Abstract
The diagnosis of infections caused by Plasmodium species is critical for understanding the nature of malarial disease, treatment efficacy, malaria control, and public health. The demands of field-based epidemiological studies of malaria will require faster and more sensitive diagnostic methods as new antimalarial drugs and vaccines are explored. We have developed a multiplex PCR-ligase detection reaction (LDR) assay that allows the simultaneous diagnosis of infection by all four parasite species causing malaria in humans. This assay exhibits sensitivity and specificity equal to those of other PCR-based assays, identifying all four human malaria parasite species at levels of parasitemias equal to 1 parasitized erythrocyte/μl of blood. The multiplex PCR-LDR assay goes beyond other PCR-based assays by reducing technical procedures and by detecting intraindividual differences in species-specific levels of parasitemia. Application of the multiplex PCR-LDR assay will provide the sensitivity and specificity expected of PCR-based diagnostic assays and will contribute new insight regarding relationships between the human malaria parasite species and the human host in future epidemiological studies.
PCR diagnosis of malaria species infections exhibits sensitivity and specificity superior to those of blood smear microscopy, which is considered the “gold standard,” and antigen-capture rapid diagnostic tests (RDTs) (22, 32). Additional practical strengths of the PCR include the ability to evaluate samples that have been archived as whole blood or on microscope slides (25) under various conditions for years and compatibility with the widely used 96-well plate, automation-ready formats.
The superior sensitivity of the PCR has greatly expanded the capability to understand malaria parasite parasitism beyond the limits of blood smear microscopy. In clinical settings equipped with appropriate instrumentation, PCR-based diagnostic strategies enable Plasmodium species identification, despite parasitemias at levels below blood smear sensitivity limits. The results obtained by these more sensitive assays are often useful in making specific treatment decisions to kill species (Plasmodium vivax and P. ovale) that are capable of establishing dormant liver stages and subsequent malarial relapses. In many epidemiological studies, evidence of low-level infection consistently reported through PCR detection of subpatent infections suggests that the prevalence of malaria parasite infection is higher than that estimated by evaluation of blood smears (2, 3, 17, 20, 29, 33). Similar observations of this nature have suggested that infection by all four parasite species causing malaria in humans is not uncommon in some regions where malaria is endemic (20, 29) and that the prevalence of P. malariae and P. ovale is higher than that estimated previously (20, 29, 33). Because of its increased sensitivity, PCR diagnostic assays detect the persistence of chronic subpatent infection during seasons with low levels of malaria parasite transmission to reveal epidemiological patterns of infection different from those understood prior to the development and application of PCR-based malaria diagnostic strategies (1, 26, 34). Additional PCR-based diagnostic studies have reported that vertical transmission of Plasmodium species infections is common in regions where malaria is endemic (21, 30). Of further epidemiological significance, PCR has enabled wide-ranging investigations of antimalarial drug resistance polymorphisms (6, 18, 23) and polymorphic antigen-based strain diversity in the context of malaria vaccine trials (8, 16).
Despite the advantages of PCR, it is unlikely to be useful outside of well-equipped laboratories where a reliable source of electricity and expensive equipment are available. These limitations exclude PCR from consideration as a field-ready rapid diagnostic test for malaria (22, 32). Also, although many nucleic acid-based diagnostic tests (4, 10, 13, 24) are able to produce quantitative data that correspond to the levels of parasitemia, numerous factors influencing field sample collection, storage, and processing can make it difficult to determine the levels of parasitemia; present nucleic acid-based diagnostic tests do not differentiate developmental stages of the parasite's erythrocytic life cycle. Finally, as with all diagnostic techniques, PCR methods encounter limits of detection, based on the amount of sample that can be evaluated in a single reaction. Although concentration of erythrocytes or DNA can enhance diagnostic sensitivity, the limit of PCR-based detection of Plasmodium from whole blood is approximately 1 to 5 parasitized erythrocytes/5 × 106 erythrocytes/μl, which translates to a parasitemia level of 0.0001%.
Although many of these factors could limit the role that PCR diagnosis can play in clinical settings in most regions of the developing world where malaria is endemic, it is clear that PCR diagnosis of malaria will play an increasing role in epidemiology as the technology evolves and the scale of field studies grows. In the present study we have modified an earlier PCR-based diagnostic strategy through incorporation of a post-PCR, multiplex, species-specific ligase detection reaction (LDR). The multiplex assay significantly reduces technical manipulations and the time to results compared with those for other PCR-based approaches. Additionally, as the multiplex LDR screens each sample for all four Plasmodium species simultaneously, in contrast to methods that detect each species individually (19, 29), we were interested in evaluating the relative differences in LDR product intensity as a means of providing further insight into the relative species-specific parasite densities in individual samples.
MATERIALS AND METHODS
Blood sample collection and culturing of P. falciparum laboratory strains.
Blood samples were obtained from individuals who volunteered to participate in malaria prevalence surveys conducted in the Wosera (East Sepik Province, Papua New Guinea). Blood samples were collected in K+-EDTA-coated Vacutainer tubes and stored at −20°C until DNA extraction could be performed. Ethical approval for this study and the procedures used to retrieve oral informed consent were obtained from the Medical Research and Advisory Council of Papua New Guinea and from the Institutional Review Board for Human Investigation of Case Western Reserve University and the University Hospitals of Cleveland, Cleveland, Ohio. P. falciparum laboratory strains 3D7, HB3, FCR, Dd2, 7G8, 106/1, 3B-D5, Pf120, and ItG2 were provided by MR4, American Type Culture Collection (Manassas, Va.). P. vivax (SalI, Thai 3), P. malariae (Uganda I/CDC strain), and P. ovale (Nigeria I/CDC strain) strains were provided by W. E. Collins (Centers for Disease Control and Prevention, Atlanta, Ga.). P. falciparum ItG2 was propagated in vitro in human red blood cells (type O positive) by standard malaria parasite culturing techniques (31). Briefly, the culture medium (RPMI 1640 with 25 mM HEPES) was supplemented with 0.5% AlbuMax II (lipid-rich bovine serum albumin, Gibco-Invitrogen), 0.25% sodium bicarbonate, 0.01 mg of gentamicin per ml, and 0.05 mg of hypoxanthine per ml. Parasites were grown to a density of 2% (mixed developmental stage infection) in the presence of a 5% hematocrit before they were harvested by centrifugation at 800 × g in a Sorvall GLC-1 centrifuge for 5 min. All serial dilutions were performed with blood at 50% hematocrit in RPMI 1640.
