Abstract Abstract
Endomyocardial fibrosis (EMF) is a restrictive cardiomyopathy of unknown origin, affecting predominantly the right side of the heart. Its highest prevalence is in poor regions of sub-Saharan Africa, where it is usually found in children and adolescents. In areas where it is endemic, EMF is a major cause of heart failure and premature death. EMF of the right ventricle has unique clinical, electrocardiographic, and echocardiographic signs. Several features of advanced disease are not fully understood, the most striking being the presence of severe ascites with little or no pedal edema. Echocardiography is the main diagnostic tool and supports management of the disease in most patients, as it allows assessment of the severity and extension of endocardial fibrosis, the quality of the right atrioventricular valve, and the presence of intracardiac thrombi. Pulmonary hypertension in the setting of right EMF is related to chronic thromboembolism, through the chronic thrombi present in the severely enlarged right atrium found in advanced disease. The management of right EMF is difficult. Treatment of symptomatic patients should be surgical. However, as the disease is usually detected in late stages, with severe and advanced lesions, surgery cannot be performed without a very high risk of death and complications. New surgical approaches addressing several components of the structural cardiac abnormalities have been attempted, with promising results. EMF remains one of the most neglected diseases worldwide, and research into its pathophysiological mechanisms will probably improve outcomes and alter the natural history of the disease. This requires improvement in the health systems in areas of endemicity as well as the design of international collaborative research projects.
Keywords: pulmonary hypertension, endomyocardial fibrosis, sub-Saharan Africa
Endomyocardial fibrosis (EMF) is a restrictive cardiomyopathy of unknown etiology that affects primarily children and adolescents in underserved areas of sub-Saharan Africa, Latin America, and Asia.1 It is the most prevalent form of restrictive cardiomyopathy worldwide, showing marked variation in geographic distribution.2-4 The hypotheses postulated to explain the etiology of EMF have not been proven and cannot explain its occurrence worldwide. They include cerium deficiency, magnesium deficiency, cassava, malnutrition, parasitic diseases, viral diseases, autoimmunity, allergy, and ethnicity.3
The right ventricle is the cardiac chamber most frequently affected in EMF, either in isolation or as part of bilateral disease.5 This may reflect better clinical tolerance of right-heart forms of the disease, which allows the patients to reach health facilities. Patients with right EMF are relatively asymptomatic, despite marked structural and hemodynamic abnormalities, when assessed by ultrasound.6 Isolated or predominant left EMF causes earlier deaths, usually as a result of severe pulmonary hypertension and systemic thromboembolism.
Natural history
The endocardial lesions in EMF are said to evolve in 3 successive stages, namely, necrosis, thrombosis, and fibrosis.7,8 The rate and progression of EMF are variable and have been described in hospital-based studies,7,8 most of them performed before echocardiography was available. Most patients have rapidly progressing heart failure, leading to death within 2 years of the initial insult.5 However, some patients may have a steady period without any clinical deterioration, and others may experience remission of the clinical signs, with no further progression, without seeking specialized medical care. This might explain why there has been little improvement in our knowledge of the natural course of the illness.
EMF patients usually die from complications of progressive chronic heart failure leading to protein-losing enteropathy, as well from cardiac cirrhosis complicating with hepatic failure. They may also have sudden death caused by massive pulmonary embolism, systemic embolic events, or arrhythmia.8,9
Pathology
The basic lesion of EMF is ventricular endocardial fibrosis. There seems to be no primary involvement of other organs, but a recent study has suggested concomitant effects on the skeletal muscle.10 Macroscopically, there is cardiomegaly due to severe atrial dilatation that is related to both impedance of ventricular filling and massive atrioventricular valve regurgitation. The fibrotic endocardium is characteristically prominent in the right ventricle, particularly the apex. The scar tissue may be massive, engulfing and fusing the trabeculae carneae, obliterating the apex of the right ventricle, and fixing and obliterating the tricuspid valve apparatus, thus resulting in a severely dilated tricuspid annulus and free tricuspid regurgitation.11,12 The pulmonary valve and arteries are spared from the fibrotic process.
