Abstract
Pseudomonas aeruginosa is the major pathogenic bacteria in cystic fibrosis and other forms of bronchiectasis. Growth in antibiotic resistant biofilms contributes to the virulence of this organism. Sodium nitrite has antimicrobial properties and has been tolerated as a nebulized compound at high concentrations in human subjects with pulmonary hypertension; however, its effects have not been evaluated on biotic biofilms or in combination with other clinically useful antibiotics. We grew P. aeruginosa on the apical surface of primary human airway epithelial cells to test the efficacy of sodium nitrite against biotic biofilms. Nitrite alone prevented 99% of biofilm growth. We then identified significant cooperative interactions between nitrite and polymyxins. For P. aeruginosa growing on primary CF airway cells, combining nitrite and colistimethate resulted in an additional log of bacterial inhibition compared to treating with either agent alone. Nitrite and colistimethate additively inhibited oxygen consumption by P. aeruginosa. Surprisingly, while the antimicrobial effects of nitrite in planktonic, aerated cultures are nitric oxide (NO) dependent, antimicrobial effects in other growth conditions are not. The inhibitory effect of nitrite on bacterial oxygen consumption and biofilm growth did not require NO as an intermediate as chemically scavenging NO did not block growth inhibition. These data suggest an NO-radical independent nitrosative or oxidative inhibition of respiration. The combination of nebulized sodium nitrite and colistimethate may provide a novel therapy for chronic P. aeruginosa airway infections, because sodium nitrite, unlike other antibiotic respiratory chain ‘poisons’, can be safely nebulized at high concentration in humans.
Keywords: Pseudomonas aeruginosa, biofilm, sodium nitrite, colistimethate, colistin, polymyxin
Introduction
In cystic fibrosis (CF), chronic airway infection results in bronchiectasis and cycles of airway inflammation that ultimately lead to early death from respiratory failure. Pseudomonas aeruginosa is the most common pathogen in teenagers and adults with CF. Once chronic airway infection is established, P. aeruginosa becomes very difficult to eradicate because of multiple resistance mechanisms including intrinsic antibiotic tolerance, selection of antibiotic-resistant subpopulations by decades of antibiotic exposure, and bacterial growth in biofilms (1-3).
The high metabolic activity of P. aeruginosa and neutrophils in and around mucus plaques depletes oxygen at the airway surface, such that biofilm growth is largely anaerobic, with bacteria subsisting through denitrification (4). Ex vivo evidence of denitrification in the airway was recently established by detecting nitrous oxide, a unique product of bacterial denitrification, in sputum samples from patients with CF (5). Anaerobic growth confers resistance to many antibiotics that clinicians commonly use against P. aeruginosa, in part through alterations in efflux pump expression (1, 6). Further complicating the search for new antimicrobial approaches to P. aeruginosa is the difference in behavior of biofilms grown in the presence of airway cells compared to those grown on abiotic surfaces (glass or plastic). The former, known as “biotic” biofilms, can be >100-fold more resistant to antibiotics than predicted by conventional susceptibility testing. This makes extrapolation of data derived from planktonic experiments difficult to translate to performance against biotic biofilms and limits our understanding of the in vivo activity of existing drugs (2).
Sodium nitrite has long been known to have antimicrobial properties as a food preservative. Nitrite may also contribute to host defense against Helicobacter pylori and Clostridium difficile (7, 8). The antimicrobial action of the nitrite moiety is due in part through generation of NO, inactivation of Fe-S containing proteins, and inhibition of bacterial respiration (9-11). Within the lung, inhaled nitrite salts are converted to NO through reductive reactions with heme- and molybdenum-containing enzymes such as hemoglobin and xanthine oxidoreductase, and potentially through bacterial metabolism. NO has a half-life of milliseconds in vivo (reviewed in (12,13)). There are ongoing attempts to adapt inhaled NO as an antimicrobial agent, but the short half-life makes delivery cumbersome (14,15). Nitrite has a half-life of 50-60 minutes in vivo when delivered intravenously, allowing intermittent dosing (16). An ongoing Phase 2b clinical trial is currently evaluating the safety and efficacy of nebulized sodium nitrite in pulmonary hypertension; inhaled nitrite has thus far been well tolerated at concentrations near 1 molar (ClinicalTrials.gov locator NCT01431313, M. Gladwin personal communication). The availability of pharmaceutical-grade sodium nitrite for inhalation improves the feasibility of using nitrite as an antimicrobial agent in CF airway infection.
Previous work has shown that sodium nitrite has pH-dependent antimicrobial activity against P. aeruginosa, with activity at pH 6.5 being best studied (hereafter “nitrite” refers to sodium nitrite in solution at pH 6.5)(17). At micromolar concentrations, nitrite is a potential substrate for anaerobic energy generation through denitrification, however at millimolar concentrations it inhibits anaerobic growth (11,18). Beyond direct bactericidal effects, nitrosative stress also affects virulence of P. aeruginosa by inactivating pyocyanin and increasing production of alginate (19, 20). Additionally, nitrite has antimicrobial activity against a wide variety of other pathogens found in CF, including Burkholderia cepacia complex and Staphylococcus aureus (21, 22). When nitric oxide reductase activity is lost, nitrite becomes growth inhibitory in P. stutzeri (23). Whether nitrite itself or the NO produced from nitrite can prevent biotic biofilm growth is unknown. The interactions between nitrite and commonly used antibiotics are also poorly understood. The goals of this study were to determine the effect of sodium nitrite on P. aeruginosa biotic biofilms and screen nitrite for interactions with other commonly used antibiotics in the CF population. We focused primarily on colistin because previous work has shown that it targets the inner core of highly structured abiotic biofilms where the oxygen tension is lowest, suggesting activity against anaerobically growing P. aeruginosa (24, 25). The effect of polymyxins on P. aeruginosa clinical isolates grown under anaerobic conditions has not been extensively studied but available data suggest decreased susceptibility when compared to aerobic growth (6).
Polymyxins are polycationic lipopeptide antibiotics that interact with negatively charged lipopolysaccharide at the outer membrane of Gram-negative bacteria. Polymyxins initially increase outer membrane permeability and, after diffusing across the periplasmic space, disrupt the inner (cytoplasmic) membrane. Bacterial death ensues within minutes (26). Colistimethate is a prodrug of colistin (polymyxin E) that can be administered by inhalation for the treatment of chronic airway infections in both CF and non-CF bronchiectasis (27). In 2005, 9% of CF patients in the United States routinely inhaled an aerosol form of intravenous colistimethate for the treatment of chronic airway infection, and as of 2012, approximately 11% of adult CF patients were doing so (28, 29). A dry powder colistimethate inhaler approved to treat CF airway infection in Europe is designed to make delivery more convenient (30).
The primary objective of this study was to investigate the role of nitrite in preventing biotic biofilm growth and the potential interactions between nitrite and colistimethate. We tested the hypotheses (1) that the inhibition of biofilm growth by nitrite is NO-independent and (2) that the cooperative interaction between nitrite and colistimethate is due to NO independent respiratory inhibition.