Diagnosis by use of blood smears.
Thick and thin blood smears were stained with 4% Giemsa and examined by Papua New Guinea Institute of Medical Research-trained microscopists under oil immersion (magnification, ×100). Parasite species and species-specific densities were identified and recorded while the number of microscope fields containing 200 leukocytes was counted. On the basis of an average leukocyte count of 8,000/μl (7), the same blood volume would contain approximately 125,000 erythrocytes. If one parasitized erythrocyte were observed during this evaluation, the lower limit of parasitemia would be 0.001% (1/125,000; 40/5 × 106 erythrocytes/μl). Ten percent of the blood smear results were subjected to reanalysis by the senior expert microscopist to confirm the accuracy of the results. Additional validation to rule out translation errors between microscopists' tally sheets and the electronic database was performed for all samples with discordant results.
DNA extraction.
DNA was extracted from whole blood from study participants and parasite cultures (200 μl) by using QIAamp 96 spin blood kits and a QIAamp DNA blood mini kit (Qiagen, Valencia, Calif.), respectively.
PCR amplification.
Amplification of a small-subunit (SSU) rRNA gene fragment (491 to 500 bp) spanning the V7 and V8 regions (14) was performed with a single set of Plasmodium genus-specific primers: upstream primer 5′-TTC AGA TGT CAG AGG TGA AAT TCT-3′ and downstream primer 5′-AAT TAG CAG GTT AAG ATC TCG TTC-3′ (Integrated DNA Technologies, Coralville, Iowa). All amplification reactions were performed in the PCR mixtures described previously (20) with a PTC-225 thermocycler (MJ Research, Watertown, Mass.). Two-step PCR amplification conditions were 92°C for 2 min (1 cycle), 92°C for 30 s and 63°C for 2 min (35 cycles), and 63°C for 5 min (1 cycle). To evaluate the overall amplification efficiency, PCR products were electrophoresed on 2% agarose gels, stained with SYBR Gold (Molecular Probes, Eugene, Oreg.), and visualized on a Storm 860 fluorescence scanner with ImageQuant (version 5.2) software (Molecular Dynamics, Sunnyvale, Calif.).
SSOP hybridization (SSOPH) dot blot assay.
Plasmodium genus PCR products were prepared for dot blot analysis by heating 10 μl of the amplicon solution to 95°C for 2 min, followed by addition of an equal volume (10 μl) of 20× SSC (3.0 M NaCl plus 0.3 M sodium citrate [pH 7.0]). Two microliters of this solution was then spotted onto Hybond N+ filters (Amersham Pharmacia Biotech, Piscataway, N.J.). After the nylon filters were dried, they were bathed first in denaturing buffer (0.4 N NaOH) for 10 min and second in neutralizing buffer (1.8 M NaCl, 0.1 M NaH2PO4, 0.01 M EDTA) for 1 min. The filters were air dried and cross-linked by UV light exposure in a UV Stratalinker 2400 instrument (Stratagene, La Jolla, Calif.). Filters prepared in this manner were incubated in 10 ml of hybridization buffer (0.75 M NaCl, 0.075 M sodium citrate, 0.1% sodium dodecyl sulfate, 5% liquid block [Amersham Pharmacia Biotech], 30% dextran sulfate) in 50-ml conical tubes for 1 h before labeled sequence-specific oligonucleotide probes (SSOPs) were added. Species-specific SSOP sequences, oligonucleotide probe labeling, hybridization and high-stringency washing conditions, signal amplification, and probe detection have previously been described in detail (19).
Plasmodium species-specific post-PCR multiplex LDR.
The LDR analysis used to identify the P. falciparum, P. vivax, P. malariae, and P. ovale amplicons specifically was designed as follows. LDR probes consisted of five species-specific probes and two fluorescently labeled conserved-sequence probes (Table 1). The conserved-sequence probes were phosphorylated at the 5′ end and labeled with cyanine 5 (Cy5) at the 3′ end by the supplier (Integrated DNA Technologies). Each of the species-specific probes was synthesized to vary in length by 3 nucleotides (LDR product sizes were as follows: for P. falciparum (P. falciparum1 and P. falciparum2, 60 nucleotides; for P. vivax, 63 nucleotides; for P. malariae, 57 nucleotides; for P. ovale, 54 nucleotides) so that the LDR products would migrate to different positions following denaturing polyacrylamide gel electrophoresis.
TABLE 1.
SSU rRNA gene-specific LDR probes for differentiation of human malaria parasite species
| Probea | Probe sequenceb | Lengthc |
|---|---|---|
| Polymorphica | ||
| Pf1 | 5′-TGT AGC ATT TCT TAG GGA ATG TTG ATT TTA TAT-3′ | 33 |
| Pf2 | 5′-agg ccc ggc AAA AGT CAT CTT TCG AGG TGA CTT-3′ | 33 |
| Pv | 5′-gcc cgA AAA TAA GAA TTT TCT CTT CGG AGT TTA TTC-3′ | 36 |
| Pm | 5′-AAG AGA CAT TCT TAT ATA TGA GTG TTT CTT-3′ | 30 |
| Po | 5′-TAA GAA AAT TCC TTT CGG GGA AAT TTC-3′ | 27 |
| Conserved | ||
| Common1 | 5′-/Phos/TAG AAT TGC TTC CTT CAG TAC CTT ATG-3′ Cy5 | 27 |
| Common2 | 5′-/Phos/TTA GAT WGC TTC CTT CAG TRC CTT ATG-3′ Cy5 | 27 |
Polymorphic probe sequences are based on GenBank accession nos. M19173 for P. falciparum1 (Pf1), M19172 for P. falciparum2 (Pf2), U07367 for P. vivax (Pv), M54897 for P. malariae (Pm), and L48987 for P. ovale (Po). Conserved probe sequences are based on GenBank accession no. M19173 for Common1 and GenBank accession nos. M19172, U07367, M54897, and L48987 for Common2.
