Abstract
The severity of Helicobacter pylori-related disease is correlated with the presence of a cag pathogenicity island (PAI). Genetic diversity within the cag PAI may have a modifying effect on the pathogenic potential of the infecting strain. We analyzed the complete cag PAI sequences of 11 representative Japanese strains according to their vacA genotypes and clinical effects and examined the relationship between the diversity of the cag PAI and clinical features. The cag PAI genes were divided into two major groups, a Western and a Japanese group, by phylogenetic analysis based on the entire cag PAI sequences. The predominant Japanese strains formed a Japanese cluster which was different from the cluster formed by Western strains. The diversity of the cag PAI was associated with the vacA and cagA genotypes. All strains with the s1c vacA genotype were in the Japanese cluster. In addition, all strains with the East Asian-type cagA genotype were also in the Japanese cluster. Patients infected with the Japanese-cluster strain had high-grade gastric mucosal atrophy. These results suggest that a distinct diversity of the cag PAI of H. pylori is present among Japanese strains and that this diversity may be involved in the development of atrophic gastritis and may increase the risk for gastric cancer.
Helicobacter pylori is a gram-negative microaerophilic bacterium that chronically colonizes the gastric epithelium of more than half of all people worldwide. It is a human pathogen responsible for chronic active gastritis; and infection with this organism is an important risk factor for peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma (20, 22, 28, 29). The CagA protein, encoded by the cagA gene, is one of the most studied virulence factors of H. pylori and is highly immunogenic. The cagA gene is one of several genes in a pathogenicity island (PAI) known as the cag PAI. The presence of cagA is considered a marker of the presence of cag PAI (10). The cag PAI is a 40-kb locus in the chromosomal glutamate racemase gene. Its G+C content (35%) differs from the G+C content of the rest of the genome (39%), suggesting that it was acquired from another organism by horizontal transfer (8, 11, 25). At some point during evolution, IS605, a mobile sequence encoding two transposases, entered the H. pylori genome and in some strains interrupted, multiplied, or deleted parts of the PAI (8). The severity of H. pylori-related disease is correlated with the presence of the cag PAI. Infection with cag PAI-positive H. pylori is statistically associated with duodenal ulceration, gastric mucosal atrophy, and gastric cancer (7, 8, 10). The cag PAI contains 31 genes, and 6 of the cag genes are thought to encode a putative type IV secretion system that specializes in the transfer of a variety of multimolecular complexes across the bacterial membrane to the extracellular space or into other cells (11). Recent studies have indicated that CagA is delivered into epithelial cells by the cag type IV secretion system, where it is phosphorylated on tyrosine residues and connected to eukaryotic signal transduction pathways, and is likely to play a major role in H. pylori-host cell interactions and pathogenesis (3, 21, 23, 24). Moreover, it was recently discovered (17) that translocated CagA forms a physical complex with the SRC homology 2 domain-containing tyrosine phosphatase SHP-2, which is known to play an important positive role in mitogenic signal transduction, and stimulates phosphatase activity. On the basis of the sequence constituting the SHP-2 binding site, CagA proteins can be subclassified into East Asian and Western types. The East Asian-type CagA possesses stronger SHP-2 binding and transforming activities than the Western-type CagA (16).
H. pylori exhibits a large degree of genomic and allelic diversity. Strain-specific diversity has been proposed to be involved in the organism's ability to cause different diseases. There are also indications of significant geographical differences among strains. Only one-half to two-thirds of Western isolates carry the cag PAI. In contrast, nearly all East Asian strains carry the cag PAI (19, 26). It has also been reported that large sequence differences distinguish the cagA gene fragments from Asian strains and those from other strains. The lineage of H. pylori isolates infecting Asian subjects may be different from that of isolates in other parts of the world, or a specific strain may have accumulated in the Asian population (1). The variable genetic structure of the cag PAI may influence the clinical outcome of H. pylori infection.
Vacuolating cytotoxin (VacA) is another virulence factor. The vacA gene is present in all H. pylori strains and contains at least two variable parts. The s region (which encodes the signal peptide) coexists as s1 or s2 allelic types. Subtypes s1a, s1b, and s1c have been identified among type s1 strains. The m (middle) region occurs as the m1 or m2 allelic type (4, 26, 27, 30). Production of the vacuolating cytotoxin is related to the mosaic structure of vacA. In general, type s1/m1 and s1/m2 strains produce high and moderate levels of toxin, respectively, whereas s2/m2 strains produce little or no toxin (4). Because most vacA s1 strains are cagA positive, these two markers are closely related. It has been reported that the vacA s1- and cagA-positive genotype is significantly associated with a more severe clinical outcome, such as gastric cancer (26).
The incidence of gastric cancer and the rate of death due to gastric cancer in Japan are high compared with those in other developed countries. However, large intracountry differences in the rates of death from gastric cancer have been reported (18). Fukui is a typical rural prefecture located on the central Japanese mainland (Honshu), while Okinawa consists of islands in the southwestern part of Japan and has a history and food culture different from those in other parts of Japan. The prevalence of atrophic gastritis, a precursor lesion of gastric cancer, is more frequent in Fukui, and the rate of death from gastric cancer is more than 2.4 times higher in Fukui (43.7 per 100,000 population in 1999) than in Okinawa (18.2 per 100,000 population in 1999). In this study, we selected 11 Japanese strains according to their vacA genotypes, clinical effects, and geographical locations and determined the complete cag PAI gene sequences in order to examine the association between the diversity of genes in the cag PAI and clinical outcomes.
MATERIALS AND METHODS
H. pylori strains.
H. pylori clinical isolates were obtained during upper gastroduodenal endoscopy at the Second Department of Internal Medicine, Faculty of Medical Science, University of Fukui, Fukui, Japan, and Okinawa Chubu Hospital, Okinawa, Japan, respectively. A total of four biopsy specimens were obtained from each patient: two from the greater curvature of the gastric antral mucosa and two from the greater curvature of the gastric fundic mucosa. One specimen each from the antral and fundic mucosae was fixed in 10% buffered formalin (pH 7.2) and subjected to histological analysis. The other antral and fundic mucosal specimens were subjected to culture for H. pylori. These studies were performed according to the principles of the Declaration of Helsinki, and consent was obtained from each individual after he or she was given a full description of the nature and protocol of the study.
Histological analysis.
Biopsy specimens were embedded in paraffin and stained with hematoxylin-eosin. The specimens were examined without knowledge of the experimental results. The histological features of chronic gastritis in terms of lymphocyte infiltration (inflammation), neutrophil infiltration (activity of gastritis), and mucosal atrophy were graded from 0 to 3 according to the Updated Sydney system (12).
H. pylori culture.
Gastric biopsy specimens from each patient were inoculated onto a Trypticase soy agar II (TSA-II)-5% sheep blood plate and cultured under microaerobic conditions (O2, 5%; CO2, 15%; N2, 80%) at 37°C for 5 days. A single colony was picked from each primary culture plate, inoculated onto a fresh TSA-II plate, and cultured under the conditions described above. A few colonies were picked from each plate and transferred into 20 ml of brucella broth liquid culture medium containing 10% fetal calf serum and cultured for 3 days under the same conditions described above. A part of the liquid culture sample was stored at −80°C in 0.01 M phosphate-buffered saline (PBS) containing 20% glycerol. DNA from each H. pylori isolate was extracted from the pellet of the liquid culture sample by the protease-phenol-chloroform method, suspended in 300 μl of TE buffer (10 mM Tris HCl, 1 mM EDTA), and stored at 4°C until PCR analysis and nucleotide sequencing.
Complete cag PAI nucleotide sequences of nine representative Japanese strains.
The complete cag PAIs of two Japanese strains, strains F32 and OK107, were sequenced previously (5). We selected nine additional Japanese strains according to their vacA genotypes, clinical effects, and geographical locations. The characteristics of the selected strains are listed in Table 1. The region comprising the entire cag PAI of each strain was amplified with cag PAI-spanning primer sets designed from the sequence of strain 26695, as reported previously (25) (Table 2). PCR conditions were as follows: heating at 94°C for 5 min, followed by 25 cycles consisting of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The tubes were held at 72°C for 7 min before storage at 4°C. The PCR products were then purified with Centricon-100 Concentrator columns (Amicon, Beverly, Mass.). DNA sequencing was performed by the dideoxynucleotide chain termination method with a BigDye Terminator Cycle Sequencing Ready Reaction Mix (Applied Biosystems, Tokyo, Japan) in an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). According to the protocol of the manufacturer, reagent mixtures containing 5 μl of purified PCR product, 3.2 pmol of primer, 8 μl of Terminator Cycle Sequencing Ready Reaction Mix, and 5 μl of sterilized distilled water were prepared. The reaction tubes were placed in the thermal cycler, and the thermal cycling conditions were 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min, which were repeated for 25 cycles. The cycle sequencing reactions were performed for both DNA strands. The nucleotide sequences were aligned and analyzed with GENTYX-Mac software (version 10.0; Software Development Co., Tokyo, Japan). The cag PAI gene sequences of strains 26695 (25), J99 (2), and NCTC11638 (8) published previously were also included in the analysis.