Materials and Methods
Bacterial strains
The following bacterial strains were studied: Pseudomonas aeruginosa strains PA14 and PAO1 (gift of George O'Toole, Geisel School of Medicine at Dartmouth) (31), ten “late” P. aeruginosa clinical isolates from the University of Washington collection previously published in (32), 8 previously published Achromobacter sp. isolates and ten Burkholderia sp. isolates from the Cystic Fibrosis Foundation Burkholderia cepacia Research Laboratory and Repository at the University of Michigan (33) and colistin resistant P. aeruginosa isolates (described in (34)).
Reagents
Colistin sulfate, sodium nitrite, hydrogen peroxide, potassium cyanide, sodium azide, paraquat, Luria broth (LB), Luria broth agar, polymyxin B sulfate and polymyxin b nonapeptide were all obtained from Sigma (St. Louis MO), Other reagents obtained as follows: colistimethate (XGen pharmaceuticals, Lot A72649 and A99296, Big Flats NY), Carboxy-PTIO (Cayman Chemicals, Ann Arbor MI).
Bacterial Growth Methods
5ml bacterial cultures were grown on a roller drum at 100 rpm for 16-18 hours prior to the start of the experiment in LB. Unless otherwise indicated, bacteria were “returned to log phase” by diluting overnight cultures 1:100 in fresh LB and placing cultures on a roller drum at 37°C for 2 hours. During experiments combining colistimethate, colistin sulfate, azide, cyanide and CPTIO, 200μl of “log phase” cultures were grown for additional 5.5 hours in a 96 well plate at 37°C on a nutating rocker.
Biotic Biofilm Imaging
To image epithelial-bacterial co-cultures, live-cell imaging was used as described in (31). Briefly, we used a FCS2 closed system, live-cell chamber from Bioptechs (Butler, PA). The immortalized human bronchial cell line (CFBE41o-) derived from a ΔF508/ΔF508 patient with stable expression of wild-type CFTR was used (gift from Bruce Stanton, referred to as CFBE-wt). Biofilms of GFP expressing P. aeruginosa strain PAOl were grown on CFBE-wt cells seeded onto glass coverslips in a flow chamber. Bacteria were inoculated at approximately 25 MOI and allowed to attach to airway epithelial cells for two hours without media flowing. Biofilms were then grown for four hours with minimum essential media (MEM) flowing at 20 ml/hr. After a total of six hours, z-stack images were taken of 6-10 random fields from each chamber using a Nikon Ti-inverted microscope. Biofilm biomass was measured with COMSTAT image analysis software(35). Nitrite treated chambers were exposed to 15mM sodium nitrite in pH 6.5 minimal essential medium for 4 hours following bacterial attachment, while control chambers were exposed to pH 6.5 MEM alone. The experiment was done in triplicate. More detail for this protocol can be found in the descriptions by Moreau-Marquis (31, 36).
Liquid Culture Experiments
For time-kill assays to measure bactericidal activity of polymyxins with other compounds, overnight cultures were diluted in pH 6.5 LB, returned to log phase growth at 37°C, and then treated with nitrite and/or polymyxins and grown for an additional 5.5 hours. The number of live bacteria was then determined by plating in a colony forming unit assay (CFU assay). For nitrite pretreatment assays: bacteria were grown as above in 15mM nitrite and after 5.5 hours the cultures were pelleted at 6000×g for 5 min and re-suspended in media containing colistimethate. The cultures were then incubated for 60 minutes and bacteria were counted in a CFU assay.
Co-culture experiments
Conducted as previously described (31) with the following modifications:. Primary human bronchial epithelial cells (HBE) were cultured from explanted lungs of patients with CF, under an Institutional Review Board approved protocol at the University of Pittsburgh (PRO11070367 and IRB970946). Cells were enzymatically dissociated, expanded in growth media, and seeded onto Transwell inserts at air-liquid interface. Cultures were used when well polarized and differentiated (37). For other experiments, the cell line CFBE-wt stably expressing wild-type CFTR were used as described (31). CFBE-wt cells were seeded onto Transwell filters, grown at air-liquid interface, and used 7-10 days after seeding. Both cell lines were used in P. aeruginosa – epithelial co-culture experiments as follows (described in detail in (31)). Biofilm prevention assays were done as follows: overnight cultures of PAO1 were rinsed once and added to the apical surface of the epithelial cells in 500μl of minimal essential media with glutamate at an MOI of 25. After the 60 minute bacterial attachment period, developing biofilms were treated with 300 mM sodium nitrite, 20μg/ml colistimethate, both agents or 300mM sodium chloride as a tonicity control for the subsequent 5 hours. At the end of this period, biofilms were removed with 0.1% Triton-X 100 and live bacteria were plated in a colony forming unit assay.
Agar Dilution MIC under aerobic and anaerobic conditions
Agar dilution minimum inhibitory concentration (MIC) experiments were used to screen multiple clinical isolates for nitrite/polymyxin cooperativity. The MIC protocol by Wiegand et al. was used with the following modifications (38). Checkerboards included two-fold dilutions of colistin sulfate from 0.125μg/ml to 16μg/ml. Nitrite concentrations from 0.5mM to 32mM were used. The pH of molten agar was adjusted to pH 6.5 with HC1. The agar was cooled to 50°C and increasing concentrations of colistin sulfate and/or nitrite were added. Plates were poured and used immediately. For aerobic experiments, plates were read 18 -20 hours later. For highly colistin resistant isolates, plates were grown for 48-72 hours because control growth of these organisms is very slow. To determine anaerobic MICs for Pseudomonas aeruginosa clinical isolates, MHB plates were made as described above with the addition of 1% potassium nitrate (Sigma). An anaerobic environment was created using GasPak EZ Anaerobe Container System and BBL Dry Anaerobic Indicator Strips (BD Franklin Lakes, NJ) were used for every experiment to assure an anaerobic environment. Plates were incubated for 2 days for anaerobic MIC determinations.
Oxygen Consumption measurements
Bacteria were diluted in LB and treated with nitrite and/or colistimethate for 30 min at 37°C. Oxygen consumption was measured with a Clark-type Instech Oxygen Electrode, model #125-05 with a YSE Biological Oxygen Monitor, model 5300A and calculated from the slope. Measurements were done in triplicate on different days and data are displayed as % control oxygen consumption with all points from each day normalized to the control oxygen consumption from that day. Representative tracings show the raw data. The tracing have been overlayed for easier visual comparison.
Cytotoxicity Assays
Lactate dehydrogenase release assays were done using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega Madison, WI), following the manufacturer's instructions.
Transepithelial Electrical Resistance Measurements
Transepithelial electrical resistance (TEER) was measured hourly for five hours on air-liquid interface differentiated CFBE-wt airway epithelial cells and primary human airway epithelial cells (HBE) treated with sodium nitrite or sodium chloride using a Ag/AgCl electrode (EVOM meter).
Statistics
All data are plotted as mean +/- standard deviation. Data were log transformed and tested by one-way ANOVA for an effect (or two-way ANOVA as applicable). If an effect was present, a Tukey test was used for individual comparisons using PRISM software (La Jolla, California).
