Abstract
Background
The benefit of primary tumor resection for metastatic inflammatory breast cancer (IBC) patients is unknown.
Methods
We reviewed 172 cases of metastatic IBC. All received chemotherapy with or without radiotherapy and/or surgery. Patients were classified as responders or non-responders to chemotherapy. Five-year overall (OS) and distant progression-free survival (DPFS) and local control at last follow-up were evaluated.
Results
Seventy-nine (46%) patients underwent surgery. OS and DPFS were better with surgery vs no surgery (47% vs 10%, respectively, p<0.001 and 30% vs 3%, p<0.001). Surgery plus radiotherapy was associated with better survival compared to treatment with surgery or radiotherapy alone (OS: 50% vs 25% vs 14%, respectively, DPFS: 32% vs 18% vs 15%, p<0.0001 for both). Surgery was associated with better survival for both responders (surgery vs no surgery OS: 49% vs 23%, p<0.0001, DPFS: 31% vs 8%, p<0.0001) and non-responders (surgery vs no surgery OS: 40% vs 6%, p<0.0001, DPFS: 30% vs 0, p<0.0001). On multivariate analysis, treatment with surgery plus radiotherapy and response to chemotherapy were significant predictors of better OS and DPFS. Local control at last follow-up was 4-fold more likely in patients who underwent surgery with or without radiotherapy compared to patients who received chemotherapy alone (81% vs 18%, p<.0001). Surgery and response to chemotherapy independently predicted local control on multivariate analysis.
Conclusion
This study demonstrates that for select patients with metastatic IBC, multimodality treatment including primary tumor resection may result in better local control and survival. A randomized trial is needed to validate these findings.
Keywords: breast cancer, inflammatory breast cancer, survival, local control, multimodality treatment, combined modality therapy, metastatic breast cancer
Introduction
Inflammatory breast cancer (IBC) is an aggressive form of the disease which accounts for 1-2% of all breast cancers but as much as 10% of breast cancer deaths. As many as 30% of patients with IBC present with metastatic disease.1
Multimodality therapy that includes chemotherapy, radiotherapy and modified radical mastectomy results in optimal survival and local control outcomes for non-metastatic IBC2, with single institution series demonstrating 5-year OS rates of 45-57% and >80% locoregional control rates.3-6
Reported outcomes for metastatic IBC, however, are dismal. With chemotherapy alone, 5-year OS rates of <10% have been reported.7-9 A contemporary review of the SEER database showed a 39% 2-year OS for those with metastatic disease.10 While this is an improvement over historical data, it is not clear what percentage of patients received radiation and/or surgery, and therefore benefits attributable to locoregional treatment in patients with metastatic IBC remain largely unknown.
Primary tumor resection in the setting of metastatic breast cancer remains controversial because it has never been definitively associated with improved outcomes. It is traditionally reserved for select patients and for those in need of palliation. The issue remains a matter of debate as recent literature suggests that surgery may actually result in better survival and local control outcomes in stage IV disease.11-19 Using the National Cancer Database, Kahn et al analyzed over 16,000 cases of metastatic breast cancer and identified a nearly 2-fold increase in the 3-year OS for patients undergoing mastectomy with negative margins compared to those who did not undergo surgery (35.7% vs. 17.3%, p=.01).11 In a recent review of the SEER database that included over 700 patients with stage IV IBC, primary tumor resection was associated with a 51% decreased risk of death compared to patients who did not undergo surgery.10 Furthermore, as patients with metastatic disease experience longer survival, durable chest wall control becomes a significant issue in managing their disease. This issue is particularly relevant in IBC, where local recurrence after a response to therapy may be more likely and is often more severe than in patients with non-inflammatory breast cancer.20
In this study, we evaluated our experience with surgical resection of the primary tumor in patients with metastatic IBC. It is the largest, single-institution series of stage IV IBC patients in the literature. Our objectives were to determine local control and survival rates for those who did and did not undergo surgery and to identify additional prognostic and treatment-related variables associated with improved outcomes.
Methods
We reviewed records of all patients treated for de novo stage IV IBC at our institution from 1994-2009. Patients with metastases identified within 3 months of IBC diagnosis were included. A multidisciplinary team confirmed each IBC diagnosis based on the clinical picture of rapid onset (<3months) breast enlargement and diffuse erythema affecting more than one third of the breast. Patients with secondary skin changes from locally advanced disease were not included. The initial staging work up included bilateral mammogram and ultrasound of the breasts and nodal basins, bone scan, chest X-ray and abdominal CT scan. Six patients diagnosed before the 2006 AJCC staging guideline revisions had ipsilateral supraclavicular lymph node metastases and were included. Review of data for this investigation was approved by the Institutional Review Board of our institution.