Nucleotides in lowercase are nonspecific and were added to the rRNA gene LDR probes to reach a desired specific length. Nucleotide codes W and R correspond to T or A and G or A degeneracy, respectively.
Length in nucleotides.
LDR was performed in a solution (15 μl) containing 20 mM Tris-HCl buffer (pH 7.6), 25 mM potassium acetate, 10 mM magnesium acetate, 1 mM NAD+, 10 mM dithiothreitol, 0.1% Triton X-100, 20 nM (300 fmol) each LDR probe, 1 μl of PCR product, and 2 U of Taq DNA ligase. The reaction mixtures were initially heated for 1 min at 95°C, followed by 32 thermal cycles of 95°C for 15 s (denaturation) and 58°C for 2 min (annealing and ligation). Prior to polyacrylamide gel electrophoresis, the LDR products were mixed with 5 μl of formamide loading buffer (0.05% [wt/vol] bromophenol blue, 20 mM EDTA) and denatured for 10 min at 95°C. The LDR products were separated by electrophoresis on 6% denaturing polyacrylamide gels (5.6 M urea, 32% formamide) run at a constant temperature (45°C) and voltage (1,900 V) and were visualized thereafter with a Storm 860 fluorescence scanner (Molecular Dynamics).
Statistical analysis.
The concordance between the LDR and SSOPH assays for the detection of Plasmodium species for 189 Papua New Guineans was performed by counting the number of tests that were in complete agreement divided by the total number of tests. Kappa scores were calculated for all 16 possible combinations of Plasmodium species infection (infections with single species, double species, triple species, and quadruple species and no infection). Counts for 100% agreement between LDR and SSOPH were multiplied by 1, and counts for partial agreement between LDR and SSOPH were multiplied by the appropriate fraction. For example, the counts were multiplied by 0.75 for a sample shown to be P. falciparum, P. vivax, and P. malariae positive and P. ovale negative by LDR but P. falciparum and P. vivax positive and P. malariae and P. ovale negative by SSOPH, as the test results were concordant for three (P. falciparum, P. vivax, and P. ovale) of the four species and discordant for one species (P. malariae). The counts were multiplied by 0.5 for a sample shown to be P. falciparum, P. vivax, P. malariae, and P. ovale positive by LDR but P. falciparum and P. vivax positive and P. malariae and P. ovale negative by SSOPH, as the test results were concordant for two species (P. falciparum and P. vivax). The counts were multiplied by 0.25 for a sample shown to be P. falciparum, P. vivax, P. malariae, and P. ovale positive by LDR but P. falciparum positive and P. vivax, P. malariae, and P. ovale negative by SSOPH, as the test results were concordant for one species (P. falciparum). The weighted kappa test analysis and sensitivity and specificity calculations for detection of each species by LDR compared to detection by SSOPH were performed with SAS software (version 8.2).
RESULTS
Bioinformatic development and optimization of the Plasmodium species-specific PCR-LDR diagnostic assay.
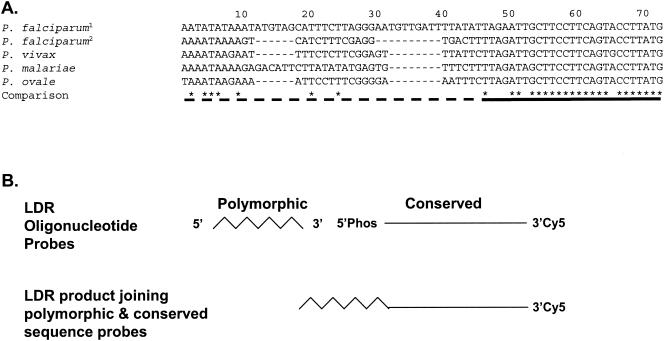
Initial surveys of the SSU rRNA gene sequence used previously to develop a P. falciparum, P. vivax, P. malariae, and P. ovale PCR-SSOPH diagnostic assay were performed with the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST) and the ClustalW program (http://clustalw.genome.ad.jp). Within this sequence, significant species-specific polymorphism (<20% identity [8 of 46 nucleotides]) was observed directly upstream of the conserved sequence (>80% identity [22 of 27 nucleotides]), providing target sites for LDR probe development (Fig. 1). Here species-specific LDR probes shared virtually no sequence similarity with each other that would promote nonspecific annealing. Polymorphisms downstream within the conserved LDR probe annealing sites were accommodated by synthesizing one LDR probe based on the P. falciparum (GenBank accession no. M19173) target sequence (Common1); a second degenerate LDR probe (Common2) was synthesized to accommodate polymorphisms at positions 7 (T or A = W) and 20 (A or G = R) within the P. malariae and P. vivax sequences, specifically. The species-specific oligonucleotide probes were synthesized to vary in length by 3 nucleotides (Table 1) and to hybridize to the target template immediately upstream from the Cy5-labeled conserved-sequence probes (Fig. 1). LDRs were therefore designed to join adjacent species-specific oligonucleotides of various lengths with fluorescently labeled, conserved-sequence oligonucleotides of identical lengths (Fig. 1B). The products formed during the LDR assay could be easily separated from one another by electrophoresis on standard denaturing polyacrylamide gels and visualized after fluorescence scanning. The LDR products for P. falciparum, P. vivax, P. malariae, and P. ovale were 60, 63, 57, and 54 nucleotides, respectively.
FIG. 1.