Results
Nitrite prevents P. aeruginosa biofilm formation on airway epithelial cells
To test the ability of nitrite to prevent biofilm formation on airway epithelial cells, immortalized human airway cells were grown as a confluent monolayer on glass coverslips. The P. aeruginosa strain PAOl stably expressing green fluorescent protein (PAOl-GFP) was added to the surface of these cells in a flow chamber and biofilm development was imaged as described (31). Under control conditions, robust biofilm growth (seen in green in Figures 1A and 1C) is seen. In the presence of 15mM sodium nitrite, biofilm growth was nearly absent (Figure 1B and 1D). Quantification of biofilm biomass revealed a 90% reduction in biomass (numbers on panels 1C and ID). P. aeruginosa biofilms grown in this model have been previously characterized for expression of biofilm marker genes (tolA), repression of planktonic marker genes (fliC), exopolysaccharide production and increased antibiotic resistance (31). To define the dose-response relationship, a static co-culture system was used where P. aeruginosa biofilms were grown on airway epithelial cells at an air-liquid interface for five hours (31). Dose-dependent prevention of biofilm growth was observed in the range of 1.5 and 50 mM nitrite, with an effect ceiling at 50 mM nitrite (Figure 1E). Transepithelial electrical resistance (TEER), a measure of epithelial barrier integrity, decreased in CFBE-wt cells at nitrite doses exceeding 50 mM (Figure 1F), but there was no consistent LDH release at doses up to 150 mM (data not shown). Further CFBE-wt experiments were conducted with nitrite concentrations below 50mM. Of note, comparable decreases in transepithelial electrical resistance were seen with sodium chloride at the same concentrations, so the decrease in TEER may be due to hypertonicity rather than nitrite per se.
Figure 1. Nitrite prevents P. aeruginosa biofilm formation on airway epithelial cells.
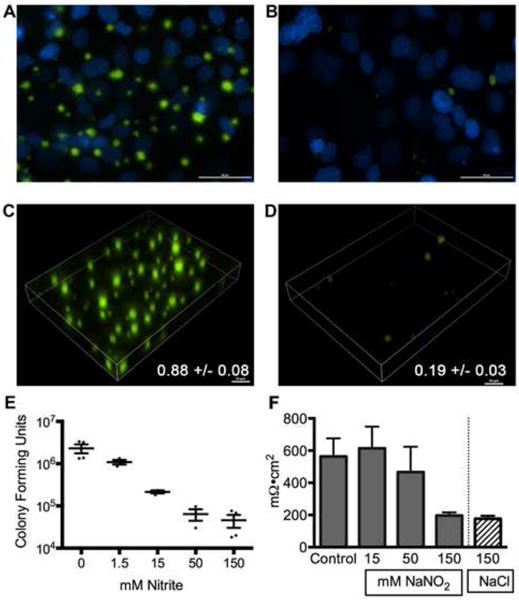
To test if nitrite prevented biotic biofilm formation, Pseudomonas aeruginosa biofilms were grown on CFBE-wt cells seeded onto coverslips and imaged by live cell microscopy after 6 hours of growth. DAPI (blue) staining shows the epithelial cell nuclei. (A) Untreated biofilms showed abundant growth of (GFP)-P. aeruginosa in characteristic clusters. (B) Biofilms treated with 15mM showed very few bacteria present. Volumetric projections of control (C) vs 15 mM (D) treated biofilms demonstrate the decreased bacterial biomass with 15 mM nitrite exposure. COMSTAT biomass quantification showed an 80% reduction in biomass (numbers on panels). (E) A static co-culture model was used to establish a dose-response relationship for nitrite in biofilm prevention. Increasing concentrations of sodium nitrite were added to epithelial - P. aeruginosa co-cultures following an attachment period. Bacterial colony forming units (CFU) were counted 5 hours later. All concentrations caused a statistically significant decrease in bacteria (one-way ANOVA followed by Tukey test). (F) Transepithelial electrical resistance measurements were used to assess epithelial integrity in the presence of nitrite. Resistance was not significantly different from control at 15mM and 50 mM nitrite (one-way ANOVA followed by Tukey test, p>0.05).
Colistin sulfate demonstrates cooperative antimicrobial activity with nitrite against biotic biofilms
Colistimethate is in wide clinical use as an inhaled antibiotic, and previous work has shown antimicrobial activity against abiotic biofilms (24, 25). The magnitude of activity of polymyxins against biotic biofilms (i.e. biofilms grown in association with airway epithelial cells) is not known, so we used the static co-culture system to test the ability of colistin to disrupt established biofilms. Figure 2A shows that 2 μg/ml colistin sulfate disrupted one-half log of mature biofilm, while 20 μg/ml colistin sulfate disrupted 3 logs (or 99.9%) of established biofilms. The airway concentration of active polymyxin achieved through colistimethate nebulization are poorly characterized, however estimated concentrations of colistin are near 10-20 μg/ml (39, 40). In comparison to reported data using the identical model system, the biotic biofilm antimicrobial activity of colistin sulfate was 2 logs better than that reported for imipenem, 1 log better than ciprofloxacin and comparable to tobramycin at doses that are achieved in the airway (31).
Figure 2. Nitrite sensitizes P. aeruginosa to polymyxins.
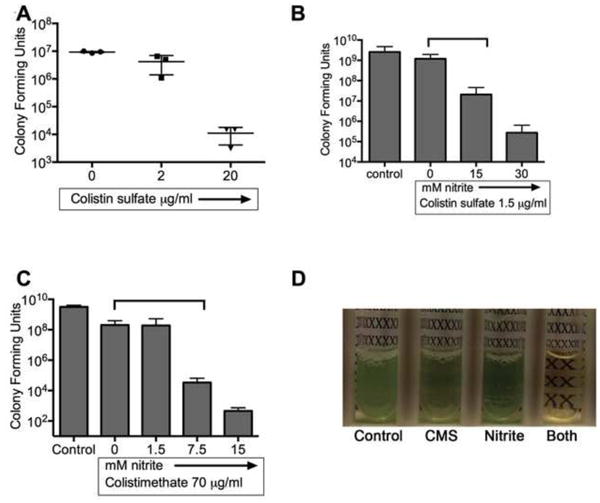
(A) The static co-culture model was used to determine the efficacy of colistin sulfate at disrupting established P. aeruginosa biotic biofilms. P. aeruginosa was added to the apical surface of CFBE-wt cells. After 6 hours of maturation, the biofilms were treated with colistin sulfate for 90 minutes and the number of bacteria was enumerated with a CFU dilution assay. 20μg/ml colistin sulfate caused a 3-log reduction in CFUs. Planktonic time-kill assays were used to determine the interaction between nitrite and polymyxins. Subinhibitory concentrations of (B) colistin sulfate (1.5μg/ml) and (C) colistimethate (70μg/ml) showed increased antimicrobial activity against P. aeruginosa strain PA14 when combined with subinhibitory concentrations of acidified sodium nitrite. p<0.05 for polymyxin vs polymyxin + nitrite by two-way ANOVA. (D) Representative cultures showed reduced bacterial growth (turbidity) when both CMS and nitrite are present.
We next examined if nitrite could improve the antimicrobial activity of polymyxins. To determine the interaction between polymyxins and nitrite, modified time-kill assays were used. Robust growth was seen in the presence of up to 30 mM nitrite. No bacterial killing was seen with 70μg/ml colistimethate (CMS) or 1.5 μg/ml colistin sulfate (COL) in well aerated cultures (doses that are subinhibitory in P. aeruginosa PA14 under these conditions). However, when nitrite was combined with subinhibitory concentrations of colistin sulfate (Figure 2B) or colistimethate (Figure 2C) up to 4 additional logs of bacteria were killed. Representative images of cultures grown with 15mM nitrite, 20μg/ml colistimethate or both agents are shown in Figure 2D, demonstrating large differences in turbidity. Similarly, increased bacterial killing was seen with polymyxin B and 12mM nitrite at sub-inhibitory concentrations of both compounds (Figure 4B). These results suggest that nitrite and polymyxins may have cooperative antimicrobial activity.