The dataset was comprised of 172 patients. Demographic, tumor and treatment-related variables including race, Eastern Cooperative Oncology Group (ECOG) performance status score at diagnosis, menopausal status, hormone and HER2 receptor status, tumor grade, histologic type, lymphovascular space invasion (LVSI), pathologic response of the primary tumor to systemic therapy, clinical response to systemic therapy at sites of distant disease, number of distant disease sites, surgical intent, margin status and locoregional treatment were evaluated.
All patients were treated with primary, anthracycline-based chemotherapy, and most received a taxane as well. Patients with refractory disease were treated with additional/ alternative therapy at the discretion of their medical oncologists. Endocrine therapy was given to patients with hormone receptor positive disease as reported in the results. Trastuzumab was given to all HER2 positive patients treated after 2001. Additional adjuvant systemic therapy was given to surgical patients at the discretion of the treating medical oncologist. Clinical response to systemic therapy was assessed at distant disease sites using physical exam and radiographic findings which were classified according to response evaluation criteria in solid tumors (RECIST) guidelines.21 Patients were classified either as responders who had a complete or partial response to systemic therapy or as non-responders who demonstrated stable or progressive disease. For patients in the no surgery group, response to chemotherapy was assessed at the time of maximum clinical response and for patients in the surgery group, response was assessed at the time of surgery.
Surgical margins ≥2mm were considered negative and <2mm were considered close. Skin margins were not routinely assessed. A pathologic complete response (pCR) was defined as no residual invasive disease in the primary tumor bed and resected lymph nodes. The surgical intent was curative if there was no clinical evidence of active distant disease at the time of surgery or if all distant disease was eradicated by concurrent metastasectomy. For patients with active distant disease at the time of surgery, the intent was considered to be for local control.
The target volume for radiotherapy was the chest wall and draining lymphatics. The most common regimen delivered 51Gy in twice daily 1.5-Gy fractions. Patients in the surgery group also received a 15-Gy chest wall boost.
Statistical Methods
End points were death, distant disease progression and local control at last follow-up. OS was calculated from diagnosis to date of death and DPFS was calculated from diagnosis to date of distant disease progression or death. Local control was defined as no clinically appreciable chest wall disease. The presence of a mass, erythema or skin thickening was considered minimal chest wall disease. Dermal nodules, rash, superficial tumor implants or pain in the setting of any local skin involvement were classified as moderate. Severe chest wall involvement included fungating lesions, ulceration or drainage. OS and DPFS were calculated using Kaplan-Meier analysis. The log-rank test was used to compare differences in survival between groups. Cox proportional hazards models were used to estimate hazard ratios (HRs) and to correlate outcomes with risk variables. Subgroups were compared using the X2 test and the Wilcoxon rank sum test where appropriate. Severity of local skin involvement was compared using the Fisher’s exact test. All P values were 2-sided, and P ≤0.05 was considered significant.
Results
Seventy-nine patients (46%) underwent primary tumor resection. Patient characteristics are shown in table 1. In 94 (55%) patients, metastatic disease was limited to one site. This was bone in 34 patients, 24 of whom (71%) were in the surgery group, and ipsilateral supraclavicular lymph nodes in 6 patients, 5 of whom (83%) were in the surgery group. Median time to surgery was 7.5 months from date of diagnosis; 19% of patients underwent surgery within 6 months of diagnosis, 61% between 6 months and one year and 20% underwent surgery more than1 year after diagnosis. The surgical procedures performed were modified radical mastectomy (n=74, 93%), segmental mastectomy with axillary lymph node dissection (n=2, 3%) and total mastectomy (n=3, 4%).
Table 1.
Patient, disease and treatment characteristics of the entire cohort, surgery and no surgery groups.