LDR probe target sites in the SSU rRNA genes of the four human Plasmodium parasite species. (A) Sequence alignments of rRNA gene-specific LDR probe target sites for P. falciparum1 (GenBank accession no. M19173; nucleotide coordinates 1176 to 1235), P. falciparum2 (GenBank accession no. M19172; nucleotide coordinates 1132 to 1182), P. vivax (GenBank accession no. U07367), P. malariae (GenBank accession no.M54897), and P. ovale (GenBank accession no. L48987) were performed with the ClustalW program (http://clustalw.genome.ad.jp). The identity (indicated by an asterisk) and nonidentity (indicated by an empty space) at each nucleotide position is reported below the alignment. A dash at a nucleotide position represents a deletion in comparison to the P. falciparum1 (GenBank accession no. M19173) sequence. The dashed line below the alignment identifies the region where the species- and sequence-specific LDR probes hybridize to target PCR amplicons; the solid line identifies the region where conserved-sequence-specific LDR probes hybridize. (B) Interaction of polymorphic and conserved-sequence probes to form LDR products. Phos, phosphorylated.
For optimization of the LDR signal intensity, cloned plasmid-based SSU rRNA gene sequences (20) and genomic DNA from laboratory-adapted P. falciparum strains and P. vivax, P. malariae, and P. ovale strains propagated in nonhuman primates were used. To produce LDR signal intensities of equal strengths from control reactions with the individual species, we found that it was necessary to include both P. falciparum1 and P. falciparum2 probe combinations (data not shown). Figure 2 shows the results of a control experiment in which a master mixture containing all seven LDR probes was used to interrogate species-specific polymorphisms in PCR products from reactions representing all possible combinations of the four human malaria parasite species. Although the master mixture contained probes capable of generating all four species-specific LDR products, the sequences of the individual reaction products were in complete concordance with the input target template sequence(s), and no extra, non-template-directed products were produced.
FIG. 2.
LDR products for all combinations of the four human Plasmodium parasite species: P. vivax (Pv), P. falciparum (Pf), P. malariae (Pm), and P. ovale (Po). In step one of the PCR-LDR assay, PCR products for all possible combinations of the four human malaria parasite species were amplified from cloned plasmid-based templates. In step two of the PCR-LDR assay, the PCR products were used as templates for the multiplex species-specific LDR. Input templates for each lane were as follows: lane 1, P. vivax and P. falciparum; lane 2, P. falciparum and P. malariae; lane 3, P. falciparum and P. ovale; lane 4, P. vivax and P. malariae; lane 5, P. vivax and P. ovale; lane 6, P. malariae and P. ovale; lane 7, P. vivax, P. falciparum, and P. malariae; lane 8, P. vivax, P. falciparum, and P. ovale; lane 9, P. falciparum, P. malariae, and P. ovale; lane 10, P. vivax, P. malariae, and P. ovale; lane 11, P. vivax, P. falciparum, P. malariae, and P. ovale; lane 12, P. falciparum; lane 13, P. vivax; lane 14, P. malariae; lane 15, P. ovale. The LDR products identified on the right were 63 nucleotides (P. vivax), 60 nucleotides (P. falciparum), 57 nucleotides (P. malariae), and 54 nucleotides (P. ovale).
Evaluating the quantitative limits of PCR-LDR detection of human Plasmodium species DNA.
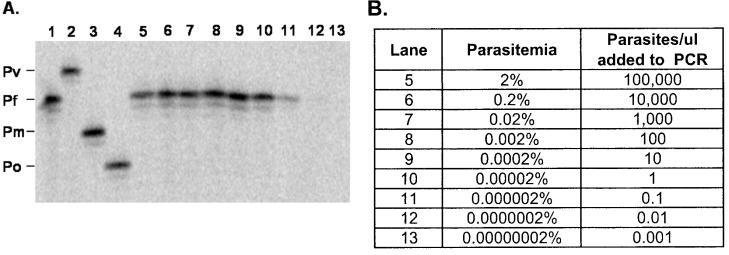
The first experiment to assess the quantitative limits of the PCR-LDR assay was performed with a 10-fold dilution series of an in vitro culture of P. falciparum (ItG2). Parasites were grown in vitro to a parasitemia of 2%. Tenfold dilutions were performed with 50% erythrocytes in RPMI 1640 (pH 7.4) to an end point of 0.00000002% (0.001 parasite/μl). Pipette tips were changed between each dilution point to avoid carryover of parasites between successive samples. Following DNA extraction of all dilution points, PCR-LDR analysis was performed with the complete multiplex LDR master mixture. Figure 3 shows that the LDR assay detected only P. falciparum and that visible P. falciparum LDR bands were observed to the 0.000002% (0.1 parasite/μl) dilution point; this experiment, initiated with different parasite cultures, was performed three times and produced identical results each time.
FIG. 3.
Sensitivity of LDR-based detection of P. falciparum following 10-fold serial dilution. (A) A genus-specific fragment of the SSU rRNA gene was amplified by PCR from serial dilutions of a P. falciparum culture following genomic DNA extraction. The combined PCR-LDR result following polyacrylamide gel (6%) electrophoresis shows that the assay's limit of detection is approximately 1 parasite/μl (lanes 10 and 11; see panel B). The controls in lanes 1 to 4 represent LDR products from P. falciparum (Pf), P. vivax (Pv), P. malariae (Pm), and P. ovale (Po), respectively. Lanes 5 to 13 contain LDR products from serial dilutions of P. falciparum (ItG2) cultures grown to a concentration of 2% (lane 5). (B) By using the parasitemia and 10-fold serial dilutions of the starting in vitro culture (column 2), the numbers of parasites (column 3) contributing to the LDR band intensities were estimated. Identical results were produced in three independent experiments.