Figure 4. Increased bacterial killing with nitrite and polymyxins is not due to increased intracellular availability of nitrite.
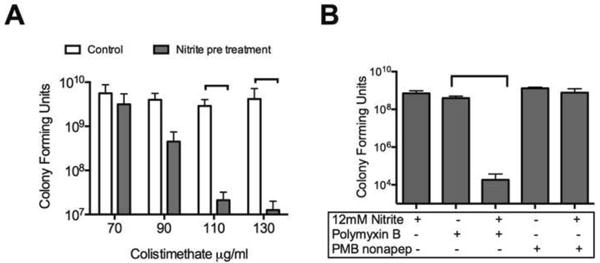
(A) Bacteria were grown in the presence of 15 mM nitrite for 5.5 hours, rinsed and exposed to colistimethate in liquid aerobic culture for 60 minutes. Nitrite pretreatment sensitized P. aeruginosa to colistimethate in planktonic culture. (B) Planktonic cultures were exposed to polymyxin b (PMB), polymyxin b nonapeptide (PMBN) and 12 mM nitrite for 5.5 hours. No significant difference was seen by one-way ANOVA between PMBN treatment with or without nitrite. PMB + nitrite was significantly lower than PMB or nitrite alone by one-way ANOVA followed by Tukey test.
Because there is great diversity in the antibiotic susceptibility of bacterial clinical isolates, we used agar dilution checkerboard testing to assess for antimicrobial synergy in a collection of P. aeruginosa clinical isolates from cystic fibrosis patients. Synergy is defined as a Fractional Inhibitory Concentration (FIC) of ≤0.5. FIC is calculated by dividing the MIC of each drug when used in combination with the MIC of each drug when used alone. Anaerobic MICs were determined adding 1% KNO3 to the growth media to support denitrification and growing the plates for 48 hours prior to interpretation to account for slower growth under anaerobic conditions. Three isolates grew too slowly anaerobically to give interpretable MICs by this method. Eight of nine isolates were more susceptible to colistin under anaerobic conditions. In addition, isolates were 2-4 fold more susceptible to nitrite under anaerobic conditions, in agreement with previously published data (17). Only the lab strain PA14 grown under anaerobic conditions met the FIC cutoff of 0.5 that defines synergy. The MIC was very consistently lowered by 2-fold when nitrite and colistin were combined (corresponds to the FICs of >0.5 and <1). The FIC cutoff of 0.5 was set in part to account for the 1 dilution variation that is accepted for MIC assays. In these experiments, we consistently saw a 1-dilution decrease (and never an increase) in colistin sulfate MIC when subinhibitory nitrite was present. Despite not meeting the 0.5 FIC threshold for synergy, the consistent decrease in MIC suggested that nitrite and colistimethate might have cooperative activity against biofilms.
To determine whether nitrite would confer polymyxin susceptibility to bacteria with high innate polymyxin resistance, eight highly colistin resistant clinical isolates were also tested under aerobic conditions in the presence of nitrite. All isolates had a colistin MIC >512 μg/ml in both the presence and absence of 3 mM (subinhibitory) sodium nitrite. Achromobacter spp. and Burkholderia spp. are pathogens that are difficult to treat within the CF population because of high innate antibiotic resistance. Burkholderia spp. have high innate resistance to polymyxins. Accordingly, the MIC to colistin was >512 μg/ml with or without nitrite, although growth of these organisms was inhibited by 18 mM nitrite alone. Amongst Achromobacter spp., increased susceptibility to colistin in the presence of nitrite was seen in 4 of 8 clinical isolates (Supplementary Table 1). Taken together, among isolates of P. aeruginosa that do not have high innate polymyxin resistance, nitrite increases the susceptibility to polymyxins for a majority of isolates, and for some of the Achromobacter isolates.
Biofilms grow more robustly on CF airway cells than on those expressing wild-type CFTR (31). We have shown that nitrite causes an increase in susceptibility to colistin in planktonic culture and on nutrient agar. To determine whether nitrite augmented the antimicrobial activity of polymyxins for biotic biofilms on primary CF HBE cells, the polymyxin pro-drug colistimethate was combined with nitrite in a biofilm prevention assay (Figure 3). In this experiment, bacteria were allowed to attach to the surface of primary CF HBE cells for one hour. The developing biofilms were then treated with nitrite, colistimethate or both agents. When nitrite was not used, sodium chloride was added to control for tonicity. Nitrite alone prevented 1-2 logs of bacterial biofilm growth. Of note, primary CF HBE cells tolerate higher concentrations of nitrite than CFBE-wt cells. Transepithelial electrical resistance did not drop with up to 300 mM nitrite (control TEER 678 mΩ•cm2 vs 300 mM nitrite 597 mΩ•cm2). Colistimethate (20 μ/ml) prevented 3 logs of growth and the combination of the agents prevented 4 logs of growth. The cooperative effect of this combination in biofilm prevention led us to undertake further mechanistic studies of nitrite and colistimethate.
Figure 3. Nitrite and colistimethate additively prevent biotic biofilm formation on primary CF human airway epithelial cells.
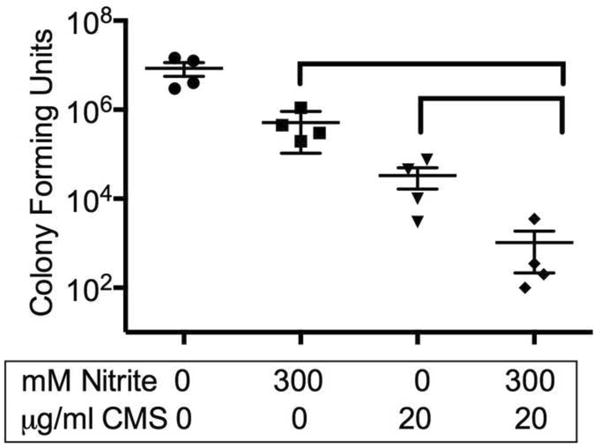
PA01 biofilms were grown on primary CF HBE cells. After attachment, developing biofilms were treated with 300mM nitrite, colistimethate 20μg/ml or a combination of the agents, p-values indicated from two-way ANOVA.
Nitrite and polymyxins additively inhibit bacterial respiration
Nitrite has many effects on bacterial physiology. In the case of P. aeruginosa, concentrations of nitrite in the 1-20 mM range inhibit aerobic respiration (as measured by NADH oxidase activity), oxygen uptake, and both aerobic and anaerobic growth (11,18, 41, 42). Polymyxins initially bind to the outer membrane and increase its permeability; subsequently they disrupt the cytoplasmic membrane (43). Polymyxins appear to inhibit bacterial respiration, presumably a consequence of damage to the cytoplasmic membrane (26). To explore the mechanism of action of combined nitrite and colistin, we tested the hypotheses that (1) polymyxins increase outer membrane permeability and thus promote increased intracellular concentrations of nitrite and (2) polymyxins and nitrite cooperatively inhibit bacterial respiration to exert antimicrobial activity.
