Rose KL, Sampson UK, Hill MF. Augmented cardiac formation of oxidatively-induced carbonylated proteins accompanies the increased functional severity of post-myocardial infarction heart failure in the setting of type 1 diabetes mellitus. Cardiovasc Pathol. 2013;22:473–480. doi: 10.1016/j.carpath.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shao CH, Tian C, Ouyang S, Moore CJ, Alomar F, Nemet I, D'Souza A, Nagai R, Kutty S, Rozanski GJ, Ramanadham S, Singh J, Bidasee KR. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol Pharmacol. 2012;82:383–399. doi: 10.1124/mol.112.078352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shao CH, Capek HL, Patel KP, Wang M, Tang K, DeSouza C, Nagai R, Mayhan W, Periasamy M, Bidasee KR. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes. 2011;60:947–959. doi: 10.2337/db10-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shao CH, Rozanski GJ, Nagai R, Stockdale FE, Patel KP, Wang M, Singh J, Mayhan WG, Bidasee KR. Carbonylation of myosin heavy chains in rat heart during diabetes. Biochem Pharmacol. 2010;80:205–217. doi: 10.1016/j.bcp.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Puthanveetil P, Zhang D, Wang Y, Wang F, Wan A, Abrahani A, Rodrigues B. Diabetes triggers a PARP1 mediated death pathway in the heart through participation of FoxO1. J Mol Cell Cardiol. 2012;53:677–686. doi: 10.1016/j.yjmcc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 151.Joshi MS, Mihm MJ, Cook AC, Schanbacher BL, Bauer JA. Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. J Diabetes. 2014 doi: 10.1111/1753-0407.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–361. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 153.Luan R, Liu S, Yin T, Lau WB, Wang Q, Guo W, Wang H, Tao L. High glucose sensitizes adult cardiomyocytes to ischaemia/reperfusion injury through nitrative thioredoxin inactivation. Cardiovasc Res. 2009;83:294–302. doi: 10.1093/cvr/cvp085. [DOI] [PubMed] [Google Scholar]
- 154.Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci (Lond) 2013;125:361–382. doi: 10.1042/CS20130065. [DOI] [PubMed] [Google Scholar]
- 155.Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, Konduri GG. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2012;302:L651–663. doi: 10.1152/ajplung.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang X, Dai Y, Ding Z, Khaidakov M, Mercanti F, Mehta JL. Regulation of autophagy and apoptosis in response to angiotensin II in HL-1 cardiomyocytes. Biochem Biophys Res Commun. 2013;440:696–700. doi: 10.1016/j.bbrc.2013.09.131. [DOI] [PubMed] [Google Scholar]
- 157.Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis. 2009;204:73–78. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 158.Garcia-Navas R, Munder M, Mollinedo F. Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy. 2012;8:1557–1576. doi: 10.4161/auto.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kwak SS, Suk J, Choi JH, Yang S, Kim JW, Sohn S, Chung JH, Hong YH, Lee DH, Ahn JK, Min H, Fu YM, Meadows GG, Joe CO. Autophagy induction by tetrahydrobiopterin deficiency. Autophagy. 2011;7:1323–1334. doi: 10.4161/auto.7.11.16627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 161.Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, Del Campo A, Toro B, Battiprolu PK, Aranguiz P, Chiong M, Yakar S, Gillette TG, Hill JA, Abel ED, Leroith D, Lavandero S. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res. 2012;93:320–329. doi: 10.1093/cvr/cvr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM. Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res. 2008;79:322–330. doi: 10.1093/cvr/cvn095. [DOI] [PubMed] [Google Scholar]
- 163.Nagareddy PR, McNeill JH, MacLeod KM. Chronic inhibition of inducible nitric oxide synthase ameliorates cardiovascular abnormalities in streptozotocin diabetic rats. Eur J Pharmacol. 2009;611:53–59. doi: 10.1016/j.ejphar.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 164.Szabo C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves JT. Part I: pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 165.Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 166.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer research. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 167.Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guo R, Liu B, Zhou S, Zhang B, Xu Y. The protective effect of fasudil on the structure and function of cardiac mitochondria from rats with type 2 diabetes induced by streptozotocin with a high-fat diet is mediated by the attenuation of oxidative stress. Biomed Res Int. 2013;2013:430791. doi: 10.1155/2013/430791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iorio F, Isacchi A, di Bernardo D, Brunetti-Pierri N. Identification of small molecules enhancing autophagic function from drug network analysis. Autophagy. 2010;6:1204–1205. doi: 10.1073/pnas.1000138107. [DOI] [PubMed] [Google Scholar]
- 170.Riad A, Unger D, Du J, Westermann D, Mohr Z, Sobirey M, Dorenkamp M, Schultheiss HP, Tschope C. Chronic inhibition of p38MAPK improves cardiac and endothelial function in experimental diabetes mellitus. Eur J Pharmacol. 2007;554:40–45. doi: 10.1016/j.ejphar.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 171.Chiu J, Farhangkhoee H, Xu BY, Chen S, George B, Chakrabarti S. PARP mediates structural alterations in diabetic cardiomyopathy. J Mol Cell Cardiol. 2008;45:385–393. doi: 10.1016/j.yjmcc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 172.Zhou ZR, Zhu XD, Zhao W, Qu S, Su F, Huang ST, Ma JL, Li XY. Poly(ADP-ribose) polymerase-1 regulates the mechanism of irradiation-induced CNE-2 human nasopharyngeal carcinoma cell autophagy and inhibition of autophagy contributes to the radiation sensitization of CNE-2 cells. Oncol Rep. 2013;29:2498–2506. doi: 10.3892/or.2013.2382. [DOI] [PubMed] [Google Scholar]
- 173.Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295–304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 174.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Picatoste B, Ramirez E, Caro-Vadillo A, Iborra C, Ares-Carrasco S, Egido J, Tunon J, Lorenzo O. Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS One. 2013;8:e78330. doi: 10.1371/journal.pone.0078330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu J, Liu Y, Chen L, Wang Y, Li J. Glucagon-Like Peptide-1 Analog Liraglutide Protects against Diabetic Cardiomyopathy by the Inhibition of the Endoplasmic Reticulum Stress Pathway. J Diabetes Res. 2013;2013:630537. doi: 10.1155/2013/630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo Z, Qi W, Yu Y, Du S, Wu J, Liu J. Effect of exenatide on the cardiac expression of adiponectin receptor 1 and NADPH oxidase subunits and heart function in streptozotocin-induced diabetic rats. Diabetol Metab Syndr. 2014;6:29. doi: 10.1186/1758-5996-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]