TABLE 1.
Characteristics of the selected strains
| H. pylori strain | Origin | Disease | vacA genotype |
|---|---|---|---|
| 26695 | United Kingdom | Gastritis | s1a/m1 |
| J99 | United States | Duodenal ulcer | s1b/m1 |
| 11638 | Australia | Gastritis | s1a/m1 |
| F16 | Fukui | Gastritis | s1c/m1 |
| F17 | Fukui | Gastritis | s1c/m1 |
| F28 | Fukui | Gastritis | s1c/m1 |
| F32 | Fukui | Gastric cancer | s1c/m1 |
| F79 | Fukui | Gastric ulcer | s1a/m1 |
| F80 | Fukui | Duodenal ulcer | s1b/m1 |
| OK101 | Okinawa | Gastritis | s1c/m1 |
| OK107 | Okinawa | Gastritis | s1a/m2 |
| OK109 | Okinawa | Gastritis | s1a/m1 |
| OK112 | Okinawa | Gastritis | s2/m2 |
| OK129 | Okinawa | Gastritis | s1c/m2 |
TABLE 2.
H. pylori cag PAI-spanning primer sets
| Primer position in strain 26695 | Designation in the following study:
|
Primer
|
Size of PCR product (bp) | |||
|---|---|---|---|---|---|---|
| Tomb et al. (25) | Censini et al. (8) | PAI primer | Nucleotide sequence | Primer set | ||
| 546899 | HP0519 | PAI-1 S | 5′-ACACTGCCAAGCCCGATGCTGTA-3′ | PAI-1 S, PAI-1 AS | 1,264 | |
| 547464 | HP0520 | cag ζ | PAI-2 S | 5′-GTGTCTTGAGCGGTGCTATG-3′ | PAI-2 S, PAI-2 AS | 1,366 |
| 547652 | HP0520 AS1 | 5′-CAGTTGGTTCGTTGGTAAC-3′ | ||||
| 547850 | HP0521 S1 | 5′-GCTGTAAGGGCGTTTTAC-3′ | ||||
| 548162 | PAI-1 AS | 5′-GATACAGCGGTTGCTAGT-3′ | ||||
| 548526 | HP0522 S4 | 5′-GACTTATGCGGATCTCATTA-3′ | ||||
| 548547 | PAI-3 S | 5′-CATCACAGGCTCATTAGAG-3′ | PAI-3 S, PAI-3 AS | 1,233 | ||
| 548829 | PAI-2 AS | 5′-ATCTCTTAGGGGCGAACACACTTC-3′ | ||||
| 549011 | HP0522 S2 | 5′-CCCAACTCGTAGCCAATGAAGAAG-3′ | ||||
| 549312 | HP0523 | cag γ | HP0522 AS3 | 5′-GATCAAAGTCCCCTCATAGC-3′ | ||
| 549703 | PAI-4 S | 5′-GGTTGCGACAATGAAGTG-3′ | PAI-4 S, PAI-4 AS | 982 | ||
| 549779 | PAI-3 AS | 5′-CTGTTGTTCAACCCTAGAGAG-3′ | ||||
| 550007 | PAI-5 S | 5′-GCGCTTACAATGGGGGAATGAA-3′ | PAI-5 S, PAI-5 AS | 1,327 | ||
| 550032 | HP0524 | cag β (virD4) | HP0523 S2 | 5′-CAACCCTAATGGCGCTTACGTGAA-3′ | ||
| 550227 | HP0524 S4 | 5′-CTTGAACCCACAGGCACTAAAGA-3′ | ||||
| 550423 | HP0524 AS6 | 5′-CCTATCAAGTGCCACAAG-3′ | ||||
| 550684 | PAI-4 AS | 5′-GGTAGGAATGGCGCTAAGAC-3′ | ||||
| 550666 | PAI-6 S | 5′-TCTTAGCGCCATTCCTACCATAACC-3′ | PAI-6 S, PAI-6 AS | 1,104 | ||
| 550866 | HP0524 AS4 | 5′-TTCTGCCAATCCATGATCCACAGTG-3′ | ||||
| 551230 | PAI-7 S | 5′-AGTTGATCCCGCTTGCCATAGAAC-3′ | PAI-7 S, PAI-7 AS | 1,503 | ||
| 551333 | PAI-5 AS | 5′-CATGTGGACTAAAAAGGGGCTTGAG-3′ | ||||
| 551653 | HP0524 S5 | 5′-CCATAGTGTCAGCTTTAGGGTCA-3′ | ||||
| 551769 | PAI-6 AS | 5′-CGTTCATTGGCTTGATTGCTCCTAC-3′ | ||||
| 552250 | HP0525 | cag α (virB11) | HP0524 AS5 | 5′-GGCTTTTGGTTGCAAGCTATAC-3′ | ||
| 552610 | PAI-8 S | 5′-GGCCAAACGGATAAACGCTTCTTCA-3′ | PAI-8 S, PAI-8 AS | 1,021 | ||
| 552732 | PAI-7 AS | 5′-ATTTTAGGGGAACTCAGAAGCAGTG-3′ | ||||
| 553114 | HP0526 | cag Z | PAI-9 S | 5′-GACAATCTGCACCCTTTCAC-3′ | PAI-9 S, PAI-9 AS | 1,122 |
| 553630 | PAI-8 AS | 5′-GTGGCGTTTCAGATCCTAGGGATAG-3′ | ||||
| 553948 | HP0526 AS2 | 5′-GATAGCAACGATCCGCAAGA-3′ | ||||
| 553930 | HP0527 | cagY (virB10) | PAI-10 S | 5′-CTTGCGGATCGTTGCTATCT-3′ | PAI-10 S, PAI-10 AS | 1,041 |
| 554235 | PAI-9 AS | 5′-ATCACCACAAGCCCCAAAGGT-3′ | ||||
| 554504 | PAI-11 S | 5′-CTACCTTTGCCAAGGCCTATGAGT-3′ | PAI-11 S, PAI-11 AS | 1,283 | ||
| 554970 | PAI-10 AS | 5′-GAAACAAGCCCTGTCAAACAGG-3′ | ||||
| 555368 | PAI-12 S | 5′-GGATAACCTTTAGCCGCCATGT-3′ | PAI-12 S, PAI-12 AS | 1,829 | ||
| 555786 | PAI-11 AS | 5′-GACAGAGCGGCTATCATGAAGTGT-3′ | ||||
| 555763 | PAI-13 S | 5′-ACACTTCATGATAGCCGCTCTGTC-3′ | PAI-13 S, PAI-13 AS | 2,931 | ||
| 557196 | PAI-12 AS | 5′-GAGAAATTGCTCACCCTTGAATC-3′ | ||||
| 558446 | PAI-14 S | 5′-CAATCTAGCGCCACTTGAAC-3′ | PAI-14 S, PAI-14 AS | 623 | ||
| 558693 | PAI-13 AS | 5′-GAGGACAAAAACCCGTTGAGAG-3′ | ||||
| 558925 | PAI-15 S | 5′-CACGATAAGAACAGCGACTAC-3′ | PAI-15 S, PAI-15 AS | 1,134 | ||
| 559068 | PAI-14 AS | 5′-CTTGACAACCCCACAGAAACTC-3′ | ||||
| 559445 | HP0528 | cagX (virB9) | PAI-16 S | 5′-GTGGGGTTGTCAAGATGATGATCTG-3′ | PAI-16 S, PAI-16 AS | 996 |
| 560058 | PAI-15 AS | 5′-CTATGGTGAATTGGAGCGTGTG-3′ | ||||
| 560207 | PAI-17 S | 5′-CAATGGCGGCATCAGTCATGCTCAA-3′ | PAI-17 S, PAI-17 AS | 977 | ||
| 560440 | PAI-16 AS | 5′-ATCAAGCAAAGGCGCTAGAGACTCA-3′ | ||||
| 560909 | PAI-18 S | 5′-CTTGCATGTCCTCTAGTCGTTCCAT-3′ | PAI-18 S, PAI-18 AS | 837 | ||
| 561183 | PAI-17 AS | 5′-ACTTATCGTAGATGCGCCTGACC-3′ | ||||
| 561561 | HP0529 | cagW (virB8) | PAI-19 S | 5′-TGCCTGCCCCATCAACAATTCCTCT-3′ | PAI-19 S, PAI-19 AS | 1,008 |
| 561745 | PAI-18 AS | 5′-GAGCGTCAATGCGATCGTTAATACC-3′ | ||||
| 561843 | PAI-20 S | 5′-TAGCAACAGAGGGCGTTATG-3′ | PAI-20 S, PAI-20 AS | 1,130 | ||
| 562568 | PAI-19 AS | 5′-TCAAAGGAGCGGACGCTGCTGTT-3′ | ||||
| 562738 | PAI-21 S | 5′-GTCCTCAACACCGCCTTTGGTAAA-3′ | PAI-21 S, PAI-21 AS | 1,024 | ||
| 562972 | HP0530 | cagV | PAI-20 AS | 5′-CACAAGTTTAGCCGCTAGCA-3′ | ||
| 563540 | PAI-22 S | 5′-GATAGCTTCTGCTCGGACTT-3′ | PAI-22 S, PAI-22 AS | 970 | ||
| 563761 | PAI-21 AS | 5′-GTCAAACGCTCCGATGCTAG-3′ | ||||
| 563925 | HP0531 | cagU | PAI-23 S | 5′-CTATCAAGGGCTATCACACC-3′ | PAI-23 S, PAI-23 AS | 1,107 |