If increased bacterial killing by nitrite and colistin were a consequence of increased intracellular nitrite concentration, then nitrite and colistin would need to be present simultaneously for increased killing to occur. To test this prediction, bacteria were treated with 15 mM nitrite, rinsed and treated with increasing concentrations of colistimethate in aerobic culture. Pretreatment with nitrite increases the susceptibility to colistimethate (Figure 4A). The timing of these effects excludes increased intracellular concentration of nitrite due to polymyxins because extracellular nitrite has been removed from the system prior to exposure to colistimethate. Polymyxin B nonapeptide, which lacks a lipid tail, increases outer membrane permeability without causing bacterial death or cytoplasmic membrane damage (44). Increased susceptibility to polymyxin b nonapeptide was not seen with nitrite (Figure 4B) in contrast to the increased bacterial killing seen with the parent compound polymyxin b and nitrite in a time-kill assay. The lack of killing in combination with polymyxin b nonapeptide and the increased susceptibility to polymyxins with nitrite pretreatment both exclude increased intracellular accessibility to nitrite as a cause of this effect.
Both nitrite and polymyxins inhibit bacterial respiration, although in the case of polymyxins this may be a secondary effect to massive cytoplasmic membrane damage. We next tested if other agents that inhibit cellular respiration sensitize P. aeruginosa to polymyxins. In time-kill assays, the combinations of colistin sulfate and colistimethate with potassium cyanide or sodium azide all showed at least 4 logs of additional killing than any of the agents alone (Figure 5A). To show more quantitatively that nitrite and polymyxins were inhibiting respiration, oxygen consumption was measured. For these experiments low concentrations of nitrite and colistimethate were chosen that did not affect overall bacterial viability in combination so that the oxygen consumption measurements would not be confounded by bacterial death. Nitrite, as previously described, inhibits oxygen consumption with a linear dose-response at concentrations below the MIC as measured using a Clark-type electrode (representative tracings shown in Figure 5B). 5mM nitrite and 30μg/ml colistimethate both cause a 20% reduction in oxygen uptake under the conditions tested. In combination the agents caused a statistically significant, 50% decrease in oxygen uptake (Figure 5C). Next, to determine if the effect was NO dependent, the scavenger CPTIO was added in modified time-kill assays. As shown in Figure 5D, CPTIO did not block the increased bacterial killing by nitrite with colistimethate. Similar results were obtained using oxyhemoglobin as the scavenger (data not shown). If respiratory blockade were part of the mechanism, then respiratory blockade in this situation should also be independent of NO. As shown in Figure 5E, treatment of cultures with CPTIO and hemoglobin did not protect P. aeruginosa against the inhibitory effects of nitrite on oxygen uptake.
Figure 5. Inhibition of respiration by nitrite and polymyxins.
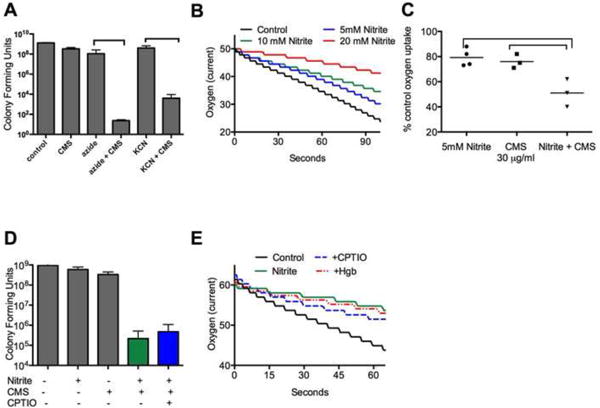
A) Inhibiting respiration with 0.4mM KCN or 5mM NaN3 increases polymyxin susceptibility of P. aeruginosa grown in aerobic LB culture. Colistin sulfate (COL) was used at 1.5μg/ml while colistimethate (CMS) was used at 20μg/ml (both sub inhibitory doses in PA14 under these conditions). (B) Representative tracings of bacterial oxygen consumption measured using a Clark-type electrode in LB with increasing concentrations of nitrite. (C) At low concentrations, both nitrite and CMS inhibit oxygen consumption by 20%. The combination of CMS and nitrite additively blocked oxygen uptake. Brackets show p values <0.05 from one-way ANOVA followed by Tukey test. Data shown as mean +/- SD. (D) Increased killing by nitrite and CMS in combination is not blocked by the addition of the scavenger CPTIO in planktonic culture. (E) Representative tracing of oxygen uptake from a Clark-type electrode. 15mM nitrite decreases oxygen consumption by 50%. Pretreatment with ImM CPTIO or 15μM hemoglobin did not prevent nitrite induced oxygen consumption blockade.
Anti-biofilm effects of nitrite are NO independent
Previous work indicates that scavenging NO prevents the anti-bacterial effects of nitrite on P. aeruginosa in planktonic culture(17). First we determined the nitrite concentrations required for growth inhibition in this system by exposing aerated, planktonic cultures of P. aeruginosa to increasing concentrations of nitrite (Figure 6A). Similar to the previous work, introduction of NO scavengers (hemoglobin and CPTIO) protected P. aeruginosa against nitrite induced growth inhibition (Figure 6B). Because the metabolic demands of biotic biofilm lifestyle differ from those of a planktonic lifestyle, we next examined whether biofilm prevention by nitrite was also dependent on NO. In contrast to the effects of nitrite under aerobic conditions, the addition of 1 mM CPTIO (far in excess of the 500 nM NO generated by nitrite in culture as reported in (17)) did not protect biotic biofilms from the activity of nitrite (Figure 6C). In conclusion, nitrite prevents P. aeruginosa biofilm formation on the apical surface of airway epithelial cells. Unlike the antibacterial activity in aerated planktonic culture, scavenging NO does not block the antibacterial activity of nitrite under biofilm conditions.
Figure 6. Biofilm cytostasis is nitric oxide independent.
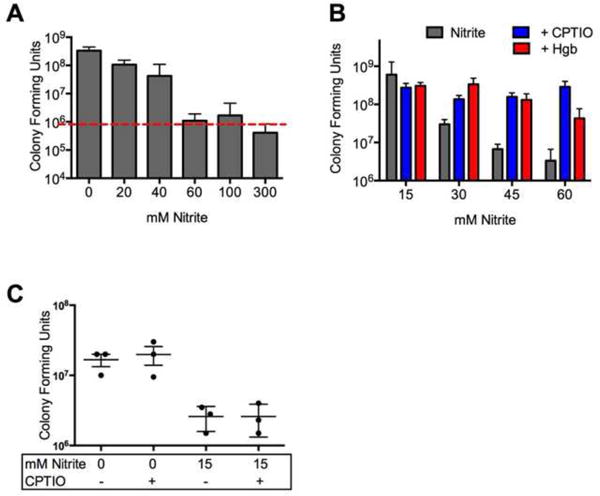
(A) Nitrite inhibited growth of PA14 in planktonic culture. (B) To confirm that nitrite induced cytostasis in planktonic cultures is nitric oxide dependent, log-phase oxygenated cultures were grown with nitrite and the nitric oxide scavengers CPTIO or oxyhemoglobin. Growth inhibition was blocked bythe addition of either agent. (C) To determine if the prevention of biofilm growth by nitrite requires nitric oxide, static epithelial- P. aeruginosa co-cultures were treated with nitrite and the nitric oxide scavenger CPTIO. Growth inhibition was unchanged by the addition of 1mM CPTIO.