The histology of the heart shows abnormalities in all 3 layers.13-15 The most prominent lesion is endocardial thickening, which is due to acellular fibrocollagen tissue deposition under a layer of normal endocardium.15-18 There is also myocytolysis of variable degree in the inner portion of the myocardium, but myocardial loss is rarely marked. In established disease, there are no intense inflammatory infiltrates, and tissue eosinophilia is rare. Small-vessel disease is unusual.14
Pathophysiology
The pathophysiology of EMF associates the restriction of diastolic filling of the ventricle caused by endocardial fibrosis with severe atrioventricular valve regurgitation due to involvement of the leaflets and the subvalvar apparatus in the fibrotic process. The endocardial scarring of the right ventricular wall not only reduces ventricular cavity size and impedes adequate filling but also impairs systolic function to a variable degree. Fibrosis of the components of the tricuspid valve (papillary muscles, chordae, and leaflets) and their fusion to the ventricular wall usually result in severe tricuspid regurgitation but may also lead to tricuspid stenosis.
On the other hand, reduced right ventricular filling and consequent decrease in stroke volume are responsible for the sustained low cardiac output, resulting in features such as finger and toe clubbing, growth retardation, testicular atrophy, failure to develop male secondary sexual characteristics, and cachexia.19-22 This can be exacerbated in the presence of pericardial effusion and/or atrial arrhythmia.
There is an increase in pressure inside the right atrium, which becomes aneurysmal, as a result of both impedance of right ventricular filling and severe tricuspid regurgitation. This high pressure is transmitted to the systemic veins and is responsible for some characteristic signs of this condition, namely, exophthalmos, elevated jugular pressure, gross hepatomegaly, and congestive splenomegaly.
The pathophysiology of some distinctive features of right EMF is not clearly understood. Central cyanosis in the absence of a patent foramen ovale, severe right heart failure with ascites but no pedal edema, marked parotid swelling, asthma-like episodes in the absence of blood hypereosinophilia, and the characteristic hyperpigmentation of lips and gums remain subjects of controversy.23,24
Right EMF is clearly associated with increased clot formation and pulmonary embolism. Early disease causes acute pulmonary embolism and sudden death from ventricular thrombi, while advanced EMF is associated with chronic pulmonary thromboembolism due to stasis and clot formation inside the right atrium.25,26 Pulmonary hypertension may occur in both cases.
Mechanism of right ventricular obliteration
Ventricular obliteration and cavity retraction are distinctive features of right EMF, clearly depicted on echocardiography. The mechanism of right ventricular cavity obliteration, studied in patients undergoing surgery, corresponds to compaction of trabeculae between the thickened fibrous endocardium and the epicardium, excluding the trabecular portion of the ventricle from circulation.27 The fibrotic process produces an “artificial floor” to the right ventricle, separating the inflow cavity from the obliterated muscular trabecular part, which does not contain thrombus or blood. The moderator band is lost, engulfed in the endocardial fibrosis. The reopening of this obliterated portion and partial reconstitution the right ventricular cavity are possible through careful mobilization and separation of the trabeculae.27
The progressive fusion of trabeculae between the thickened endocardium and the epicardium results in retraction of the ventricular cavity. There is a reduction in the distance between the epicardium and the false floor, resulting in an apical notch, a distinctive echocardiographic feature of advanced right EMF. At this stage, mobilization of trabeculae to promote reopening of the trabecular portion of the ventricular cavity is no longer possible.
Pulmonary hypertension
Pulmonary hypertension can be found in both left and right EMF, depending on the stage of the disease and the severity of the structural lesions. Severe pulmonary hypertension is common in left EMF,25 associated with the retrograde increase of pulmonary pressure caused by diastolic dysfunction and mitral valve abnormalities. In right EMF, there is pulmonary hypertension due to acute or chronic thromboembolism26 linked to acute or recurrent fragmentation of thrombi lodged in any of the cavities. The mechanisms underlying failure to resolve thrombi are still uncertain, but both ongoing inflammation and stasis may play a major role. Progression to pulmonary hypertension may occur over a short period despite anticoagulation with warfarin.26
The clinical and echocardiographic features of pulmonary hypertension in right EMF may be altered by distorted anatomy and by the reduction in ventricular output. The bulging interventricular and interatrial septa compressing the left cavities impair left ventricular filling, further contributing to retrograde pulmonary hypertension.