| 564509 | PAI-22 AS | 5′-TCTTTGCTCCCCTAAACTCC-3′ | ||||
| 564610 | HP0531 S1 | 5′-GACAAGCCAAACAGAGAATA-3′ | ||||
| 564889 | HP0532 | cagT (virB7) | PAI-24 S | 5′-GTAGCACTAACGACAAGGTGCT-3′ | PAI-24 S, PAI-24 AS | 1,087 |
| 565031 | PAI-23 AS | 5′-TGCACCGCCTTGTTTCTTTG-3′ | ||||
| 565573 | PAI-25 S | 5′-CTTGCATGGCTATGATGTGAG-3′ | PAI-25 S, PAI-25 AS | 983 | ||
| 565975 | PAI-24 AS | 5′-TAACGCCCGTTGGCGTTTCTCT-3′ | ||||
| 566268 | PAI-26 S | 5′-GGGAGCTTAGTGCCATACAA-3′ | PAI-26 S, PAI-26 AS | 842 | ||
| 566555 | PAI-25 AS | 5′-GCATACAAACAAGGGAGCGTTAG-3′ | ||||
| 566816 | PAI-27 S | 5′-CAGAGCGGTCATAATTCAAAGAGC-3′ | PAI-27 S, PAI-27 AS | 1,342 | ||
| 567109 | PAI-26 AS | 5′-TCATCTTTCACGCAGAGC-3′ | ||||
| 567090 | HP0535 S3 | 5′-CAACTCTGCGTTCAGTGTGTTGAC-3′ | ||||
| 567212 | HP0535 S2 | 5′-CCAACCAAAGCAGATCCCATGT-3′ | ||||
| 567426 | HP0535 AS2 | 5′-TTGTTGGGTGGCGGAACAAA-3′ | ||||
| 567409 | PAI-28 S | 5′-TGTTCCGCCACCCAACAAAGAA-3′ | PAI-28 S, PAI-28 AS | 1,174 | ||
| 568157 | PAI-27 AS | 5′-CTTATGGGGCAGGGGTGATTTTAG-3′ | ||||
| 568144 | HP0537 | cagM | PAI-29 S | 5′-CCCTGCCCCATAAGAAAA-3′ | PAI-29 S, PAI-29 AS | 1,160 |
| 568582 | PAI-28 AS | 5′-GTATGCGGCTTGTTGGTA-3′ | ||||
| 568783 | HP0537 AS2 | 5′-CCACATTAGCCGACAAAACTCC-3′ | ||||
| 568993 | PAI-30 S | 5′-GAGGCTCTAGAGAAAGAGAC-3′ | PAI-30 S, PAI-30 AS | 1,086 | ||
| 569303 | PAI-29 AS | 5′-GCTAATCGGCTCGCTTTT-3′ | ||||
| 569724 | HP0538 | cagN | PAI-31 S | 5′-CGTAGATAGCGATCCTATG-3′ | PAI-31 S, PAI-31 AS | 1,331 |
| 570078 | PAI-30 AS | 5′-CTCTCAAAGCGTTAGTGG-3′ | ||||
| 570169 | HP0539 | cagL | PAI-32 S | 5′-AAGCGGCTAAGCACAAAG-3′ | PAI-32 S, PAI-32 AS | 1,311 |
| 571054 | PAI-31 AS | 5′-CACAGACGCTTGTAGAAAG-3′ | ||||
| 571040 | PAI-33 S | 5′-CTACAAGCGTCTGTGAAG-3′ | PAI-33 S, PAI-33 AS | 880 | ||
| 571479 | HP0540 | cagI | PAI-32 AS | 5′-AGAGACCAACCAACAAGTGC-3′ | ||
| 571741 | HP0539 ASI | 5′-GATTTGAACGCGCTCATAG-3′ | ||||
| 571729 | PAI-34 S | 5′-GCGCGTTCAAATCTACTG-3′ | PAI-34 S, PAI-34 AS | 1,350 | ||
| 571919 | PAI-33 AS | 5′-CACAAAATGCCCCTATCTTG-3′ | ||||
| 572648 | HP0541 | cagH | HP0540 AS3 | 5′-GCTTGAACCCGCCTTAAA-3′ | ||
| 572894 | PAI-35 S | 5′-TCGCTTGAGTGTCATAGG-3′ | PAI-35 S, PAI-35 AS | 1,271 | ||
| 573078 | PAI-34 AS | 5′-CACTCCTGCATGCCCTATTG-3′ | ||||
| 573766 | HP0541 AS2 | 5′-GTGTTGCAGGGCCATTTG-3′ | ||||
| 573832 | HP0542 | cagG | PAI-36 S | 5′-GCTTGTGTACCTGCCATGTT-3′ | PAI-36 S, PAI-36 AS | 783 |
| 574164 | HP0543 | cagF | PAI-35 AS | 5′-AAATAGCGTGGGGCTTGT-3′ | ||
| 574444 | PAI-37 S | 5′-GCTTCAACGCTCATATCAG-3′ | PAI-37 S, PAI-37 AS | 1,089 | ||
| 574614 | PAI-36 AS | 5′-CATAAGCGAGGACATGCAGAAC-3′ | ||||
| 574991 | HP0544 | cagE (virB4.picB) | PAI-38 S | 5′-GAGCGGTAAGGTTTTGTTCGGTGAT-3′ | PAI-38 S, PAI-38 AS | 1,594 |
| 575324 | HP0544 S4 | 5′-GGGCTTCCATCCTGTTTGTAGAGAA-3′ | ||||
| 575532 | PAI-37 AS | 5′-GTCAGACTTGCGACTCAAAG-3′ | ||||
| 576092 | PAI-39 S | 5′-GCCGCCCAAGCAAAAGGATTTA-3′ | PAI-39 S, PAI-39 AS | 1,498 | ||
| 576161 | HP0544 AS4 | 5′-CAATGGGTGGGGAGTATGTCAAGA-3′ | ||||
| 576584 | PAI-38 AS | 5′-CCAACGCAGCGACTTTCTCTATG-3′ | ||||
| 577004 | HP0544 AS5 | 5′-TGCATGGTGGGGTGAAAGAAGTTTA-3′ | ||||
| 577255 | PAI-40 S | 5′-CATAACGGGTTCATTGAGAGTGTCT-3′ | PAI-40 S, PAI-40 AS | 1,051 | ||
| 577589 | PAI-39 AS | 5′-ATGGGGTGATCCTTACTAACAACTA-3′ | ||||
| 578096 | HP0545 | cagD | PAI-41 S | 5′-GCCACAAACACCCCTCTCTTTA-3′ | PAI-41 S, PAI-41 AS | 921 |
| 578305 | HP0546 | cagC | PAI-40 AS | 5′-CCAACAAACAACGCTGCTTTC-3′ | ||
| 578847 | PAI-42 S | 5′-CAGTCGCCTGACCTCTTTTGAT-3′ | PAI-42 S, PAI-42 AS | 1,594 | ||
| 579016 | PAI-41 AS | 5′-CAATCCTTTAATGGCGGTCACCAG-3′ | ||||
| 579342 | HP0546 S2 | 5′-CAAACCCAAGCTGATCAGAGTGAG-3′ | ||||
| 579530 | HP0546 AS1 | 5′-TTTGGTTTGTGTGTGTCATACT-3′ | ||||
| 579910 | HP0547 | cagA | PAI-43 S | 5′-AAGGAGAAACAATGACTAACGAAACTATTG-3′ | PAI-43 S, PAI-43 AS | 1,066 |
| 580440 | PAI-42 AS | 5′-CTGCAAAAGATTGTTTGGCAGA-3′ | ||||
| 580674 | PAI-44 S | 5′-ATACAAGGCTTACCGCCTG-3′ | PAI-44 S, PAI-44 AS | 1,675 | ||
| 580975 | PAI-43 AS | 5′-GTAGCCACATTGTCGCCTTGTTGG-3′ | ||||
| 581449 | HP0547 C1(+) | 5′-ATTTCAAATACACCAACGCCTCCAA-3′ | HP0547 C1(+), HP0547 L2(−) | 2,043 | ||
| 581979 | PAI-45 S | 5′-GAATTGTCTGATAAACTTGAAA-3′ | PAI-45 S, PAI-45 AS | 1,152 | ||
| 582348 | PAI-44 AS | 5′-GCTCTACCTTACTGAAATCGCC-3′ | ||||
| 582974 | HP0547 C4(−) | 5′-AGCTTCTGATACCGCTTGACTG-3′ | ||||
| 583130 | PAI-45 AS | 5′-GCGTATGTGGCTGTTAGTAGCG-3′ | ||||
| 583193 | HP0548 | cagΩ | PAI-46 S | 5′-AAACCCTGAGTGGCTCAAGCTC-3′ | PAI-46 S, PAI-46 AS | 829 |
| 583491 | HP0547 L2(−) | 5′-TCCTTTAAGATTTTTGGAAACCACCTTTTG-3′ | ||||
| 583718 | PAI-47 S | 5′-CAGCTCCCACCTAGCATTGAT-3′ | PAI-47 S, PAI-47 AS | 1,514 | ||
| 584021 | PAI-46 AS | 5′-GTTGATGCTCCCCTTCAACA-3′ | ||||
| 584386 | HP0548 S2 | 5′-GCGAAGCGATGAGAAGAA-3′ | ||||
| 584646 | HP0549 AS1 | 5′-ATTTCATGCGAGCGGCGATGTG-3′ | ||||
| 584873 | PAI-48 S | 5′-CTAGCAATTCGCCCTCTA-3′ | PAI-48 S, PAI-48 AS | 772 | ||
| 585231 | PAI-47 AS | 5′-CACTAAAGACCCCACCAC-3′ | ||||
| 585644 | PAI-48 AS | 5′-TTAAAGGCACCGGGAATAGC-3′ | ||||
Phylogenetic analysis.