| All n=172 |
Surgery n=79 |
No Surgery n=93 |
p Value¶ | |||||
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| Average | 52 | 51 | 53 | .38 | ||||
| Range | 22-85 | 22-78 | 26-85 | |||||
| Race | ||||||||
| White | 119 | 69% | 60 | 76% | 59 | 63% | 0.2 | |
| Black | 32 | 19% | 11 | 14% | 21 | 23% | ||
| Other | 21 | 12% | 8 | 10% | 13 | 14% | ||
| ECOG Performance Status | ||||||||
| 0-1 | 126 | 73% | 72 | 91% | 54 | 58% | <0.0001 | |
| >1 | 29 | 17% | 4 | 5% | 25 | 27% | ||
| Unknown | 17 | 10% | 3 | 4% | 14 | 15% | ||
| Menopausal status* | ||||||||
| Post | 100 | 58% | 44 | 56% | 56 | 60% | 0.38 | |
| Pre | 69 | 40% | 35 | 44% | 34 | 37% | ||
| Hormone receptor* | ||||||||
| Pos | 89 | 52% | 43 | 54% | 46 | 49% | 0.59 | |
| Neg | 77 | 45% | 34 | 43% | 43 | 46% | ||
| HER2 receptor* | 1 | |||||||
| Pos | 45 | 26% | 27 | 34% | 18 | 19% | 0.02 | |
| Neg | 117 | 68% | 47 | 59% | 70 | 75% | ||
| Grade* | ||||||||
| 1 | 3 | 2% | 1 | 1% | 2 | 2% | 0.57 | |
| 2 | 38 | 22% | 15 | 19% | 23 | 25% | ||
| 3 | 120 | 70% | 58 | 73% | 62 | 67% | ||
| LVSI | ||||||||
| Yes | 78 | 45% | 49 | 62% | 29 | 31% | 0.33 | |
| No | 39 | 23% | 28 | 35% | 11 | 12% | ||
| Unknown | 55 | 32% | 2 | 3% | 53 | 57% | ||
| Histology | ||||||||
| Ductal | 136 | 79% | 64 | 81% | 72 | 77% | 0.22 | |
| Lobular | 13 | 8% | 8 | 10% | 5 | 5% | ||
| Mixed | 14 | 8% | 4 | 5% | 10 | 11% | ||
| Systemic therapy | ||||||||
| Chemo only | 117 | 68% | 45 | 57% | 72 | 77% | 0.006 | |
| chemo+endocrine | 55 | 32% | 34 | 43% | 21 | 23% | ||
| Response to systemic therapy | ||||||||
| Non-responders | 69 | 40% | 10 | 13% | 59 | 63% | <.0001 | |
| Responders | 98 | 56% | 67 | 85% | 31 | 34% | ||
| Locoregional therapy | ||||||||
| None | 75 | 44% | ||||||
| Radiotherapy alone | 18 | 10% | ||||||
| Surgery alone | 11 | 6% | ||||||
| Surgery+radiotherapy | 68 | 40% | ||||||
| Surgical intent | ||||||||
| Curative | 47 | 59% | ||||||
| Local control | 32 | 41% | ||||||
| Margin status* | ||||||||
| Negative | 60 | 87% | ||||||
| Positive | 7 | 4% | ||||||
| Close | 8 | 6% | ||||||
| pCR | ||||||||
| Yes | 12 | 15% | ||||||
| No | 67 | 85% | ||||||
| # distant disease sites | ||||||||
| 1 | 94 | 55% | 59 | 75% | 35 | 38% | ||
| >1 | 78 | 45% | 20 | 25% | 58 | 62% | ||
Unknown values were omitted when they accounted for ≤6% of patients, thus percentages displayed may not total 100%.
p value is between surgery and no surgery groups
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LVSI, lymphovascular space invasion; pCR pathologic complete response
Endocrine therapy was given to 39% (n=18/46) of hormone receptor positive patients in the no surgery group and 74% (n=32/43) of hormone receptor positive patients in the surgery group. Of the 45 patients with HER2 positive tumors, 73% (n=33) received trastuzumab. Of 27 patients in the surgery group with HER2 positive tumors, 21 received neoadjuvant trastuzumab; the remaining 6 patients received trastuzumab either in the adjuvant setting (4/6) or not at all (2/6). A pCR was seen in 15% (n=12) of patients in the surgery group; seven of these (58%) were HER2 positive and 5 of the 7 had received neoadjuvant trastuzumab.
The median follow-up was 33 months (3-166 months). Five-year OS and DPFS for the entire cohort was 29% and 17%, respectively. Notably, 8 patients (5%) survived beyond 10 years. Several factors, including performance status score of 0 or 1, HER2 positivity, absence of LVSI, response to chemotherapy, pCR, and locoregional treatment with surgery and/or radiation were significant predictors of improved OS and DPFS on univariate analysis (Table 2). Black race and >1 site of distant disease were predictors for worse DPFS but did not significantly affect OS.
Table 2.