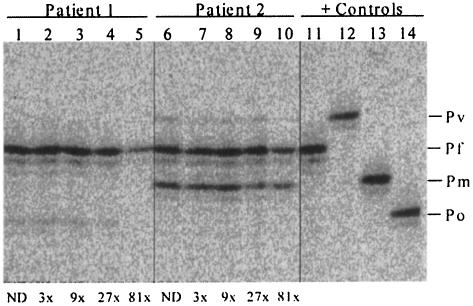
A second experiment was performed with two blood samples collected during malaria field studies in Papua New Guinea from individuals characterized by microscopy to be infected with different combinations of Plasmodium species. Blood smear analysis suggested that patient 1 was infected with P. falciparum (360 infected red blood cells [irbc]/μl; 0.01% P. falciparum parasitemia) and P. ovale (40 irbc/μl; 0.001% P. ovale parasitemia) and that patient 2 was infected with P. vivax (120 irbc/μl; 0.002% P. vivax parasitemia) P. falciparum (1,720 irbc/μl; 0.03% P. falciparum parasitemia), and P. malariae (1,040 irbc/μl; 0.02% P. malariae parasitemia). A series of threefold dilutions (no dilution and 3-, 9-, 27-, and 81-fold dilutions) of these samples were prepared to examine the relative sensitivity of detection of different Plasmodium species in individual patients. The results presented in Fig. 4 were comparable to those shown for P. falciparum alone (Fig. 3). P. ovale- and P. vivax-specific LDR bands were observed to become undetectable at the 81-fold dilution point for patients 1 and 2, respectively. Given that the blood smear results for P. ovale and P. vivax were approaching the limits of detection (0.001%) for these two patients, the sensitivity of the multiplex PCR-LDR assay was approximately 0.00001%. Further results appeared to suggest that as the relative quantities of parasite DNA fell to levels approximating 10 parasitized erythrocytes/μl, LDR band signal intensities were noticeably reduced (for patient 1, the P. falciparum-specific signal dropped at the 81-fold dilution point [360/81 = 4.5]); for patient 2 the P. falciparum-specific signal dropped at the 81-fold dilution point [1,720/81 = 21.2] and the P. malariae-specific signal dropped at the 27-fold dilution point [1,040/81 = 38.5]. Differences in band intensities above these levels were difficult to differentiate. Interestingly, the relative differences in the LDR band intensities within each sample were very similar to the differences in species-specific parasitemias determined by blood smear analysis.
FIG. 4.
Relative differences in Plasmodium species-specific LDR band intensities in individual infected patient samples. Comparison of LDR and blood smear parasitemia results for two infected Papua New Guinean study volunteers. Blood smear results for patient 1 (lanes 1 to 5) reported P. falciparum and P. ovale parasitemias of 360 and 40/μl, respectively. Blood smear results for patient 2 (lanes 6 to 10) reported P. vivax, P. falciparum, and P. malariae parasitemias of 120, 1,720, and 1,040/μl, respectively. LDR analysis of positive control samples: lane 11, P. falciparum (Pf); lane 12, P. vivax (Pv); lane 13, P. malariae (Pm); lane 14, P. ovale (Po). Dilution points are indicated below the panel for each patient (ND, no dilution) and correspond to 0.33 (dilution 3 times), 0.11 (dilution 9 times), 0.037 (dilution 27 times), and 0.0125 (dilution 81 times) of the original genomic DNA preparation. For a starting level of parasitemia in a blood smear of 0.001% (40 irbc/μl), this dilution series would detect parasite DNA to levels of 0.00033, 0.00011, 0.000037, and 0.0000125%, respectively.
Comparison of diagnosis of Plasmodium infection by PCR-LDR to that by PCR-SSOPH and conventional blood smear microscopy.
To compare the sensitivities and specificities of the two post-PCR diagnostic approaches, aliquots of the genus-specific PCR products were used to perform the species-specific SSOPH and multiplex LDR analyses. The results, summarized in Table 2, are divided into three categories: 100% concordant, discordant with the LDR assay showing greater sensitivity, and discordant with the SSOPH assay showing greater sensitivity. The overall concordance was 78.31% (148 of 189 samples) when the results of the SSOPH and LDR diagnostic assays were identical. For those samples for which discrepancies were observed (41 of 189 [21.69%]), the multiplex LDR assay appeared to be more sensitive than the SSOPH assay in 35 comparisons because it detected Plasmodium species that were not detected by the latter approach; in six comparisons the SSOPH assay detected P. ovale when LDR did not. The LDR analysis was performed twice to verify the results. The kappa score from a comparison of the LDR and SSOPH test results was 0.83 (standard error, 0.04; 95% confidence interval, 0.76 to 0.90, which indicates very good agreement between the tests). Finally, individual sensitivity and specificity analyses by comparison of LDR to SSOPH for the detection of P. falciparum (sensitivity, 1.0; specificity, 0.90; kappa score, 0.89), P. vivax (sensitivity, 1.0; specificity, 0.86; kappa score, 0.81), P. malariae (sensitivity, 1.0; specificity, 0.95; kappa score, 0.87), and P. ovale (sensitivity, 0.83; specificity, 0.96; kappa score, 0.79) consistently showed that LDR was more sensitive than the SSOPH assay.
TABLE 2.
Concordance between LDR and SSOPH for individual samples from patients from Papua New Guineaa
| Concordance | LDR | SSOPH | Countb |
|---|---|---|---|
| 100% concordant | F | F | 42 |
| V | V | 28 | |
| O | O | 2 | |
| FV | FV | 11 | |
| FM | FM | 1 | |
| FO | FO | 4 | |
| VO | VO | 2 | |
| MO | MO | 4 | |
| FVM | FVM | 2 | |
| FVO | FVO | 1 | |
| FMO | FMO | 8 | |
| VMO | VMO | 5 | |
| FVMO | FVMO | 4 | |
| NEG | NEG | 34 | |
| Discordant LDR has greater sensitivity | F | NEG | 3 |
| V | NEG | 5 | |
| M | NEG | 1 | |
| FV | F | 6 | |
| FV | V | 2 | |
| FM | F | 1 | |
| FO | F | 1 | |
| MO | O | 1 | |
| VO | NEG. | 1 | |
| FVM | FV | 1 | |
| FVM | M | 1 | |
| FVO | FV | 1 | |
| FMO | FO | 1 | |
| VMO | VM | 1 | |
| VMO | MO | 1 | |
| VMO | M | 1 | |
| FVMO | FVM | 1 | |
| FVMO | FVO | 1 | |
| FVMO | FMO | 1 | |
| FVMO | VMO | 2 | |
| FVMO | VO | 1 | |
| FVMO | O | 1 | |
| Discordant SSOPH has greater sensitivity | V | VO | 1 |
| F | FO | 1 | |
| FV | FVO | 2 | |
| FM | FMO | 2 |
A total of 189 samples were tested. Abbreviations: NEG, negative; F, P. falciparum; V, P. vivax; M, P. malariae; O, P. ovale.