To clarify the phylogenetic relationships between Japanese strains and previously characterized H. pylori strains from the West, the nucleotide sequences of the cag PAI genes were aligned by using DNASIS Pro software (Hitachi Software Engineering Co., Tokyo, Japan). A phylogenetic tree was constructed by using the unweighted pair group method and the same software.
Detection of vacA gene diversity.
Genotyping of vacA s- and m-region alleles was performed as previously described in detail (4, 18, 19). Briefly, parts of the vacA s and m regions were amplified and directly sequenced by using primers SS1-F, SS3-F, S1c-F, and VA1-R for the s region and primers VA3-F, VA4-F, VA3-R, and VA4-R for the m region. The conditions used for PCR and direct sequencing are described above.
In vitro infection.
Human gastric epithelial AGS cells were cultured in RPMI 1640 (GIBCO BRL, Grand Island, N.Y.) containing 10% fetal bovine serum (Filtron Pty Ltd., Brooklin, Australia). H. pylori (3 × 108 cells) was added to AGS cells (3 × 106 cells per 100-mm dish), which were then cultured in an antibiotic-free medium at a multiplicity of infection of 100. After incubation in a 5% CO2 atmosphere for 5 h, infected cells were washed three times with 0.01 M PBS (pH 7.5) containing 2 mM Na3VO4, lysed in ice-cold 1% Triton X-100 buffer (50 mM Tris-HCl [pH 7.4], 1% Triton X-100, 5 mM EDTA, 1 mM Na3VO4, 10 μg of leupeptin per ml, 10 μg of aprotinine per ml, 100 μM p-tosyl-l-phenylalanine chloromethyl ketone, 100 μΜ p-tosyl-l-lysine chloromethyl ketone, 1 mM phenylmethylsulfonyl fluoride), and subjected to immunoprecipitation and immunoblotting.
Antibodies.
The primary antibodies used for immunoprecipitation and immunoblotting were an anti-CagA polyclonal antibody (Austral Biologicals, San Ramon, Calif.), an antiphosphotyrosine antibody (4G10; Upstate Biotechnology Inc. Lake Placid, N.Y.), and an anti-SHP-2 antibody (C-18; Santa Cruz Biotech. Inc., Santa Cruz, Calif.).
Immunoprecipitation and immunoblotting.
The cell lysates were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant was subsequently immunoprecipitated with the anti-CagA polyclonal antibody or control normal immunoglobulin G for 30 min at 4°C, after which protein G-Sepharose beads (Amersham Pharmacia Biotech. Inc., Piscataway, N.J.) were added for 90 min at 4°C. The immunoprecipitates were washed four times with the lysis buffer and then boiled with a sodium dodecyl sulfate (SDS)-electrophoresis sample buffer (2% SDS, 10% glycerol, 6% 2-mercaptoethanol, 0.003% bromophenol blue, 50 mM Tris-HCl [pH 6.8]) for 5 min. Equal amounts of samples from homogenates or immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis (7.5% polyacrylamide) and blotted onto an Immobilon P membrane (Millipore Corp., Bedford, Mass.). The membranes were blocked with 1% bovine serum albumin or 5% skim milk in T-PBS (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5% Tween 20) and incubated with a primary antibody in T-PBS overnight at 4°C. After the membranes were washed with T-TBS, they were incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G polyclonal antibodies in T-TBS for 1 h and visualized with an enhanced chemiluminescence detection system, as directed by the manufacturer (Amersham Pharmacia Biotech. Inc.).
Nucleotide sequence accession numbers.
The DNA sequences of the complete cag PAI sequences of 11 representative Japanese strains characterized here were deposited in the DDBJ database under accession numbers AB120416 for F16, AB120417 for F17, AB120418 for F28, AB120419 for F32, AB120420 for F79, AB120421 for F80, AB120422 for OK101, AB120423 for OK107, AB120424 for OK109, AB120425 for OK112, and AB120426 for OK129.
RESULTS
Construction of cag PAI.
All 11 Japanese strains contained the complete cag PAI flanked by the same chromosomal genes and the previously described integration signal but lacked the insertion sequence IS605 elements associated with the cag PAI in strain NCTC11638. The general comparative features of the cag PAIs are shown in Table 3. The sizes of the complete cag PAI ranged from 36,534 to 38,477 bp. The numbers of cag PAI genes ranged from 27 to 33. The G+C contents of the full-length cag PAI ranged from 35.49 to 36.00%.
TABLE 3.
General comparative features of cag PAI
| H. pylori strain | Size (bp) | No. of genes | % G+C content | Presence of IS605 | CagA type |
|---|---|---|---|---|---|
| 26695 | 37,388 | 29 | 35.76 | − | Western |
| J99 | 37,729 | 27 | 35.97 | − | Western |
| 11638 | 38,477 | 33 | 35.83 | + | Western |
| F16 | 36,739 | 27 | 35.60 | − | East Asian |
| F17 | 37,119 | 27 | 35.52 | − | East Asian |
| F28 | 36,914 | 27 | 35.64 | − | East Asian |
| F32 | 36,534 | 27 | 35.72 | − | East Asian |
| F79 | 37,153 | 27 | 35.64 | − | Western |
| F80 | 37,209 | 28 | 35.89 | − | Mixed type |
| OK101 | 36,715 | 27 | 35.49 | − | East Asian |
| OK107 | 37,251 | 27 | 35.56 | − | Western |
| OK109 | 36,892 | 27 | 35.57 | − | East Asian |
| OK112 | 36,714 | 27 | 36.00 | − | Western |
| OK129 | 37,342 | 27 | 35.60 | − | East Asian |
Comparative analysis of cag PAI genes.
The nucleotide sequences of all cag PAI genes of the strains were aligned. The degrees of homology of both the nucleotide sequence of the coding regions of the entire cag PAI and the amino acid sequences between each isolate are shown in Table 4. The degrees of nucleotide sequence identity ranged from 88.26 to 96.73%, and the degrees of amino acid sequence identity ranged from 87.14 to 96.76%.
TABLE 4.