Univariate analysis for overall survival and distant progression-free survival
| No. of patients* |
5-year OS (%) |
p Value | 5-year DPFS (%) |
p Value | ||
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 119 | 30 | 0.05 | 20 | <.001 | |
| Black | 32 | 17 | 0 | |||
| Other | 21 | 38 | 27 | |||
| ECOG Performance Status | ||||||
| 0-1 | 126 | 31 | 0.01 | 18 | 0.002 | |
| >1 | 29 | 11 | 5 | |||
| Menopausal status | ||||||
| Post | 100 | 34 | 0.44 | 21 | 0.79 | |
| Pre | 69 | 27 | 14 | |||
| Hormone receptor | ||||||
| Pos | 89 | 31 | 0.12 | 15 | 0.81 | |
| Neg | 77 | 25 | 18 | |||
| HER2 receptor | ||||||
| Pos | 45 | 44 | 0.002 | 25 | <.001 | |
| Neg | 117 | 20 | 11 | |||
| Grade | ||||||
| 1 | 3 | 67 | 0.73 | 33 | 0.47 | |
| 2 | 38 | 24 | 15 | |||
| 3 | 120 | 28 | 17 | |||
| LVSI | ||||||
| Yes | 78 | 27 | <.001 | 16 | <.001 | |
| No | 39 | 52 | 40 | |||
| Unknown | 55 | |||||
| Histology | ||||||
| Ductal | 136 | 30 | 0.92 | 18 | 0.88 | |
| Lobular | 13 | 42 | 19 | |||
| Mixed | 14 | 19 | 9 | |||
| Systemic therapy | ||||||
| Chemo only | 117 | 28 | 0.63 | 17 | 0.42 | |
| Chemo+endocrine | 55 | 29 | 8 | |||
| Response to systemic therapy | ||||||
| Non-responders | 69 | 13 | <.0001 | 8 | <.0001 | |
| Responders | 98 | 41 | 24 | |||
| Locoregional therapy | ||||||
| None | 75 | 9 | <.0001 | 0 | <.0001 | |
| Radiotherapy alone | 18 | 14 | 15 | |||
| Surgery alone | 11 | 25 | 18 | |||
| Surgery+radiotherapy | 68 | 50 | 32 | |||
| Surgery | ||||||
| Yes | 79 | 47 | <.0001 | 30 | <.0001 | |
| No | 93 | 10 | 3 | |||
| Surgical intent | ||||||
| Curative | 47 | 49 | 0.66 | 35 | 0.56 | |
| Local control | 32 | 44 | 24 | |||
| Margin status | ||||||
| Negative | 60 | 48 | 0.92 | 33 | 0.38 | |
| Positive | 7 | 47 | 42 | |||
| Close | 8 | 40 | 25 | |||
| pCR | ||||||
| Yes | 12 | 60 | <.001 | 61 | <.001 | |
| No | 67 | 26 | 13 | |||
| # distant disease sites | ||||||
| 1 | 94 | 34 | 0.05 | 24 | <.001 | |
| >1 | 78 | 18 | 6 | |||
Patients with unknown data were excluded, thus totals may vary
Abbreviations: OS, overall survival; DPFS, distant progression-free survival; ECOG, Eastern Cooperative Oncology Group; LVSI, lymphovascular space invasion; pCR pathologic complete response
Five-year OS and DPFS was 47% vs 10% (p<0.0001) and 30% vs 3% (p<0.0001) for the surgery and no surgery groups, respectively, as shown in figures 1a and 1b.
Figure 1.
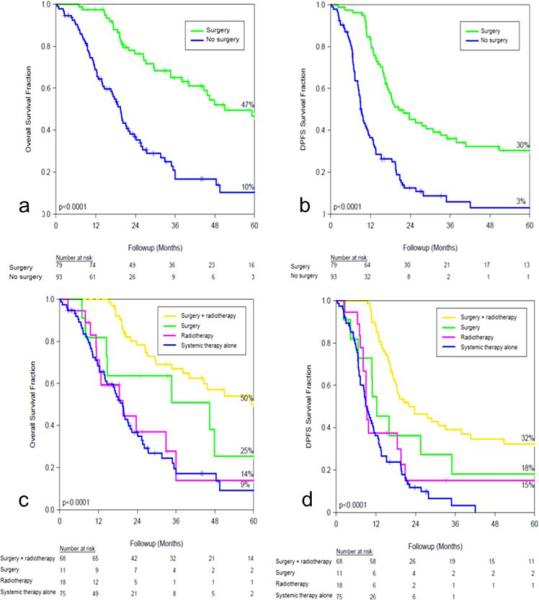
Fractional survival comparison between the surgery and no surgery groups, (a) overall survival and (b) distant progression-free survival (DPFS). Fractional survival stratified by locoregional treatment strategy, (c) overall survival and (d) DPFS.