Discussion
We have demonstrated that sodium nitrite prevents biotic biofilm formation by P. aeruginosa grown on the surface of CF primary human airway cells. While it was previously known that nitrite could inhibit growth of P. aeruginosa in planktonic conditions, in abiotic biofilms and in expectorated sputum, this is the first demonstration of antimicrobial activity against biotic biofilms. In concordance with previously published work, we found that anaerobic conditions increased the susceptibility of P. aeruginosa to nitrite. The oxygen tension in our epithelial co-culture systems is very low as the perfusate for the flow chamber is not actively oxygenated and the static co-cultures are submerged under several millimeters of media despite having >106 metabolically active bacteria/ml growing on the surface of the airway cells. While the available evidence suggests that mucus plugs within the CF airway provide an anaerobic environment for bacterial growth, regions of aerobic growth cannot be excluded, especially during periods of biofilm dispersal. It is noteworthy that nitrite effectively prevented bacterial growth under both aerobic and anaerobic conditions.
The millimolar concentrations of nitrite we used in this study are much higher than typical concentrations of currently used antibiotics, which may cause concern regarding cytotoxicity. Yoon et al clearly demonstrated that nitrite concentrations up to 300 mM do not cause epithelial cytotoxicity as measured by lactate dehydrogenase release, effect on short circuit current, permeability to dextran, or IL-8 secretion (17). In our studies, CFBE-wt cells tolerated up to 50mM nitrite without a loss of barrier function and well-differentiated primary CF HBE cells did not show loss of barrier integrity even with exposure to 300 mM sodium nitrite. Further, inhalation of approximately 1 molar sodium nitrite has been well tolerated by human subjects in phase I and II studies (M. Gladwin, personal communication). These data support the pursuit of clinical trials using nitrite as a nebulized antibiotic against P. aeruginosa in CF.
While our study investigated the interactions between colistimethate and nitrite, inhaled colistimethate is used less frequently than inhaled tobramycin and inhaled aztreonam in the CF population. Previous reports demonstrated that NO protects bacteria against a wide range of antibiotics (45). However, these reports do not address the interaction between NO and antimicrobial peptides such as the polymyxins, which have a unique mechanism of action. The possibility that nitrite may have adverse interactions with other antibiotics is a valid concern, warranting further investigation.
Despite widespread clinical use, the activity of colistimethate against biotic biofilms has not been clearly described. We have shown that colistin sulfate has activity against biotic biofilms that is at least comparable to that of ciprofloxacin. The activity of polymyxins under anaerobic conditions also has not been extensively studied. In the report by Hill et al, anaerobic growth was supported by Muller Hinton broth in the absence of exogenous nitrate, which makes the results difficult to interpret. Polymyxin susceptibility is also pH dependent. This study used pH 6.5, the reported pH of the CF airway surface liquid, thus the colistin sulfate MICs for these isolates may be slightly different in this study than when MIC testing is performed at pH 7.4. Additionally, ionic strength can affect polymyxin activity, and hypertonicity can cause additional effects on the epithelial cells used in our co-culture experiments (43). To account for this possibility, sodium chloride was used as a tonicity control in the static co-culture experiments.
The strengths of combining polymyxins with nitrite are the activity under anaerobic conditions and the activity against biotic biofilms, which are limitations of our current antibiotic approaches. Additionally, the proposed mechanism of interaction is unique. Existing antibiotics do not target bacterial respiration, although the combination of fosfomycin and tobramycin has recently been shown to suppress denitrification (46). While the current study focused on the interaction of nitrite and polymyxins in aerobic metabolism, a similar effect is possible regarding anaerobic respiration where polymyxin induced inner membrane damage may indirectly disrupt the function of the denitrification enzymes allowing accumulation of toxic intermediates. Because of the rapidity of bacterial killing by polymyxins we did not directly test the hypothesis that polymyxins inhibit denitrification. However, the proposed mechanism that nitrite and polymyxins are both inhibiting aerobic respiration is concordant with the observation that flow cell biofilms are killed by the combination of colistin and the respiratory chain uncoupler CCCP (24). The advantage of our approach is that, unlike CCCP and other respiratory chain poisons, nitrite can be safely nebulized.
While the antimicrobial effects of nitrite in planktonic, aerated culture are NO-dependent, antimicrobial effects under biofilm growth conditions are likely not NO-dependent. In these experiments, the scavenger CPTIO was added while the biofilms were microcolonies with minimal matrix production, so inability of the scavenger to penetrate the colony is unlikely to be solely responsible for these results. There are several potential explanations for the difference in NO-radical dependence of growth inhibition by nitrite. First, P. aeruginosa has multiple mechanisms for detoxifying NO including expression of flavohemoglobins and the anaerobic expression catalase (47, 48). The relative efficiency of these detoxification systems, and thus organismal exposure to NO vs nitrite, may vary with growth conditions. Second, the targets of nitrosative stress are not clearly understood in P. aeruginosa, especially when the metabolic versatility of the bacterium is considered. Nitrite and NO can cause S-nitrosation of cysteine and the inactivation of Fe-S containing proteins. Targets of S-nitrosation have been identified in Borrelia burgdorferi, Mycobacterium tuberculosis, Escherichia coli and Helicobacter pylori, but to date not in P. aeruginosa (49-51). Multiple enzymes within central metabolism are potential targets for inactivation by nitrosative stress including NADH dehydrogenase, the terminal cytochrome c oxidases, and aconitase. In eukaryotes, mitochondrial complex I is a target for cysteine S-nitrosation, which confers protection from ischemia/perfusion injury (52, 53). In contrast, work in Salmonella enterica suggested the site of respiratory inhibition by the NO donor spermine NONOate to be nitrosylation of heme d in the terminal cytochrome oxidases while bacterial NADH dehydrogenase activity remained uninhibited. The sources of nitrosative stress vary between these reports, and more importantly the role of NADH dehydrogenase in the organism vary (S. enterica is viable in the absence of both NADH dehydrogenases). Growth conditions may dictate the bacterial targets of nitrosative stress (and thus the chemical targets for nitrite or NO). The study by Ren et al is informative. E. coli growth in minimal media requires synthesis of branched chain amino acids catalyzed by dihydroxyacid dehydratase (IlvD). NO inhibits IlvD through formation of dinitrosyl iron complexes, thus inhibiting growth and rendering the bacteria dependent on supplementation of branched chain amino acids. Growth inhibition by NO in this situation is avoided by supplementing the growth media with the needed amino acids (54). One could imagine that had the experiment performed in E. coli been done in rich media, a different target of nitrosative stress might have been found (albeit with a higher stress required)(55). In the current study, the bacterial substrate usage in aerated LB is likely very different from that of a low-oxygen tension biofilm grown in apposition to an epithelial cell, which may then in turn dictate which metabolic pathways are susceptible to inhibition. Teasing out the targets of NO vs nitrite under biofilm conditions as compared to planktonic growth will be a complex task.