Counts refers to the number of blood samples in each category (e.g., 42 samples were positive for P. falciparum by both LDR and SSOPH).
Comparison of the results of LDR and blood smear microscopy showed complete concordance for the diagnosis of malaria for 70 of the 189 samples analyzed (37.0%); 32 samples were observed to be not infected, and 35 samples were observed to be infected with a single Plasmodium species. As shown for two previously selected samples (Fig. 4), complete concordance between the compositions and the relative intensities of species-specific infection (blood smear parasite counts and LDR signal strength) was observed for three samples from the population surveyed. Partial concordance, in which LDR detected each of the Plasmodium species observed by microscopy plus one or more additional species, was observed for 32 samples (16.9%). For these samples, the levels of parasitemia and the LDR signal intensities were consistent for the Plasmodium species detected by both techniques; the species detected by LDR only were characterized by lower LDR signal strengths. LDR detected Plasmodium species in 78 samples in which microscopy did not (41.3%). Finally, the results of LDR and microscopy were discordant for nine samples (4.8%).
DISCUSSION
We have developed a new approach for performing a multiplex assay for the detection of the four human malaria parasite species. The strategy uses a post-PCR LDR that, in the present assembly, joins species- and length-specific oligonucleotide probes with Cy5-labeled conserved-sequence probes of identical lengths. Individual infections are then evaluated following polyacrylamide gel electrophoresis to separate products on the basis of the species-specific product length differences. The rRNA gene sequence polymorphisms interrogated in this assay have been used successfully to develop other assays for the detection of Plasmodium species (12, 19). The overall limits of detection of this multiplex PCR-LDR assay were shown to be comparable to those of other PCR-based diagnostic assays, as it can detect 1 parasitized erythrocyte/μl for P. falciparum. Possible explanations for PCR-LDR positivity of less than 1 parasitized erythrocyte/μl may include the presence of schizonts (which have an ≈10-fold greater genomic DNA content than parasites at earlier developmental stages) in the parasite culture, individual free merozoites, nondegraded free parasite DNA, or pipetting errors.
The multiplex PCR-LDR assay developed here, as with another recently developed nested PCR multiplex assay (27), reduces technical steps by avoiding four separate procedures to assess clinical samples for P. falciparum, P. vivax, P. malariae, and P. ovale infections. An advantage to the multiplex PCR-LDR assay may be observed by the consistent separation of regularly spaced LDR products that do not overlap other species-specific bands, and the assay simplifies the evaluation of samples for infections with multiple species. Alternative strategies incorporating four different fluorescent tags onto the upstream species-specific oligonucleotides (5, 11) would further simplify this assay by eliminating the need for gel electrophoresis and would thereby increase the speed of sample processing.
We have also observed that differences in LDR band intensities are easily distinguished in intraindividual infections. As the PCR primers anneal to sequences conserved among the four human malaria parasite species, the SSU rRNA gene targets should be amplified without bias toward any particular species. This suggests that differences in LDR band intensities may allow approximation of the relative abundance of Plasmodium species in a malaria parasite-infected patient. In five patients in this study (the results for two individuals are shown in Fig. 4, and the results for three individuals are included in Table 2), these relative signal intensity differences were observed to coincide with the species-specific differences in the levels of parasitemia in an infected individual determined by blood smear microscopy. For 32 additional samples these relative LDR signal intensity differences coincided with the single or multiple Plasmodium species shown to predominate according to the blood smear microscopy results, but LDR also produced lighter bands (compared to the band intensities for the microscopy-positive species) for one or more additional species that were not observed in the blood smear. As PCR-based diagnostic methods are consistently shown to be more sensitive than blood smear microscopy, these findings are not surprising. Our LDR results were therefore consistent with those of blood smear microscopy and suggest that species-specific LDR band signal intensities may reflect differences in the predominance of parasite species in individual infections. Observations of this nature could provide insight regarding competitive relationships among the human Plasmodium parasite species and the possibility that infections with mixed species may alter the manifestations of malarial disease (15, 28).
Although it has been shown that a number of nucleic acid-based diagnostic techniques can estimate the level of malaria parasite parasitemia (4, 10, 13, 24), it is important to acknowledge that numerous biological and technical factors can limit the ability to determine the levels of parasitemia of multiple species in individual patients with malaria, particularly as they apply to field studies. DNA sequence polymorphisms in PCR primer and LDR probe annealing sites can influence DNA base pairing and the efficiency of amplification or probe hybridization and, therefore, the intensities of the species-specific LDR band signal intensities. Developmental stage differences within and between species may also contribute to errors in the nucleic acid-based quantification of parasitemia. Gravenor et al. (9) have estimated that more than 70% of P. falciparum-infected erythrocytes can be sequestered away from the blood volume sampled for diagnosis. In contrast, schizonts of P. vivax, P. malariae, and P. ovale (which contain 10 to 20 merozoites) are not considered to sequester and may contribute to estimation of levels of parasitemia higher than those observed by microscopy. Technical factors influencing sample-to-sample variation during analysis of field samples may be encountered beginning at the time that the blood is collected from each finger prick and accumulates through differences in storage, shipping, and thawing. When blood is processed, two factors that can influence the amount of parasite genomic DNA extracted are commonly encountered. First, various amounts of blood may be collected from study participants; often, the blood volume collected is less than the 200 μl specified by Qiagen DNA extraction protocols. Second, various degrees of blood clotting are observed, despite the use of K+-EDTA-coated Vacutainer tubes well within the expiration date. Both factors influence the amount of parasite genomic DNA that is extracted from individual samples and that is ready to serve as the template in nucleic acid-based diagnostic assays. As the amount of DNA extracted from individual samples would be influenced by these factors, the harvesting of equivalent amounts of DNA from all samples may not be possible and attempts to compare the levels of parasitemia between samples would be compromised. Therefore, despite procedures that enable quantitative comparisons with known amounts of a reference sequence standard or the use of real-time PCR machines that mark the kinetics of target sequence amplification, numerous factors make the consistent determination of levels of parasitemia in nucleic acid-based malaria diagnostic assays from samples collected in the field problematic.