Ten patients in the surgery group underwent metastasectomy procedures in addition to receiving trimodal therapy. These procedures included liver resection (n=5), contralateral axillary lymph node dissection (n=7), craniotomy (n=1), excision of distant dermal metastases (n=1), supraclavicular lymph node dissection (n=1) and 2 staged neuroradiosurgical procedures in one patient. This group ranged in age from 40-62 years and all had a performance status score of 1. They were further selected based on response to systemic therapy (CR or PR), distant disease burden (1-2 sites) and feasibility of achieving stage IV NED status with metastasectomy. OS for this group ranged from 10-166 months and 7 of these patients are still alive today with stable disease or in NED status. OS and DPFS analyses were repeated after excluding this subset of patients from the surgery group revealing 5-year OS of 40% vs 10% (p<0.0001) and DPFS of 29% vs 3% (p<0.0001) for surgery and no surgery groups, respectively. These patients were included in the surgery group for the remainder of the analyses.
When survival was analyzed by locoregional treatment strategy, patients receiving both surgery and radiotherapy had significantly improved survival outcomes compared to patients who received chemotherapy, radiotherapy or surgery alone (figure 1b, 1c).
Responders demonstrated significantly higher OS and DPFS compared to non-responders. The 5-year OS for responders vs non-responders was 41% versus 13%, respectively (p<0.0001). For DPFS, 5-year values for responders versus non-responders were 24% and 8%, respectively (p<0.0001). A subgroup analysis based on response to systemic therapy was performed and outcomes were compared between the surgery and no surgery groups (figure 2). On pairwise comparison, surgery was associated with significantly better OS and DPFS for both non-responders(surgery vs no surgery p<0.0001 for OS and DPFS) and responders(surgery vs no surgery OS:p=0.0029, DPFS:p=0.0015).
Figure 2.
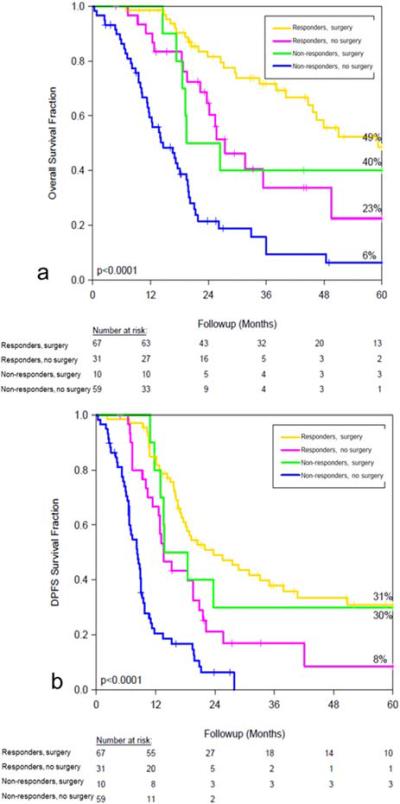
Fractional survival by response to chemotherapy (responders versus non-responders) and further stratified by surgery versus no surgery, (a) overall survival and (b) DPFS.
All factors associated with improved survival on univariate analysis were incorporated into a multivariate regression model. Response to chemotherapy (HR 0.49, CI 0.3-0.8, p=0.005) and locoregional treatment with surgery plus radiation (HR 0.9, CI 0.2-0.6, p=0.0001) reached significance for an effect on OS. Three variables were significantly associated with an independent effect on DPFS: response to chemotherapy (HR 0.59, CI 0.4-0.9, p=0.02), locoregional treatment with surgery plus radiotherapy (HR 0.37, CI 0.2-0.7, p=0.001), and black race (HR 1.96, CI 1.2-3.2, p=0.006).
Median follow-up in the surgery group was 40 months versus 22 months for the no surgery group. The status of the chest wall at last follow up was unknown in 2 patients. Fifteen patients (19%) in the surgery group had local recurrences and re-operation for local control was performed in four of them. There was a marked difference in local control based on locoregional treatment strategy (figure 3a). Treatment with surgery alone or surgery plus XRT was associated with a 4-fold greater rate of local control compared to patients who received systemic therapy alone (p<0.0001) and a greater than 2-fold increase over patients treated with chemotherapy plus radiation. Surgery was also associated with better local control irrespective of the patient’s response to systemic therapy (figure 3b). Non-responders were nearly 3-fold more likely to have severe chest wall involvement if they did not undergo primary tumor resection.
Figure 3.