Analysis of cag PAI divergence
| Strain | % Nucleotide and amino acid sequence identitya
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | |
| 26695 | 91.93 | 91.18 | 91.13 | 91.52 | 91.59 | 92.79 | 92.67 | 92.73 | 92.12 | 89.98 | 90.22 | 93.13 | 92.88 | |
| J99 | 91.29 | 91.23 | 91.15 | 89.64 | 91.05 | 91.28 | 91.73 | 92.54 | 90.77 | 90.12 | 90.34 | 93.19 | 90.54 | |
| 11638 | 91.12 | 90.78 | 89.42 | 88.26 | 89.70 | 89.10 | 90.94 | 89.91 | 88.92 | 88.50 | 88.90 | 91.67 | 89.06 | |
| F16 | 90.38 | 90.54 | 88.75 | 95.05 | 96.33 | 95.11 | 94.82 | 92.05 | 95.68 | 91.14 | 93.32 | 92.87 | 95.78 | |
| F17 | 90.72 | 88.88 | 87.14 | 95.00 | 95.45 | 94.61 | 93.64 | 92.39 | 96.20 | 90.20 | 91.74 | 90.92 | 96.16 | |
| F28 | 90.99 | 90.34 | 89.08 | 95.96 | 95.16 | 94.74 | 95.37 | 92.28 | 95.07 | 92.27 | 93.72 | 92.96 | 96.10 | |
| F32 | 92.34 | 90.80 | 88.76 | 94.88 | 94.56 | 94.75 | 93.43 | 93.10 | 96.07 | 90.24 | 92.16 | 92.58 | 94.56 | |
| F79 | 92.69 | 91.35 | 91.09 | 94.14 | 92.63 | 94.66 | 92.78 | 92.90 | 93.58 | 92.13 | 92.26 | 93.48 | 94.59 | |
| F80 | 92.29 | 92.17 | 89.50 | 91.41 | 91.65 | 91.77 | 92.73 | 92.32 | 93.05 | 89.56 | 90.10 | 91.68 | 93.31 | |
| OK101 | 91.78 | 90.24 | 88.40 | 95.72 | 95.96 | 95.03 | 96.08 | 92.91 | 92.67 | 90.02 | 92.31 | 91.91 | 96.73 | |
| OK107 | 89.29 | 88.97 | 87.89 | 90.19 | 89.12 | 91.47 | 89.63 | 91.58 | 88.67 | 89.22 | 95.43 | 92.15 | 90.92 | |
| OK109 | 89.26 | 89.08 | 88.07 | 93.14 | 91.57 | 93.89 | 92.22 | 91.58 | 89.53 | 92.46 | 94.10 | 92.24 | 93.11 | |
| OK112 | 92.95 | 92.17 | 91.34 | 91.90 | 90.21 | 92.36 | 92.02 | 93.40 | 91.30 | 91.38 | 90.76 | 90.77 | 92.22 | |
| OK129 | 92.35 | 89.83 | 88.55 | 95.57 | 96.22 | 96.21 | 96.35 | 94.01 | 92.81 | 96.76 | 90.17 | 93.20 | 91.64 | |
Values above the diagonal are for nucleotide sequences, and those below the diagonal are for amino acid sequences.
Comparison of each cag PAI gene showed minor differences among strains. Strains F16, F17, F28, F32, F79, F80, OK101, and OK129 did not have HP0521. Strains OK107 and OK109 contained an HP0521 open reading frame (ORF) (348 bp, compatible with ORF7 of 11638) but did not have a stop codon until the end of HP0522. Therefore, HP0521 was combined with HP0522 in these strains. Strain OK112 did not have HP0533. The orientation of HP0535 was reversed in strains F16, F17, F28, F79, OK101, OK107, OK109, and OK129. None of the Japanese strains except strain F80 had HP0548. The lengths of HP0527 (virB10) and HP0547 (cagA) varied among the strains (Table 5). To analyze the divergence in each cag PAI gene, the nucleotide and amino acid sequence identities were calculated for the strains. The nucleotide and amino acid sequence identities of each cag PAI gene were more than 90%, except for HP0523 (cag; 73 to 99%) (Table 6), HP0527 (virB10; 77 to 95%) (Table 7), HP0535 (cagQ; 70 to 100%) (Table 8), and HP0547 (cagA; 72 to 97%) (Table 9).
TABLE 5.
Length of each H. pylori cag PAI gene
| Gene designation by Tomb et al. (25) | Gene orientation |
cag PAI gene length (bp)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | ||
| HP0520 | + | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 | 345 |
| HP0521 | + | 240 | 657 | 348 | 1,833 | 1,791 | 735 | ||||||||
| HP0522 | + | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | 1,443 | ||
| HP0523 | + | 507 | 507 | 507 | 507 | 507 | 507 | 507 | 507 | 507 | 507 | 420 | 507 | 507 | 507 |
| HP0524 | − | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 | 2,244 |
| HP0525 | − | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 | 990 |
| HP0526 | − | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 |
| HP0527 | − | 5,781 | 5,457 | 5,169 | 5,388 | 5,460 | 5,385 | 6,006 | 5,439 | 5,682 | 5,430 | 5,391 | 5,304 | 5,406 | 5,781 |
| HP0528 | − | 1,566 | 1,566 | 1,566 | 1,563 | 1,563 | 1,563 | 1,563 | 1,563 | 1,566 | 1,563 | 1,566 | 1,566 | 1,566 | 1,566 |
| HP0529 | − | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 | 1,605 |
| HP0530 | − | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 | 756 |
| HP0531 | + | 654 | 654 | 648 | 645 | 645 | 645 | 645 | 645 | 654 | 645 | 648 | 645 | 648 | 645 |
| HP0532 | + | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 | 840 |
| HP0533 | − | 87 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 87 | |||
| HP0534 | − | 588 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 | 597 |
| HP0535 | + | 309 | 303 | 303 | 309 | 378 | 303 | 303 | 303 | ||||||
| − | 378 | 378 | 378 | 378 | 303 | 378 | |||||||||
| HP0536 | − | 342 | 342 | 330 | 342 | 342 | 342 | 342 | 342 | 342 | 342 | 321 | 342 | 342 | 342 |
| HP0537 | + | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 | 1,128 |
| HP0538 | + | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 | 918 |
| HP0539 | − | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 711 | 705 |
| HP0540 | − | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 | 1,143 |
| HP0541 | − | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 | 1,110 |
| HP0542 | − | 426 | 426 | 426 | 429 | 426 | 429 | 426 | 426 | 426 | 429 | 426 | 429 | 426 | 426 |
| HP0543 | − | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 | 804 |
| HP0544 | − | 2,949 | 2,949 | 2,943 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 | 2,949 |
| HP0545 | − | 621 | 624 | 627 | 621 | 621 | 621 | 621 | 621 | 621 | 621 | 621 | 621 | 621 | 621 |
| HP0546 | − | 345 | 345 | 345 | 345 | 342 | 345 | 345 | 345 | 345 | 345 | 342 | 345 | 345 | 345 |
| HP0547 | + | 3,558 | 3,501 | 3,441 | 3,516 | 3,825 | 3,714 | 3,519 | 3,648 | 3,666 | 3,516 | 3,753 | 3,534 | 3,540 | 3,516 |
| HP0548 | + | 825 | 381 | 381 | |||||||||||
TABLE 6.