Within-sample comparisons are susceptible to many of the same biological and technical problems identified above. However, as there should be no bias toward the extraction of DNA from any one of the human malaria parasite species, the use of within-sample comparisons may allow the sample-to-sample differences caused by underlying differences in DNA extraction efficiency to be avoided. While the PCR-LDR results presented here have shown relative differences in the levels of infection in individual samples, caution should be exercised, as the signal intensity appears to plateau when the level of parasitemia approaches 100 parasitized erythrocytes/μl. In future studies it may be useful to incorporate reference standard target sequences to optimize PCR and LDR cycle numbers and assist in further evaluation of the LDR signal intensity differences observed.
Finally, as acknowledged in previous studies (18, 19), we observed discordant results, in that the PCR-LDR assay did not detect parasites identified by microscopy in nine samples. False negativity of this nature has previously been attributed to DNA degradation, the presence of sufficient quantities of PCR inhibitors in the genomic DNA sample, or DNA polymorphism in the targeted DNA template (18). The discordance between the results of LDR and SSOPH for six samples may be the result of variability between assays when the quantity of DNA approaches the limits of detection.
In conclusion, the multiplex PCR-LDR detection assay for the four human Plasmodium parasite species provides important advantages for large-scale malaria diagnosis in epidemiological studies. The procedures are robust and easily adapted to routine use in the context of well-equipped laboratories where a reliable source of electricity and equipment for DNA extraction, PCR, and polyacrylamide gel electrophoresis are available. The strategies described here are also easily adapted to the detection of other pathogens characterized by species- and or strain-specific DNA sequence polymorphisms. Finally, by expanding the PCR-LDR sequences targeted, these multiplex assay strategies should be able to diagnose simultaneous infections caused by coendemic protozoan, viral, or helminthic pathogens that are present in a blood sample and that may produce similar, nonspecific clinical symptoms (32).
Acknowledgments
We thank Forrest O. Gulden, Rajeev Mehlotra, Shannon Bruse, and Hisashi Fujioka for technical assistance and advice during the course of this study and Allen Hightower for assistance with kappa score, sensitivity, and specificity calculations. We thank the Wosera community for their willing participation in our ongoing malaria field studies; Kerry Lorry for malaria microscopy; and Benson Kiniboro, Will Kastens, and Moses Bockarie for conducting and supervising the malaria field studies in Papua New Guinea. We also thank James Kazura for helpful comments and criticisms during the preparation of the manuscript.
This study was supported by grants from the National Institutes of Health (grants AI-46919-01A2 and AI-523213-01).
REFERENCES
- 1.Babiker, H. A., A. M. Abdel-Muhsin, L. C. Ranford-Cartwright, G. Satti, and D. Walliker. 1998. Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern Sudan where malaria transmission is markedly seasonal. Am. J. Trop. Med. Hyg. 59:582-590. [DOI] [PubMed] [Google Scholar]
- 2.Barker, R. H., Jr., T. Banchongaksorn, J. M. Courval, W. Suwonkerd, K. Rimwungtragoon, and D. F. Wirth. 1994. Plasmodium falciparum and P. vivax: factors affecting sensitivity and specificity of PCR-based diagnosis of malaria. Exp. Parasitol. 79:41-49. [DOI] [PubMed] [Google Scholar]
- 3.Bottius, E., A. Guanzirolli, J. F. Trape, C. Rogier, L. Konate, and P. Druilhe. 1996. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 90:15-19. [DOI] [PubMed] [Google Scholar]
- 4.Cheesman, S. J., J. C. de Roode, A. F. Read, and R. Carter. 2003. Real-time quantitative PCR for analysis of genetically mixed infections of malaria parasites: technique validation and applications. Mol. Biochem. Parasitol. 131:83-91. [DOI] [PubMed] [Google Scholar]
- 5.Day, D. J., P. W. Speiser, P. C. White, and F. Barany. 1995. Detection of steroid 21-hydroxylase alleles using gene-specific PCR and a multiplexed ligation detection reaction. Genomics 29:152-162. [DOI] [PubMed] [Google Scholar]
- 6.Djimde, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourte, A. Dicko, X. Z. Su, T. Nomura, D. A. Fidock, T. E. Wellems, C. V. Plowe, and D. Coulibaly. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 7.Genton, B., F. al-Yaman, H. P. Beck, J. Hii, S. Mellor, A. Narara, N. Gibson, T. Smith, and M. P. Alpers. 1995. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Ann. Trop. Med. Parasitol. 89:359-376. [DOI] [PubMed] [Google Scholar]
- 8.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 9.Gravenor, M. B., A. L. Lloyd, P. G. Kremsner, M. A. Missinou, M. English, K. Marsh, and D. Kwiatkowski. 2002. A model for estimating total parasite load in falciparum malaria patients. J. Theor. Biol. 217:137-148. [DOI] [PubMed] [Google Scholar]
- 10.Hermsen, C. C., D. S. Telgt, E. H. Linders, L. A. van de Locht, W. M. Eling, E. J. Mensink, and R. W. Sauerwein. 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol. Biochem. Parasitol. 118:247-251. [DOI] [PubMed] [Google Scholar]