In conclusion, nitrite shows promise as an antimicrobial agent targeting biotic biofilms in the lungs of patients with P. aeruginosa infection. Combining nitrite with colistimethate may increase the effectiveness of the latter. Even in isolation, inhaled nitrite may be a useful addition to our antimicrobial armamentarium because of its broad range of activity, including activity against anaerobically growing P. aeruginosa, anti-biofilm activity, and activity against other pathogens such as Achromobacter sp. and Burkholderia sp. for which few effective antimicrobials are available. This study provides further motivation to pursue human trials of nebulized nitrite as an antimicrobial in patients with airway colonization by resistant organisms.
Supplementary Material
Table 1.
Checkerboard MIC testing under aerobic and anaerobic conditions for 12 isolates of P. aeruginosa. MIC was determined by agar dilution with pH 6.5 MHB agar. 1% KNO3 was added for anaerobic growth and anaerobic plates were incubated for 48 hours. Three isolates (33-2, 66-2, and 74-2) grew too poorly under anaerobic conditions to obtain interpretable MICs. The MIC for both colistin sulfate and nitrite are lower under anaerobic conditions. Nitrite and colistin sulfate were synergistic for one strain (PA14) under anaerobic conditions (FIC<0.5).
| Strain | Aerobic MIC COL μg/ml | Anaerobic MIC COL μg/ml | Aerobic MIC mM Nitrite | Anaerobic MIC mM Nitrite | Aerobic FIC | Anaerobic FIC |
|---|---|---|---|---|---|---|
| PA14 | 2 | 2 | 16 | 4 | 0.75 | 0.375 |
| PAOl | 2 | 1 | 16 | 4 | 0.75 | 0.75 |
| 31-2 | 2 | 0.5 | 16 | 4 | 0.75 | 0.75 |
| 36-3 | 2 | 0.5 | 16 | 4 | 0.75 | 0.75 |
| 41-2 | 4 | 0.5 | 8 | 4 | 1.5 | 0.625 |
| 47-3 | 1 | 0.25 | 8 | 2 | 1 | 1 |
| 60-3 | 4 | 1 | 16 | 4 | 0.625 | 0.75 |
| 62-3 | 1 | 0.25 | 8 | 4 | 1 | 1 |
| 71-2 | 4 | 1 | 16 | 4 | 0.625 | 0.75 |
| 33-2 | 4 | n.d. | 16 | n.d. | 0.75 | n.d. |
| 66-2 | 8 | n.d. | 8 | n.d. | 0.625 | n.d. |
| 74-2 | 0.5 | n.d. | 8 | n.d. | 1 | n.d. |
Highlights.
Nitrite prevents P. aeruginosa biofilms on human CF airway epithelial cells.
Nitrite and polymyxins additively inhibit bacterial respiration.
Nitrite and colistimethate additively inhibit biofilm growth on airway cells.
Acknowledgments
The authors would like to thank Lauren Lashua, Stefanie Brown, and Catherine Corey for technical assistance. Strains kindly provided by Dr. John LiPuma (B. cepacia Research Laboratory and Repository; supported by the Cystic Fibrosis Foundation).
Funding: ACZ 2T32HL007563-26
JLB, JMP P30DK072506,
JMB R00HL098342-01, Breathe Pennsylvania Research Grant
JMP Cystic Fibrosis Foundation
MTG: R01HL098032, RO1HL096973, and P01HL103455, T32 HL110849, the Institute for Transfusion, Medicine and the Hemophilia Center of Western Pennsylvania.
SMM: R01AI067653 and Cystic Fibrosis Foundation MOSKOW13P0
Footnotes
Conflict of interest: Dr. Gladwin is listed as a co-inventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Mast-Aires Pharmaceuticals on the development of a phase II proof of concept trial using inhaled nitrite for pulmonary arterial hypertension.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Schaible B, Taylor CT, Schaffer K. Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob Agents Chemother. 2012;56:2114–2118. doi: 10.1128/AAC.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O'Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan M, McClean S. Bacterial host interactions in cystic fibrosis. Curr Opin Microbiol. 2012;15:71–77. doi: 10.1016/j.mib.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, McDermott TR, Mason RP, Wozniak DJ, Hancock RE, Parsek MR, Noah TL, Boucher RC, Hassett DJ. Pseudomonas aeruginosa anaerobic respiration in biofilms: Relationships to cystic fibrosis pathogenesis. Dev Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 5.Kolpen M, Kuhl M, Bjarnsholt T, Moser C, Hansen CR, Liengaard L, Kharazmi A, Pressler T, Hoiby N, Jensen PO. Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLoS One. 2014;9:e84353. doi: 10.1371/journal.pone.0084353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill D, Rose B, Pajkos A, Robinson M, Bye P, Bell S, Elkins M, Thompson B, Macleod C, Aaron SD, Harbour C. Antibiotic susceptabilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J Clin Microbiol. 2005;43:5085–5090. doi: 10.1128/JCM.43.10.5085-5090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykhuizen RS, Fraser A, McKenzie H, Golden M, Leifert C, Benjamin N. Helicobacter pylori is killed by nitrite under acidic conditions. Gut. 1998;42:334–337. doi: 10.1136/gut.42.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham R, Mustoe E, Spiller L, Lewis S, Benjamin N. Acidified nitrite: A host defence against colonization with C. difficile spores? J Hosp Infect. 2014;86:155–157. doi: 10.1016/j.jhin.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, Vazquez-Torres A. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem. 2008;283:7682–7689. doi: 10.1074/jbc.M708845200. [DOI] [PubMed] [Google Scholar]
- 10.Reddy D, Lancaster JR, Jr, Cornforth DP. Nitrite inhibition of Clostridium botulinum: Electron spin resonance detection of iron-nitric oxide complexes. Science. 1983;221:769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- 11.Rowe JJ, Yarborough JM, Rake JB, Eagon RG. Nitrite inhibition of aerobic bacteria. Current Microbiology. 1979;2:51–54. [Google Scholar]
- 12.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide. 2013 doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno M, Wang J, Mora AL, Gladwin MT. Nitrite signaling in pulmonary hypertension: Mechanisms of bioactivation, signaling, and therapeutics. Antioxid Redox Signal. 2013;18:1797–1809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C, McMullin B, Ghaffari A, Stenzler A, Pick N, Roscoe D, Ghahary A, Road J, Av-Gay Y. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide. 2009;20:16–23. doi: 10.1016/j.niox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Miller C, Miller M, McMullin B, Regev G, Serghides L, Kain K, Road J, Av-Gay Y. A phase i clinical study of inhaled nitric oxide in healthy adults. J Cyst Fibros. 2012;11:324–331. doi: 10.1016/j.jcf.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Hon YY, Sun H, Dejam A, Gladwin MT. Characterization of erythrocytic uptake and release and disposition pathways of nitrite, nitrate, methemoglobin, and iron-nitrosyl hemoglobin in the human circulation. Drug Metab Dispos. 2010;38:1707–1713. doi: 10.1124/dmd.110.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, Mortensen JE, Burns JL, Speert D, Boucher RC, Hassett DJ. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest. 2006;116:436–446. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T. Mechanism of nitrite inhibition of cellular respiration in Pseudomonas aeruginosa. Current Microbiology. 1985;12:35–40. [Google Scholar]
- 19.Reszka KJ, Xiong Y, Sallans L, Pasula R, Olakanmi O, Hassett DJ, Britigan BE. Inactivation of the potent Pseudomonas aeruginosa cytotoxin pyocyanin by airway peroxidases and nitrite. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1044–1056. doi: 10.1152/ajplung.00172.2011. [DOI] [PubMed] [Google Scholar]