Analysis of HP0523 (cag γ) gene divergence
| Strain | % Nucleotide and amino acid sequence identitya
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | |
| 26695 | 93.14 | 92.35 | 90.20 | 89.80 | 88.63 | 89.02 | 89.41 | 89.80 | 89.80 | 76.67 | 92.55 | 91.18 | 89.22 | |
| J99 | 94.12 | 90.39 | 87.25 | 87.25 | 86.47 | 87.06 | 87.06 | 87.25 | 87.25 | 74.90 | 90.00 | 88.82 | 86.67 | |
| 11638 | 92.94 | 90.59 | 87.84 | 87.84 | 87.65 | 88.04 | 88.04 | 91.57 | 87.65 | 76.67 | 92.16 | 93.73 | 87.65 | |
| F16 | 93.53 | 91.18 | 90.59 | 96.08 | 95.29 | 94.12 | 95.29 | 90.59 | 95.29 | 76.08 | 92.35 | 90.00 | 95.49 | |
| F17 | 94.12 | 91.76 | 91.18 | 98.24 | 96.27 | 96.27 | 98.43 | 93.33 | 98.82 | 75.69 | 92.35 | 89.02 | 99.02 | |
| F28 | 92.35 | 90.59 | 89.41 | 96.47 | 95.88 | 97.65 | 95.29 | 90.98 | 95.29 | 77.06 | 92.35 | 88.63 | 95.29 | |
| F32 | 91.18 | 90.00 | 89.41 | 95.88 | 95.29 | 97.65 | 96.47 | 91.76 | 96.08 | 75.69 | 91.57 | 88.24 | 95.29 | |
| F79 | 92.35 | 90.59 | 90.59 | 97.65 | 98.24 | 95.29 | 97.06 | 92.94 | 98.04 | 75.88 | 92.35 | 89.02 | 98.63 | |
| F80 | 92.35 | 88.24 | 92.35 | 97.35 | 92.94 | 91.18 | 91.18 | 93.53 | 93.73 | 77.45 | 93.14 | 91.37 | 92.35 | |
| OK101 | 93.53 | 91.18 | 90.59 | 97.65 | 99.41 | 95.29 | 95.88 | 98.82 | 93.53 | 75.88 | 92.35 | 89.02 | 97.84 | |
| OK107 | 73.53 | 73.53 | 74.71 | 75.29 | 74.71 | 75.88 | 74.12 | 74.12 | 74.12 | 74.12 | 82.75 | 78.04 | 75.49 | |
| OK109 | 92.94 | 91.76 | 92.35 | 95.88 | 95.29 | 94.71 | 93.53 | 94.71 | 92.94 | 94.71 | 78.24 | 93.53 | 92.16 | |
| OK112 | 95.29 | 91.76 | 93.53 | 92.35 | 92.94 | 91.18 | 90.00 | 91.18 | 92.94 | 92.35 | 75.29 | 95.29 | 89.61 | |
| OK129 | 93.53 | 91.18 | 90.59 | 97.65 | 99.41 | 95.29 | 94.71 | 97.65 | 92.35 | 98.82 | 74.12 | 94.71 | 92.35 | |
Values above the diagonal are for nucleotide sequences, and those below the diagonal are for amino acid sequences.
TABLE 7.
Analysis of HP0527 (cagY) gene divergence
| Strain | % Nucleotide and amino acid sequence identitya
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | |
| 26695 | 83.47 | 85.06 | 84.54 | 89.85 | 88.77 | 91.72 | 88.59 | 89.76 | 90.04 | 88.45 | 87.59 | 89.61 | 95.18 | |
| J99 | 80.70 | 87.06 | 84.06 | 80.17 | 87.42 | 82.82 | 87.29 | 83.90 | 81.26 | 87.18 | 87.46 | 87.35 | 82.98 | |
| 11638 | 84.34 | 85.67 | 86.34 | 82.03 | 90.45 | 81.64 | 91.02 | 82.33 | 82.65 | 90.87 | 91.06 | 90.09 | 85.24 | |
| F16 | 82.10 | 82.65 | 84.40 | 85.16 | 91.92 | 84.14 | 88.86 | 81.12 | 83.54 | 90.34 | 89.84 | 90.06 | 85.57 | |
| F17 | 88.43 | 77.61 | 78.68 | 84.64 | 84.98 | 87.95 | 84.45 | 87.01 | 93.65 | 84.42 | 84.16 | 83.51 | 92.10 | |
| F28 | 88.38 | 84.66 | 90.04 | 90.08 | 85.06 | 87.11 | 93.11 | 83.55 | 85.50 | 95.23 | 94.40 | 94.20 | 90.28 | |
| F32 | 90.92 | 81.32 | 80.98 | 82.75 | 87.43 | 87.22 | 85.50 | 87.14 | 88.45 | 86.21 | 85.15 | 85.93 | 83.30 | |
| F79 | 87.66 | 85.85 | 90.90 | 86.84 | 83.67 | 93.34 | 84.83 | 84.16 | 83.32 | 93.86 | 91.48 | 91.75 | 89.41 | |
| F80 | 86.98 | 82.96 | 79.78 | 78.47 | 84.94 | 81.88 | 85.83 | 81.92 | 88.26 | 83.34 | 84.92 | 83.26 | 90.31 | |
| OK101 | 89.26 | 79.52 | 80.68 | 82.71 | 92.12 | 85.45 | 88.07 | 82.77 | 86.93 | 83.88 | 84.78 | 83.64 | 91.43 | |
| OK107 | 88.02 | 84.81 | 90.55 | 88.34 | 84.39 | 95.61 | 86.18 | 93.73 | 81.62 | 83.39 | 93.48 | 94.10 | 89.48 | |
| OK109 | 86.63 | 85.17 | 89.94 | 87.70 | 82.22 | 94.61 | 85.12 | 90.75 | 83.49 | 84.13 | 93.33 | 92.71 | 88.19 | |
| OK112 | 88.90 | 84.07 | 89.52 | 87.57 | 82.73 | 93.90 | 85.08 | 90.94 | 80.79 | 82.77 | 93.79 | 91.91 | 88.47 | |
| OK129 | 93.83 | 80.25 | 84.49 | 83.62 | 92.01 | 90.51 | 92.96 | 88.75 | 88.69 | 90.72 | 89.52 | 87.87 | 87.81 | |
Values above the diagonal are for nucleotide sequences, and those below the diagonal are for amino acid sequences.
TABLE 8.
Analysis of HP0535 (cagQ) gene divergence
| Strain | % Nucleotide and amino sequence identitya
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | |
| 26695 | 97.38 | 97.90 | 74.28 | 72.44 | 73.23 | 97.90 | 73.49 | 79.00 | 89.24 | 72.97 | 73.23 | 97.90 | 73.23 | |
| J99 | 96.85 | 97.90 | 74.80 | 72.44 | 73.23 | 98.43 | 73.49 | 77.69 | 90.03 | 72.97 | 73.23 | 97.90 | 73.23 | |
| 11638 | 97.64 | 97.64 | 75.07 | 72.70 | 73.49 | 98.43 | 73.75 | 77.95 | 90.03 | 73.23 | 73.49 | 100.00 | 73.49 | |
| F16 | 73.23 | 73.23 | 74.02 | 96.79 | 96.47 | 74.28 | 97.12 | 88.78 | 81.10 | 95.51 | 96.47 | 75.07 | 96.47 | |
| F17 | 70.87 | 71.65 | 71.65 | 96.15 | 98.37 | 72.44 | 94.87 | 89.87 | 79.27 | 97.39 | 98.37 | 72.70 | 97.71 | |
| F28 | 70.87 | 71.65 | 71.65 | 95.19 | 97.06 | 73.23 | 95.51 | 90.85 | 79.00 | 98.37 | 98.69 | 73.49 | 98.69 | |
| F32 | 97.64 | 99.21 | 98.43 | 72.44 | 70.87 | 70.87 | 73.49 | 78.48 | 89.24 | 72.97 | 73.23 | 98.43 | 73.23 | |
| F79 | 71.65 | 71.65 | 72.44 | 95.19 | 92.31 | 94.23 | 70.87 | 87.82 | 78.74 | 93.91 | 94.87 | 73.75 | 94.87 | |
| F80 | 77.95 | 75.59 | 75.59 | 84.62 | 85.29 | 85.29 | 76.38 | 82.69 | 72.70 | 90.52 | 90.85 | 77.95 | 90.85 | |
| OK101 | 85.83 | 86.61 | 86.61 | 81.10 | 78.74 | 77.95 | 85.83 | 77.17 | 69.29 | 78.22 | 79.53 | 90.03 | 79.00 | |
| OK107 | 70.08 | 70.87 | 70.87 | 94.23 | 96.08 | 97.06 | 70.08 | 91.35 | 84.31 | 77.17 | 97.71 | 73.23 | 97.71 | |
| OK109 | 72.44 | 73.23 | 73.23 | 97.12 | 97.06 | 98.04 | 72.44 | 94.23 | 87.25 | 79.53 | 97.06 | 73.49 | 99.02 | |
| OK112 | 97.64 | 97.64 | 100.00 | 74.02 | 71.65 | 71.65 | 98.43 | 72.44 | 75.59 | 86.61 | 70.87 | 73.23 | 73.49 | |
| OK129 | 71.65 | 72.44 | 72.44 | 96.15 | 96.08 | 97.06 | 71.65 | 93.27 | 86.27 | 78.74 | 96.08 | 99.02 | 72.44 | |
Values above the diagonal are for nucleotide sequences, and those below the diagonal are for amino acid sequences.
TABLE 9.