- 11.Iannone, M. A., J. D. Taylor, J. Chen, M. S. Li, P. Rivers, K. A. Slentz-Kesler, and M. P. Weiner. 2000. Multiplexed single nucleotide polymorphism genotyping by oligonucleotide ligation and flow cytometry. Cytometry 39:131-140. [PubMed] [Google Scholar]
- 12.Kimura, M., O. Kaneko, Q. Liu, M. Zhou, F. Kawamoto, Y. Wataya, S. Otani, Y. Yamaguchi, and K. Tanabe. 1997. Identification of the four species of human malaria parasites by nested PCR that targets variant sequences in the small subunit rRNA gene. Parasitol. Int. 46:91-95. [Google Scholar]
- 13.Lee, M. A., C. H. Tan, L. T. Aw, C. S. Tang, M. Singh, S. H. Lee, H. P. Chia, and E. P. Yap. 2002. Real-time fluorescence-based PCR for detection of malaria parasites. J. Clin. Microbiol. 40:4343-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, J., R. A. Wirtz, G. A. McConkey, J. Sattabongkot, A. P. Waters, M. J. Rogers, and T. F. McCutchan. 1995. Plasmodium: genus-conserved primers for species identification and quantitation. Exp. Parasitol. 81:182-190. [DOI] [PubMed] [Google Scholar]
- 15.Luxemburger, C., F. Ricci, F. Nosten, D. Raimond, S. Bathet, and N. J. White. 1997. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans. R. Soc. Trop. Med. Hyg. 91:256-262. [DOI] [PubMed] [Google Scholar]
- 16.Masinde, G. L., D. J. Krogstad, D. M. Gordon, and P. E. Duffy. 1998. Immunization with SPf66 and subsequent infection with homologous and heterologous Plasmodium falciparum parasites. Am. J. Trop. Med. Hyg. 59:600-605. [DOI] [PubMed] [Google Scholar]
- 17.May, J., F. P. Mockenhaupt, O. G. Ademowo, A. G. Falusi, P. E. Olumese, U. Bienzle, and C. G. Meyer. 1999. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am. J. Trop. Med. Hyg. 61:339-343. [DOI] [PubMed] [Google Scholar]
- 18.Mehlotra, R. K., H. Fujioka, P. D. Roepe, O. Janneh, L. M. Ursos, V. Jacobs-Lorena, D. T. McNamara, M. J. Bockarie, J. W. Kazura, D. E. Kyle, D. A. Fidock, and P. A. Zimmerman. 2001. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. USA 98:12689-12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehlotra, R. K., L. J. Kasehagen, M. Baisor, K. Lorry, J. W. Kazura, M. J. Bockarie, and P. A. Zimmerman. 2002. Malaria infections are randomly distributed in diverse holoendemic areas of Papua New Guinea. Am. J. Trop. Med. Hyg. 67:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehlotra, R. K., K. Lorry, W. Kastens, S. M. Miller, M. P. Alpers, M. Bockarie, J. W. Kazura, and P. A. Zimmerman. 2000. Random distribution of mixed species malaria infections in Papua New Guinea. Am. J. Trop. Med. Hyg. 62:225-231. [DOI] [PubMed] [Google Scholar]
- 21.Mockenhaupt, F. P., U. Ulmen, C. von Gaertner, G. Bedu-Addo, and U. Bienzle. 2002. Diagnosis of placental malaria. J. Clin. Microbiol. 40:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plowe, C. V., A. Djimde, M. Bouare, O. Doumbo, and T. E. Wellems. 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 52:565-568. [DOI] [PubMed] [Google Scholar]
- 24.Polanco, J. C., J. A. Rodriguez, V. Corredor, and M. A. Patarroyo. 2002. Plasmodium vivax: parasitemia determination by real-time quantitative PCR in Aotus monkeys. Exp. Parasitol. 100:131-134. [DOI] [PubMed] [Google Scholar]
- 25.Reeder, J. C., K. H. Rieckmann, B. Genton, K. Lorry, B. Wines, and A. F. Cowman. 1996. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am. J. Trop. Med. Hyg. 55:209-213. [DOI] [PubMed] [Google Scholar]
- 26.Roper, C., W. Richardson, I. M. Elhassan, H. Giha, L. Hviid, G. M. Satti, T. G. Theander, and D. E. Arnot. 1998. Seasonal changes in the Plasmodium falciparum population in individuals and their relationship to clinical malaria: a longitudinal study in a Sudanese village. Parasitology 116(Pt 6):501-510. [DOI] [PubMed] [Google Scholar]
- 27.Rubio, J. M., R. J. Post, W. M. van Leeuwen, M. C. Henry, G. Lindergard, and M. Hommel. 2002. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S199-S204. [DOI] [PubMed] [Google Scholar]
- 28.Smith, T., B. Genton, K. Baea, N. Gibson, A. Narara, and M. P. Alpers. 2001. Prospective risk of morbidity in relation to malaria infection in an area of high endemicity of multiple species of Plasmodium. Am. J. Trop. Med. Hyg. 64:262-267. [DOI] [PubMed] [Google Scholar]
- 29.Snounou, G., S. Viriyakosol, W. Jarra, S. Thaithong, and K. N. Brown. 1993. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283-292. [DOI] [PubMed] [Google Scholar]
- 30.Tobian, A. A., R. K. Mehlotra, I. Malhotra, A. Wamachi, P. Mungai, D. Koech, J. Ouma, P. Zimmerman, and C. L. King. 2000. Frequent umbilical cord-blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J. Infect. Dis. 182:558-563. [DOI] [PubMed] [Google Scholar]
- 31.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2000. New perspectives in malaria diagnosis. Report WHO/MAL/2000.1091. World Health Organization, Geneva, Switzerland.
- 33.Zhou, M., Q. Liu, C. Wongsrichanalai, W. Suwonkerd, K. Panart, S. Prajakwong, A. Pensiri, M. Kimura, H. Matsuoka, M. U. Ferreira, S. Isomura, and F. Kawamoto. 1998. High prevalence of Plasmodium malariae and Plasmodium ovale in malaria patients along the Thai-Myanmar border, as revealed by acridine orange staining and PCR-based diagnoses. Trop. Med. Int. Health 3:304-312. [DOI] [PubMed] [Google Scholar]
- 34.Zwetyenga, J., C. Rogier, A. Spiegel, D. Fontenille, J. F. Trape, and O. Mercereau-Puijalon. 1999. A cohort study of Plasmodium falciparum diversity during the dry season in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Trans. R. Soc. Trop. Med. Hyg. 93:375-380. [DOI] [PubMed] [Google Scholar]