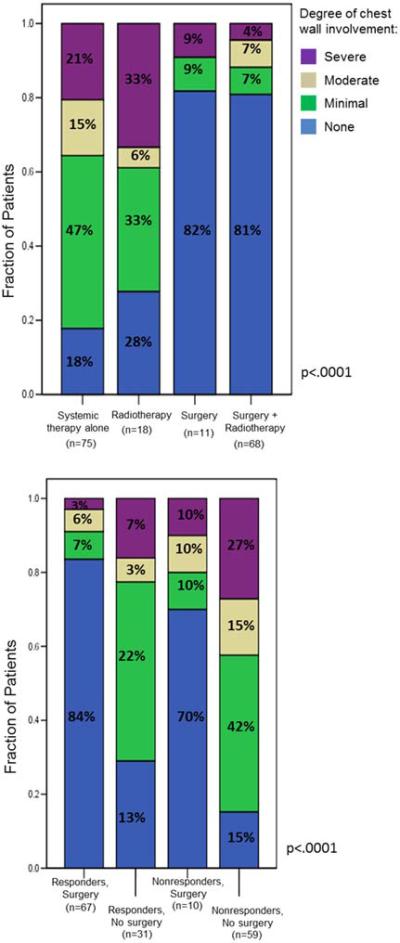
Chest wall disease status at last follow-up, (a) by locoregional treatment strategy and (b) by response to chemotherapy, further stratified by surgery versus no surgery.
On univariate analysis, four factors were significant predictors for local control: response to chemotherapy, pCR, surgery and treatment with surgery plus radiotherapy (table 3). Notably, all 12 patients with a pCR had local control at last followup and therefore an odds radio could not be generated for this variable. Intent of surgery and margin status did not affect local control. Of the 15 surgical patients with close or positive margins, 11 (73%) of them were free of chest wall involvement at the time of last follow up.
Table 3.
Univariate analysis for local control at last follow-up.
| OR | 95% CI | p value | |
|---|---|---|---|
| Black race | 1.93 | 0.82, 4.52 | 0.13 |
| Post-menopausal status | 1.64 | 0.77, 3.46 | 0.20 |
| Hormone Receptor positive |
0.96 | 0.47 ,1.95 | 0.90 |
| HER2 positive | 0.4 | 0.15, 1.03 | 0.06 |
| LVSI | 0.28 | 0.08, 1.02 | 0.05 |
| Lobular histology | 0.86 | 0.22, 3,29 | 0.82 |
| Responders | 0.24 | 0.11, 0.51 | 0.0002 |
| Locoregional therapy with: Radiotherapy alone Surgery alone Surgery +radiotherapy |
1.15 0.18 0.24 |
0.4, 3.33 0.02, 1.49 0.1, 0.58 |
0.80 0.11 0.002 |
| Surgery | 0.23 | 0.1, 0.51 | 0.0003 |
| Curative intent | 0.83 | 0.21, 3.38 | 0.80 |
| pCR | NA* | 0.04 | |
| Close or positive margins | 2.75 | 0.57,13.11 | 0.19 |
| >1 distant disease site | 1.61 | 0.8, 3.24 | 0.19 |
pCR was associated with local control in 100% of cases, therefore an odds ratio could not be generated.
Abbreviations: OR, odds ratio; LVSI, lymphovascular space invasion; pCR, pathologic complete response
On multivariate analysis, surgery (HR=0.25, CI 0.08-0.82, p=0.02) and response to chemotherapy (HR=0.38, 95% CI 0.16-0.92, p=0.03) were significantly associated with local control. Based on results from the univariate analysis, it is clear that pCR is strongly associated with local control, but it could not be included in the multivariate model due to statistical limitations associated with a zero event rate for chest wall disease among patients with a pCR.
Discussion
Primary tumor resection in the management of metastatic, non-inflammatory breast cancer is controversial; its benefit in the treatment of metastatic IBC is even less clear. However, treatment issues specific to inflammatory carcinoma, such as its propensity for severe and sometimes painful local disease, resistance to standard chemotherapy, and its high recurrence rate after treatment20 make the issue of primary tumor resection particularly relevant and led us to review our own experience.
In this analysis, we demonstrated a 29% 5-year OS and 17% 5-year DPFS for patients diagnosed with metastatic IBC. This is significantly higher than the 10% 5-year OS reported elsewhere in the literature and the 6 month median survival previously published from our institution.7, 8, 22 These higher survival rates can be attributed to recent advances in breast cancer therapy, including more effective chemotherapeutic regimens, increased use of endocrine therapy, targeted therapies such as trastuzumab and lapatinib and the use of aggressive and individualized radiotherapy regimens. It is likely that more patients in this recent cohort received multimodality treatment, a factor which has been clearly associated with better survival in IBC 3, 23. In our series, 56% of patients received some form of locoregional therapy and 40% received surgery as well as radiation. The primary focus of this analysis was to determine what benefit, if any, can be attributed to aggressive locoregional therapy and surgery in particular.