Possible role of infection, prothrombotic factors, and endothelial dysfunction
The climatic restriction of areas where EMF is endemic and the reports of sporadic cases in foreigners after short visits to these areas suggest a role of infectious agents in the pathogenesis of the disease.28,29 Studies from Africa and South America have implicated Schistosoma species in the pathogenesis of EMF because they induce severe hypereosinophilia and were found in pathology specimens from some patients with EMF.30-33 However, a community study in an area of Egypt where schistosomiasis is endemic failed to find cases of EMF in increased numbers.34
The use of traditional medicines has been suggested as a trigger for pulmonary hypertension in Africa.35 Increased levels of interleukin-6, endothelial cell activation, and increased fibrinolysis were found in patients with recent-onset EMF, strongly suggesting that inflammation, endothelial injury, and procoagulant changes are major players in the pathogenesis of this condition.36 They may also play a role in determining progression to pulmonary hypertension.
Diagnosis
Patients with right EMF often present distinctive features, such as exophthalmos, jaundice, peripheral cyanosis, finger clubbing, and ascites without pedal edema.22,37,38 The pulse may be irregular because of the presence of atrial fibrillation. The evaluation of the chest may reveal a heave at the left upper parasternal area, corresponding to a dilated and pulsatile right ventricular outflow tract. The cardiac auscultation can be unremarkable when free atrioventricular regurgitation is present and the tricuspid leaflets are immobile, but a third sound is usually heard.
Chest X-ray
On chest X-ray, there is cardiomegaly as a result of severe right atrial enlargement and/or pericardial effusion. A bulge over the left heart border may be depicted, related to infundibulum dilatation, and the lung fields are usually hypoperfused.
Electrocardiography
Electrocardiographic abnormalities include atrial arrhythmia, low-voltage QRS complexes, nonspecific ST–T wave changes, and conduction disturbances. In severe right EMF, the electrocardiogram shows a tall and broad right atrial wave and a characteristic ‘‘QR’’ pattern in V3R or V1.3 In advanced disease, atrial fibrillation and ventricular ectopic beats are common.
Echocardiography and Doppler
This is the tool used most for diagnosis of EMF in areas where the disease is endemic in Africa, assessing the structural and hemodynamic abnormalities in this condition and allowing pre- and postoperative management in most patients.39,40 Echocardiography detects the presence of a thickened endocardium in the ventricular wall, evaluates the cavity dimensions, and assesses the abnormalities of the atrioventricular valve and subvalvar apparatus.41 In right EMF, there is obliteration of the trabecular portion of the right ventricle, with reduction in cavity volume, tricuspid annulus dilatation, and regurgitation. In advanced disease, an apical notch (due to right ventricular retraction) is usually found, associated with free tricuspid regurgitation, spontaneous contrast, multiple right atrial thrombi, and pericardial effusion (Fig. 1). There is usually marked dilatation of the hepatic veins, with little change in diameter with respiration.
Figure 1.
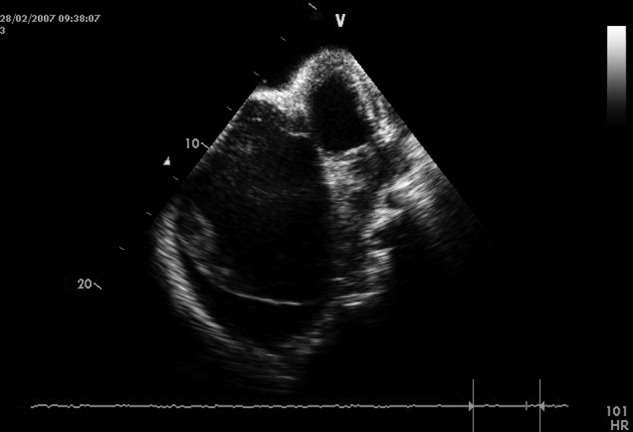
Echocardiographic features of bilateral endomyocardial fibrosis, with predominance of right-heart lesions. Notice the massive reduction of the dimension of the right ventricle, retraction of the leaflets of the tricuspid valve, and aneurysmal dilatation of the right atrium. A typical right apical notch and pericardial effusion confirm advanced disease. Mural thrombi and spontaneous contrast are seen in the right atrium.
The assessment of pulmonary artery pressure is particularly important for determining subsequent management.42 In the presence of severe right EMF, this assessment may be hampered by the absence of gradient across the tricuspid valve, by the presence of large right atrial thrombi deviating the regurgitant jet, or by major distortion of heart anatomy.15 In such cases, pulmonary regurgitation may be used to estimate the mean and diastolic pulmonary pressures (Fig. 2).
Figure 2.