Analysis of HP0547 (cagA) gene divergence
| Strain | % Nucleotide and amino acid sequence identitya
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26695 | J99 | 11638 | F16 | F17 | F28 | F32 | F79 | F80 | OK101 | OK107 | OK109 | OK112 | OK129 | |
| 26695 | 90.77 | 92.12 | 86.15 | 81.70 | 83.76 | 86.11 | 92.83 | 87.74 | 86.00 | 86.70 | 86.72 | 94.50 | 85.88 | |
| J99 | 85.82 | 89.88 | 85.83 | 80.89 | 82.76 | 86.00 | 88.24 | 85.59 | 85.77 | 87.31 | 85.75 | 91.28 | 85.71 | |
| 11638 | 89.91 | 84.06 | 84.61 | 79.78 | 80.90 | 84.55 | 90.21 | 86.59 | 84.38 | 83.70 | 84.45 | 92.81 | 84.24 | |
| F16 | 78.51 | 78.17 | 77.78 | 89.63 | 91.88 | 97.79 | 84.93 | 87.69 | 97.19 | 83.85 | 96.10 | 85.14 | 97.24 | |
| F17 | 74.16 | 73.22 | 73.32 | 88.56 | 92.18 | 89.25 | 81.66 | 84.05 | 89.26 | 83.08 | 88.79 | 80.26 | 89.50 | |
| F28 | 75.84 | 75.44 | 74.02 | 90.15 | 88.63 | 91.72 | 84.74 | 87.76 | 91.88 | 84.80 | 91.33 | 82.22 | 91.98 | |
| F32 | 78.00 | 78.17 | 77.61 | 96.59 | 88.18 | 89.92 | 84.86 | 87.88 | 97.08 | 83.88 | 96.58 | 85.29 | 97.30 | |
| F79 | 89.77 | 82.64 | 87.77 | 77.75 | 73.01 | 76.40 | 77.18 | 90.83 | 84.63 | 88.59 | 85.08 | 92.23 | 84.34 | |
| F80 | 82.99 | 79.80 | 82.86 | 82.27 | 77.47 | 81.72 | 82.03 | 85.95 | 87.50 | 87.96 | 87.34 | 88.48 | 87.77 | |
| OK101 | 78.59 | 78.34 | 78.03 | 96.16 | 88.32 | 90.15 | 95.91 | 77.26 | 82.35 | 83.51 | 96.18 | 85.07 | 98.01 | |
| OK107 | 81.67 | 83.01 | 78.79 | 77.31 | 74.42 | 77.23 | 76.91 | 82.56 | 81.75 | 76.91 | 84.13 | 85.56 | 83.80 | |
| OK109 | 78.69 | 77.84 | 77.87 | 94.57 | 87.48 | 89.44 | 94.91 | 77.19 | 81.86 | 95.08 | 77.00 | 85.16 | 96.58 | |
| OK112 | 90.99 | 86.33 | 90.10 | 77.25 | 72.44 | 74.00 | 77.17 | 88.82 | 83.66 | 77.34 | 79.43 | 77.10 | 84.84 | |
| OK129 | 78.93 | 78.42 | 78.11 | 96.16 | 88.95 | 90.56 | 96.34 | 77.59 | 82.60 | 97.70 | 77.47 | 95.00 | 77.51 | |
Values above the diagonal are for nucleotide sequences, and those below the diagonal are for amino acid sequences.
Phylogenetic analysis.
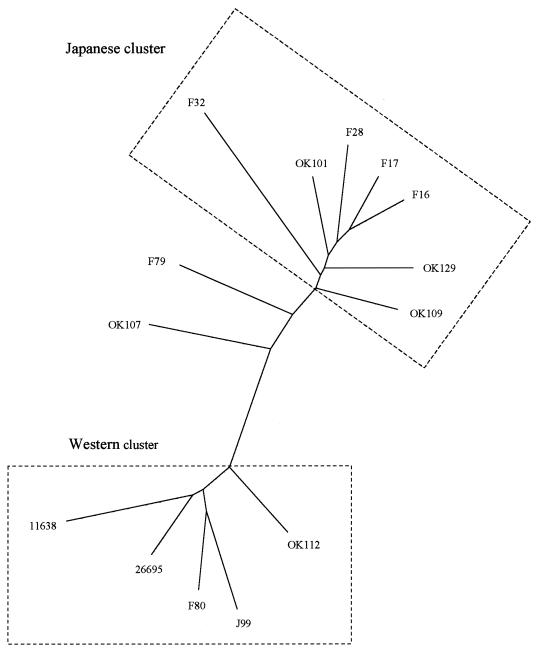
The phylogenetic tree of the full-length cag PAI demonstrated the genetic relationship among the 11 Japanese strains and 3 previously described strains from the West, strains 26695, J99, and NCTC11638 (Fig. 1). The cag PAI genes were divided into two major groups: a Western group and a Japanese group. Two strains, strains F79 and OK107, formed an intermediate type. Two Japanese strains, strains F80 and OK112, were included in the same cluster as the Western strains.
FIG. 1.
Phylogenetic tree of the full-length cag PAI gene. The cag PAI genes were divided into two major groups, a Western group and a Japanese group. Two strains, strains F79 and OK107, formed an intermediate type. Two Japanese strains, strains F80 and OK112, were included in the same cluster as the Western group.
A phylogenetic tree constructed on the basis of the cag PAI sequences was analyzed according to the vacA or cagA genotype. All strains with the s1c genotype in the vacA signal sequence, which is considered the characteristic genotype in East Asia, were in the Japanese cluster, as determined by cag PAI gene sequencing. In addition, all strains with the East Asian-type cagA genotype were also in the Japanese cluster. Two intermediate strains, strains F79 and OK107, and all strains in the Western cluster except strain F80 had the Western-type cagA genotype (strain F80 had a mixed-type CagA and a mixed Western and East Asian type).
The phylogenetic tree was analyzed according to the clinical features. Although statistical analysis could not be applied to the small number of patients (n = 11) evaluated in this study, patients infected with strains in the Japanese cluster tended to have high-grade inflammation and a high level of gastritis activity compared to those for patients infected with strains in the Western cluster. All patients infected with Japanese-cluster strains had severe gastric mucosal atrophy. In contrast, patients infected with Western-cluster strains had mild gastric mucosal atrophy (Table 10).
TABLE 10.
Relationship between histological features of gastritis and diversity of cag PAI
| Histological feature and location | Grade of histological feature
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Japanese-cluster strain
|
Intermediate strain
|
Western-cluster strain
|
|||||||||
| F16 | F17 | F28 | F32 | OK101 | OK109 | OK129 | F79 | OK107 | F80 | OK112 | |
| Inflammation | |||||||||||
| Antrum | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 3 |
| Body | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 1 | 1 | 1 |
| Activity | |||||||||||
| Antrum | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 2 |
| Body | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 |
| Atrophy | |||||||||||
| Antrum | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 |
| Body | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 |
CagA translocation, phosphorylation, and SHP-2 binding activity.
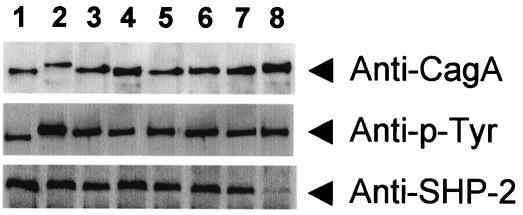
All strains in the Japanese cluster possessed CagA translocation and phosphorylation activities and bound to SHP-2. Strains in the Japanese cluster, based on the phylogenetic tree constructed by using the full-length cag PAI sequences (strains F16, F17, F28, F32, OK101, OK109, and OK129), had SHP-2 binding activities stronger than that of a Western-cluster strain, strain OK112 (Fig. 2).
FIG. 2.
Immunoblot analysis of AGS cells infected with H. pylori by use of anti-CagA, anti-phosphotyrosine (Anti-p-Tyr), and anti-SHP-2 antibodies. Although the band intensities for CagA phosphorylation were similar among the strains, the intensity of the CagA-SHP-2 complex was much higher for Japanese-cluster strains F16 (lane 1), F17 (lane 2), F28 (lane 3), F32 (lane 4), OK101 (lane 5), OK109 (lane 6), and OK129 (lane 7) on the basis of the entire cag PAI than for Western-cluster strain OK112 (lane 8).