First, we showed in a direct comparison of surgery versus no surgery, that primary tumor resection is associated with a nearly 5-fold increase in OS and 10-fold increase in DPFS in patients with metastatic IBC. Importantly, the surgery group was more than twice as likely to have had a measurable response to systemic therapy. This factor has been associated with improved survival in stage IV disease and it is likely that it represents a confounding variable in our study as well as a basis for selection bias.3, 14, 24, 25 Other factors which have been associated with improved survival in advanced breast cancer include having a more favorable performance status score and a lower burden of distant disease. While both of these factors were more common in the surgery group, they did not significantly impact survival on multivariate analysis. This suggests that while these variables may have been a basis for selection bias, they do not appear to have contributed significantly to the better outcomes seen among surgery patients in our cohort. The same is also likely true for HER2 positive receptor status. While the surgery group had proportionately more patients with HER2 positive disease, a factor which was also associated with improved outcome in our study population, survival analysis among the subset of HER2 positive patients showed that OS was still significantly higher in the surgery group compared to the no surgery group (5-year OS 62% versus 13%, respectively, p<0.0001, data not shown).
There were 10 highly select patients in the surgery group who underwent one or more metastasectomy procedures in addition to receiving trimodal therapy for their primary tumor sites. This subset of patients was included in the analysis based on our objective of reporting outcomes for a contemporary cohort of stage IV IBC patients. Though this group of 10 patients did contribute to the more favorable outcomes seen in the surgery group as a whole, both OS and DPFS remained significantly higher for the surgery group after excluding them from a repeat survival analysis (reported in results section above).
Consistent with IBC studies performed in the non-metastatic setting, the highest survival rates in our series were seen in patients treated with trimodality therapy.3-6 While the addition of either locoregional treatment modality improved survival over chemotherapy alone, only treatment with surgery plus radiation was associated with a significant increase in survival on multivariate analysis. In fact, trimodality therapy resulted in a greater than 5-fold increase in OS compared to patients treated with systemic therapy alone. Within our selected surgery cohort, it is important to note that surgery plus radiotherapy was associated with improved outcomes over surgery alone, suggesting that further improvement in outcomes could, at least in part, be based on additional treatment and independent of aforementioned selection biases.
Not surprisingly, clinical response to systemic treatment was a significant and independent prognosticator for both survival and local control. Patients with response to systemic therapy had a three-fold increase in OS and DPFS over non-responders. In an effort to better define any survival benefit attributable to surgery, we compared outcomes between the surgery and no surgery groups after stratifying by response to systemic therapy. In a similar analysis of non-metastatic IBC patients, Fleming et al demonstrated no difference in survival among patients who failed to respond to chemotherapy but among 103 patients with a complete or partial response, 5-year disease-specific survival increased from 43% to 62% with the addition of mastectomy.24 In our series, surgery was associated with improved OS and DPFS both for responders as well as non-responders. It is possible that responders in our cohort who did not undergo surgery were selected out because of a poor response at the primary tumor site, a variable not included in this analysis but one that warrants further investigation. In fact, some contemporary studies suggest that surgery may be reserved for those with residual disease after a partial response to chemotherapy or those with local recurrences after a complete response to chemotherapy and radiation without significantly compromising survival.26, 27
Another area for further research centers around margin status and its impact on outcomes. Close or positive surgical margins have frequently been associated with higher local failure rates in non-metastatic IBC.3, 9, 12 Given that margin status did not affect survival or local control in our series, we postulate that the addition of individualized, typically comprehensive high-dose radiotherapy in 68% of our surgical patients likely negated any impact on outcomes from a positive or close margin. It is also possible, however, that the small number of patients with positive margins was insufficiently powered to show an effect that really does exist.
As patients with metastatic IBC experience longer survival, the issue of maintaining chest wall control becomes more relevant. We demonstrate rates of chest wall control after surgery alone or surgery plus radiation that far exceed those seen after radiation or chemotherapy alone. Thus, surgical consultation for local control, especially for those patients with responsive distant disease, is reasonable to consider. Furthermore, in a subgroup analysis of non-responders, chest wall involvement was nearly 3-fold more likely among patients who did not undergo surgery. Future investigations into quality of life improvements associated with chest wall disease control may help guide surgical decision making in this subgroup of patients who do not have responsive distant disease and therefore may have significantly shorter survival.