- 20.Wood SR, Firoved AM, Ornatowski W, Mai T, Deretic V, Timmins GS. Nitrosative stress inhibits production of the virulence factor alginate in mucoid Pseudomonas aeruginosa. Free Radic Res. 2007;41:208–215. doi: 10.1080/10715760601052610. [DOI] [PubMed] [Google Scholar]
- 21.Major TA, Panmanee W, Mortensen JE, Gray LD, Hoglen N, Hassett DJ. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother. 54:4671–4677. doi: 10.1128/AAC.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlag S, Nerz C, Birkenstock TA, Altenberend F, Gotz F. Inhibition of staphylococcal biofilm formation by nitrite. J Bacteriol. 2007;189:7911–7919. doi: 10.1128/JB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun C, Zumft WG. Marker exchange of the structural genes for nitric oxide reductase blocks the denitrification pathway of Pseudomonas stutzeri at nitric oxide. J Biol Chem. 1991;266:22785–22788. [PubMed] [Google Scholar]
- 24.Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexab-oprm genes. Mol Microbiol. 2008;68:223–240. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 25.Chiang WC, Pamp SJ, Nilsson M, Givskov M, Tolker-Nielsen T. The metabolically active subpopulation in Pseudomonas aeruginosa biofilms survives exposure to membrane-targeting antimicrobials via distinct molecular mechanisms. FEMS Immunol Med Microbiol. 2012;65:245–256. doi: 10.1111/j.1574-695X.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 26.LaPorte DC, Rosenthal KS, Storm DR. Inhibition of Escherichia coli growth and respiration by polymyxin b covalently attached to agarose beads. Biochemistry. 1977;16:1642–1648. doi: 10.1021/bi00627a019. [DOI] [PubMed] [Google Scholar]
- 27.Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2014;189:975–982. doi: 10.1164/rccm.201312-2208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CFF. Patient registry: Annual data report. 2012 [Google Scholar]
- 29.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, Konstan MW, Wagener JS. Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 30.Schuster A, Haliburn C, Doring G, Goldman MH. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (colobreathe dpi) in patients with cystic fibrosis: A randomised study. Thorax. 2013;68:344–350. doi: 10.1136/thoraxjnl-2012-202059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O'Toole GA, Stanton BA. The deltaf508-cftr mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L25–37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spilker T, Vandamme P, Lipuma JJ. A multilocus sequence typing scheme implies population structure and reveals several putative novel Achromobacter species. J Clin Microbiol. 2012;50:3010–3015. doi: 10.1128/JCM.00814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, Miller SI, Hoiby N, Moskowitz SM. Phoq mutations promote lipid a modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother. 2011;55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program comstat. Microbiology. 2000;146(Pt 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 36.Moreau-Marquis S, Redelman CV, Stanton BA, Anderson GG. Co-culture models of Pseudomonas aeruginosa biofilms grown on live human airway cells. J Vis Exp. 2010 doi: 10.3791/2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasgado-Flores H, Krishna Mandava V, Siman H, Van Driessche W, Pilewski JM, Randell SH, Bridges RJ. Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors. Am J Physiol Cell Physiol. 2013;305:C1114–1122. doi: 10.1152/ajpcell.00166.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (mic) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 39.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 40.S WSY, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, Mcintosh MP. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob Agents Chemother. 2014;58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DR, Rowe JJ, Romero P, Eagon RG. Denitrifying Pseudomonas aeruginosa: Some parameters of growth and active transport. Appl Environ Microbiol. 1978;36:257–263. doi: 10.1128/aem.36.2.257-263.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez DD, F M, Rowe JJ. Nitrate transport and its regualtion by 02 in Pseudomonas aeruginosa. Archives of Biochemistry and Biophysics. 1991;286:159–163. doi: 10.1016/0003-9861(91)90022-b. [DOI] [PubMed] [Google Scholar]
- 43.Daugelavicius R, Bakiene E, Bamford DH. Stages of polymyxin b interaction with the Escherichia coli cell envelope. Antimicrob Agents Chemother. 2000;44:2969–2978. doi: 10.1128/aac.44.11.2969-2978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champlin FR, Ellison ML, Bullard JW, Conrad RS. Effect of outer membrane permeabilisation on intrinsic resistance to low triclosan levels in Pseudomonas aeruginosa. Int J Antimicrob Agents. 2005;26:159–164. doi: 10.1016/j.ijantimicag.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaughey G, Gilpin DF, Schneiders T, Hoffman LR, McKevitt M, Elborn JS, Tunney MM. Fosfomycin and tobramycin in combination downregulate nitrate reductase genes narg and narh, resulting in increased activity against Pseudomonas aeruginosa under anaerobic conditions. Antimicrob Agents Chemother. 2013;57:5406–5414. doi: 10.1128/AAC.00750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon SS, Karabulut AC, Lipscomb JD, Hennigan RF, Lymar SV, Grace SL, Herr AB, Howell ML, Kiley PJ, Schurr MJ, Gaston B, Choi KH, Schweizer HP, Hassett DJ. Two-pronged survival strategy for the major cystic fibrosis pathogen, Pseudomonas aeruginosa, lacking the capacity to degrade nitric oxide during anaerobic respiration. EMBO J. 2007;26:3662–3672. doi: 10.1038/sj.emboj.7601787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su S, Panmanee W, Wilson JJ, Mahtani HK, Li Q, Vanderwielen BD, Makris TM, Rogers M, McDaniel C, Lipscomb JD, Irvin RT, Schurr MJ, Lancaster JR, Jr, Kovall RA, Hassett DJ. Catalase (kata) plays a role in protection against anaerobic nitric oxide in Pseudomonas aeruginosa. PLoS One. 2014;9:e91813. doi: 10.1371/journal.pone.0091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu W, Zhou Y, Sun Y, Fang M, Yu H, Li W, Liu Z, Zeng J, Chen C, Gao C, Jia J. Identification of s-nitrosylation of proteins of Helicobacter pylori in response to nitric oxide stress. J Microbiol. 2011;49:251–256. doi: 10.1007/s12275-011-0262-7. [DOI] [PubMed] [Google Scholar]
- 50.Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF. S-nitroso proteome of Mycobacterium tuberculosis: Enzymes of intermediary metabolism and antioxidant defense. Proc Natl Acad Sci U S A. 2005;102:467–472. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourret TJ, Boylan JA, Lawrence KA, Gherardini FC. Nitrosative damage to free and zinc-bound cysteine thiols underlies nitric oxide toxicity in wild-type borrelia burgdorferi. Mol Microbiol. 2011;81:259–273. doi: 10.1111/j.1365-2958.2011.07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by s-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren B, Zhang N, Yang J, Ding H. Nitric oxide-induced bacteriostasis and modification of iron-sulphur proteins in Escherichia coli. Mol Microbiol. 2008;70:953–964. doi: 10.1111/j.1365-2958.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowman LA, McLean S, Poole RK, Fukuto JM. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv Microb Physiol. 2011;59:135–219. doi: 10.1016/B978-0-12-387661-4.00006-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