DISCUSSION
H. pylori is believed to exhibit a large degree of genomic and allelic diversity. Strain-specific diversity has been proposed to be involved in the organism's ability to cause different diseases (4, 27, 30). The severity of H. pylori-related disease is correlated with the presence of the cag PAI. Infection with cag PAI-positive H. pylori is statistically associated with duodenal ulceration, gastric mucosal atrophy, and gastric cancer (7, 8, 10). The cag PAI contains genes constituting a type IV secretion system, as well as the cagA gene, which encodes the CagA protein. During the bacterium-gastric epithelial cell interaction, H. pylori injects CagA directly into the attached cells by means of the bacterial type IV secretion system. The translocated CagA protein undergoes tyrosine phosphorylation in the host cells via an Src family protein, tyrosine kinase (3, 21, 23, 24). It has recently been discovered (17) that translocated CagA forms a physical complex with SRC homology 2 domain-containing tyrosine phosphatase SHP-2 in a phosphorylation-dependent manner and stimulates the phosphatase activity. SHP-2 is known to play an important positive role in mitogenic signal transduction (14). Also, SHP-2 is actively involved in the regulation of spreading, migration, and adhesion of cells (15, 33). Deregulation of SHP-2 by CagA may induce abnormal proliferation and movement of gastric epithelial cells, a cellular condition that eventually leads to severe gastritis and gastric carcinoma. In Japan, nearly 100% of the strains possess CagA (19), and the incidence of atrophic gastritis and gastric cancer is quite high compared with that in Western countries (9). In the present study, we determined the complete cag PAI sequences of 11 representative Japanese strains from patients with different pathophysiological conditions in order to examine the association between the diversity of the genes in the cag PAI and the clinical outcomes. We demonstrated that the cag PAI genes were divided into two major groups, a Western and a Japanese cluster, based on the complete cag PAI sequences. Predominant Japanese strains formed a Japanese cluster which was different from the cluster formed by three previously reported Western strains: 26695 from a patient with gastritis in the United Kingdom, J99 from patient with duodenal ulcer in the United Sates, and NCTC11638 from a patient with duodenal ulcer in Australia. The lineage of H. pylori isolates infecting Japanese subjects may be different from that of isolates infecting subjects in the West, or a specific strain may have accumulated in the Japanese population. Our results were consistent with those of a recent report by Falush et al. (13). Their analysis of global H. pylori samples with the linkage model defined five ancestral populations (Africa 1, Africa 2, East Asia, Europe 1, and Europe 2) (13). The variable genetic structure of the cag PAI may influence the clinical outcome of H. pylori infection.
We also demonstrated that the diversity of the cag PAI is associated with the cagA genotype. The CagA protein is highly immunogenic and has a molecular mass of 128 to 140 kDa. The variation in the size of the protein has been correlated with the presence of a variable number of repeat sequences in the 3′ region of the gene (5, 10, 31, 32). The phosphorylation sites are located in the repeat region of CagA (6). Recently, we also discovered that the predominant CagA proteins isolated in East Asia have sequences, which we designated the “East Asian CagA-specific, SHP-2 binding sequence (ESS),” that are distinct from those of CagA proteins isolated in the West. This East Asian-specific sequence conferred stronger SHP-2 binding and transforming activities than the Western CagA sequence by in vitro transfection experiments with a human gastric cancer cell line, AGS (16). In this study, all strains in the Japanese cluster based on the complete cag PAI sequences had the East Asian-type cagA genotype and had stronger SHP-2 binding activities than Western-type CagA-positive strains. The potential of CagA to disturb host cell functions as a virulence factor could be determined by the degree of SHP-2 binding activity. The diversity of the CagA phosphorylation site, which collectively determines the affinity of binding of CagA to SHP-2, may be an important variable in determining the clinical outcome of infection by different H. pylori strains. Because SHP-2 plays an important role in both cell growth and motility, deregulation of SHP-2 by CagA may be involved in the induction of abnormal proliferation and movement of gastric epithelial cells, a cellular condition that eventually leads to gastritis and gastric cancer.
The diversity of cag PAI was also associated with the vacA genotype. All strains with s1c in the signal sequence for the vacA genotype, which is considered the characteristic genotype in East Asia (26), were in the Japanese cluster, as determined by cag PAI sequencing. Production of vacuolating cytotoxin is related to the mosaic structure of vacA. In general, type s1/m1 and s1/m2 strains produce high and moderate levels of toxin, respectively, whereas s2/m2 strains produce little or no toxin (4). It was previously reported (18) that cytotoxin activity is associated with the grade of atrophy. Although there is a genetic linkage between the cag PAI and vacA, there is a substantial genetic distance between the two genes on the bacterial genome. Thus, the selection of a vacA s1c/Japanese cag PAI-positive genotype may have a functional basis. In this study, H. pylori infection with the Japanese-cluster strain possessing the East Asian CagA and the vacA s1c genotype was associated with gastric mucosal atrophy.
In conclusion, our results indicate that cag PAI genes are classified into two major groups, a Western group and a Japanese group, and that the structural differences in the cag PAIs are substantially associated with the vacA s1c genotype and the East Asian cagA genotype. The distinct distribution of cag PAI diversity in Japan may be involved in the development of atrophic gastritis and may increase the risk of gastric cancer.
Acknowledgments
This work was supported by the Special Coordination Fund for Promoting Science and Technology, Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a grant-in-aid for Scientific Research (B) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 2.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. de Jonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Asahi, M., T. Azuma, S. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton, J. C., P. Cao, R. M. Peek, M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 5.Azuma, T., A. Yamakawa, S. Yamazaki, K. Fukuta, M. Ohtani, Y. Ito, M. Dojo, Y. Yamazaki, and M. Kuriyama. 2002. Correlation between variation of 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 186:1621-1630. [DOI] [PubMed] [Google Scholar]
- 6.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobcter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 8.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa, P. 1988. A human model of gastric carcinogenesis. Cancer Res. 48:3554-3560. [PubMed] [Google Scholar]
- 10.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 13.Falush, D., T. Wirth, B. Linz, J. K. Pritchard, M. Stephens, M. Kidd, M. J. Blaser, D. Y. Graham, S. Vacher, G. I. Perez-Perez, Y. Yamaoka, F. Megraud, K. Otto, U. Reichard, E. Katzowitsch, X. Wang, M. Achtman, and S. Suerbaum. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582-1585. [DOI] [PubMed] [Google Scholar]
- 14.Feng, G., C. Hui, and T. Pawson. 1993. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science 259:1607-1611. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, R., J. Plutzky, and B. G. Neel. 1992. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc. Natl. Acad. Sci. USA 89:11239-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashi, H., R. Tsutsumi, A. Fujita, S. Yamazaki, M. Asaka, T. Azuma, and M. Hatakeyama. 2002. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 99:14428-14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 18.Ito, S., T. Azuma, H. Murakita, M. Hirai, H. Miyaji, Y. Ito, Y. Ohtaki, Y. Yamazaki, M. Kuriyama, Y. Keida, and Y. Kohli. 1996. Profile of Helicobacter pylori cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. Gut 39:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, Y., T. Azuma, S. Ito, H. Miyaji, M. Hirai, Y. Yamazaki, F. Sato, T. Kato, Y. Kohli, and M. Kuriyama. 1997. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J. Clin. Microbiol. 35:1710-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall, B. J., C. S. Goodwin, J. R. Warren, R. Murray, E. D. Blincow, S. J. Blackbourn, M. Phillips, T. E. Waters, and C. R. Sanderson. 1988. Prospective double blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet ii:1437-1442. [DOI] [PubMed] [Google Scholar]
- 21.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 22.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:11127-11131. [DOI] [PubMed] [Google Scholar]
- 23.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 26.van Doorn, L. J., C. Figueiredo, F. Megraud, S. Pena, P. Midolo, D. M. Queiroz, F. Carneiro, B. Vanderborght, M. D. Pegado, R. Sanna, W. De Boer, P. M. Schneeberger, P. Correa, E. K. Ng, J. Atherton, M. J. Blaser, and W. G. Quint. 1999. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 116:823-830. [DOI] [PubMed] [Google Scholar]
- 27.van Doorn, L. J., C. Figueiredo, R. Sanna, S. Pena, P. Midolo, E. K. Ng, J. C. Atherton, M. J. Blaser, and W. G. Quint. 1998. Expanding allelic diversity of Helicobacter pylori vacA. J. Clin. Microbiol. 36:2597-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren, J. R., and B. J. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273-1275. [PubMed] [Google Scholar]
- 29.Wotherspoon, A. C., C. Ortiz-Hidalgo, M. R. Falzon, and P. G. Isaacson. 1991. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338:1175-1176. [DOI] [PubMed] [Google Scholar]
- 30.Xiang, Z., S. Censini, P. F. Bsyeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of cagA and vacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that cagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 63:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaoka, Y., H. M. T. El-Zimaity, O. Gutierrez, N. Figura, J. G. Kim, T. Kodama, K. Kashima, D. Y. Graham, and J. K. Kim. 1999. Relationship between the cagA 3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology 117:342-349. [DOI] [PubMed] [Google Scholar]
- 32.Yamaoka, Y., T. Kodama, K. Kashima, D. Y. Graham, and A. R. Sepulveda. 1998. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J. Clin. Microbiol. 36:2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, D., C. Qu, O. Henegariu, X. Lu, and G. Feng. 1998. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273:21125-21131. [DOI] [PubMed] [Google Scholar]