This retrospective data suggests that surgery may impact outcomes for patients with metastatic IBC. However, factors such as favorable tumor biology, response to systemic therapy, and lower distant disease burden have undoubtedly biased these results. Clinical recommendation for surgery should be highly selective and include a thorough discussion of the limitations of supporting studies such as the present one as well as an acknowledgement of the lack of data from a randomized trial.
Acknowledgments
There was no specific funding for this work.
Footnotes
The authors have no financial disclosures or disclaimers to report.
This research was presented in part in June, 2012 at the annual meeting of the American Society of Clinical Oncology in Chicago, IL.
REFERENCES
- 1.Wingo PA, Jamison PM, Young JL, Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15(3):321–8. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515–23. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72(2):474–84. doi: 10.1016/j.ijrobp.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehman S, Reddy CA, Tendulkar RD. Modern Outcomes of Inflammatory Breast Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Harris EE, Schultz D, Bertsch H, Fox K, Glick J, Solin LJ. Ten-year outcome after combined modality therapy for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1200–8. doi: 10.1016/s0360-3016(02)04201-3. [DOI] [PubMed] [Google Scholar]
- 6.Liauw SL, Benda RK, Morris CG, Mendenhall NP. Inflammatory breast carcinoma: outcomes with trimodality therapy for nonmetastatic disease. Cancer. 2004;100(5):920–8. doi: 10.1002/cncr.20083. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland S, Ashley S, Walsh G, Smith IE, Johnston SR. Inflammatory breast cancer--The Royal Marsden Hospital experience: a review of 155 patients treated from 1990 to 2007. Cancer. 116(11 Suppl):2815–20. doi: 10.1002/cncr.25178. [DOI] [PubMed] [Google Scholar]
- 8.Elias EG, Vachon DA, Didolkar MS, Aisner J. Long-term results of a combined modality approach in treating inflammatory carcinoma of the breast. Am J Surg. 1991;162(3):231–5. doi: 10.1016/0002-9610(91)90076-p. [DOI] [PubMed] [Google Scholar]
- 9.Curcio LD, Rupp E, Williams WL, et al. Beyond palliative mastectomy in inflammatory breast cancer--a reassessment of margin status. Ann Surg Oncol. 1999;6(3):249–54. doi: 10.1007/s10434-999-0249-3. [DOI] [PubMed] [Google Scholar]
- 10.Dawood S, Ueno NT, Valero V, et al. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2012;23(4):870–5. doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 11.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132(4):620–6. doi: 10.1067/msy.2002.127544. discussion 26-7. [DOI] [PubMed] [Google Scholar]
- 12.Hazard HW, Gorla SR, Scholtens D, Kiel K, Gradishar WJ, Khan SA. Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer. 2008;113(8):2011–9. doi: 10.1002/cncr.23870. [DOI] [PubMed] [Google Scholar]
- 13.Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol. 2007;14(8):2187–94. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 14.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24(18):2743–9. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247(5):732–8. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 16.Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14(12):3345–51. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 17.Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat. 2009;115(1):7–12. doi: 10.1007/s10549-008-0101-7. [DOI] [PubMed] [Google Scholar]
- 18.Shien T, Kinoshita T, Shimizu C, et al. Primary tumor resection improves the survival of younger patients with metastatic breast cancer. Oncol Rep. 2009;21(3):827–32. [PubMed] [Google Scholar]
- 19.Ruiterkamp J, Ernst MF, van de Poll-Franse LV, Bosscha K, Tjan-Heijnen VC, Voogd AC. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol. 2009;35(11):1146–51. doi: 10.1016/j.ejso.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Cristofanilli M, Valero V, Buzdar AU, et al. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007;110(7):1436–44. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Dawood S, Ueno NT, Valero V, et al. Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann Oncol. 2010;21(12):2348–55. doi: 10.1093/annonc/mdq239. [DOI] [PubMed] [Google Scholar]
- 23.Dawood S, Ueno NT, Valero V, et al. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 24.Fleming RY, Asmar L, Buzdar AU, et al. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4(6):452–61. doi: 10.1007/BF02303668. [DOI] [PubMed] [Google Scholar]
- 25.Ueno NT, Buzdar AU, Singletary SE, et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40(4):321–9. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 26.Bates T, Williams NJ, Bendall S, Bassett EE, Coltart RS. Primary chemo-radiotherapy in the treatment of locally advanced and inflammatory breast cancer. Breast. 2012;21(3):330–5. doi: 10.1016/j.breast.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Abrous-Anane S, Savignoni A, Daveau C, et al. Management of inflammatory breast cancer after neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2011;79(4):1055–63. doi: 10.1016/j.ijrobp.2009.12.009. [DOI] [PubMed] [Google Scholar]