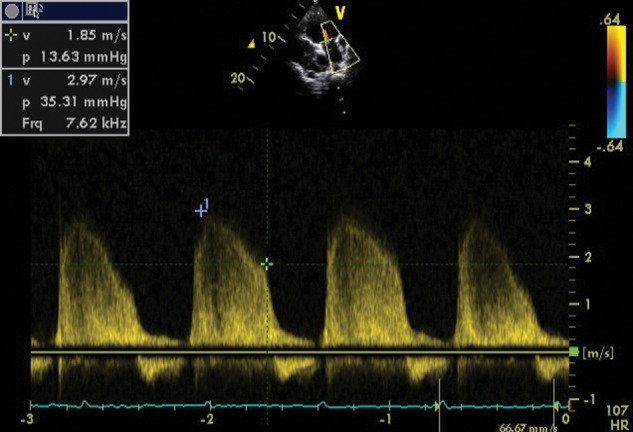
Doppler image of pulmonary regurgitation that allows the estimation of pulmonary pressures in a patient with free tricuspid regurgitation.
In severe disease with restriction of tricuspid leaflet movement and severe pulmonary valve ring dilatation, the right side of the heart may act physiologically as a single chamber (with equal pressures from the pulmonary artery to the right atrium), making it impossible to estimate the pulmonary pressure from either tricuspid or pulmonary regurgitation. Indirect signs of pulmonary hypertension, such as myocardial function, may also be difficult to evaluate, because endocardial fibrosis restricts the motion of the underlying myocardium.
Magnetic resonance imaging (MRI)
There are many advantages of MRI over other diagnostic techniques, mainly in patients with severe structural abnormalities leading to distorted anatomy, but it is an expensive technique and therefore not readily available in most areas where EMF is endemic. MRI is the ideal tool for management, allowing monitoring of the response to medical and surgical treatment.43 It confirms the existence of thrombi or calcifications, allows an exact delineation of hypoperfused areas that correspond to fibrosis, and provides hemodynamic information.44 Myocardial suppression scans, acquired after injection of gadolinium, allow exact appreciation of the disease extension of fibrosis by delayed hyperenhancement of the pathologic areas.43-45
Cardiac catheterization
In poor areas where EMF is endemic, cardiac catheterization is rarely used for the diagnosis of EMF, since complete noninvasive assessment is possible in most patients. On the other hand, cardiac catheterization is technically challenging and dangerous in advanced disease and may be misleading in localized or mild forms. Advanced right EMF is characterized by pressures that are equal in form and amplitude in the right atrium, right ventricle, and pulmonary artery. The right ventricular angiogram shows loss of trabeculated pattern, a flattened apex, reduction of ventricular volume, a dilated hypercontractile infundibulum, free tricuspid reflux to an aneurysmal right atrium, and dilated cava veins.46,47
There are several reasons to avoid cardiac catheterization in patients with right EMF, mainly those with large ascites and low basal cardiac output. Because of high systemic venous pressure, bleeding from venous puncture sites is a major concern. Access to the right ventricle and pulmonary artery is difficult because of marked dilatation of the right atrium, tricuspid regurgitation, and the small right ventricular cavity, as well as distortion and displacement of the right ventricular outflow tract. The risk of pulmonary thromboembolism from right atrial thrombi cannot be neglected, and acute hemodynamic deterioration during the procedure due to stimulation of atrial tachyarrhythmia is a real threat. Nevertheless, cardiac catheterization must be considered before surgery to add hemodynamic or structural information, in particular to confirm pulmonary pressures and the size of the right ventricle.15
Management and Prognosis
There is no effective treatment for EMF. Medical therapy is used to control symptoms and reduce the progression to irreversible changes in the liver and pulmonary vessels.
Although surgery is indicated for all symptomatic patients,46 the majority of patients are not operated on because of late presentation and/or lack of expertise and infrastructure for the surgical treatment. The surgical mortality varies between 15% and 30%,3 particularly when extensive endocardial resection or tricuspid valve prostheses are needed. The rate of relapse after surgical correction is not clear.48
The disease has a poor prognosis, with high morbidity and mortality related to refractory heart failure, thromboembolism, and arrhythmia. In early phases of right EMF, surgery may be needed to prevent the development of pulmonary embolism and pulmonary hypertension (Fig. 3).26 On the other hand, recurrent stasis and clot formation on the right atrium may determine progression of chronic thromboembolic pulmonary hypertension despite surgery.
Figure 3.
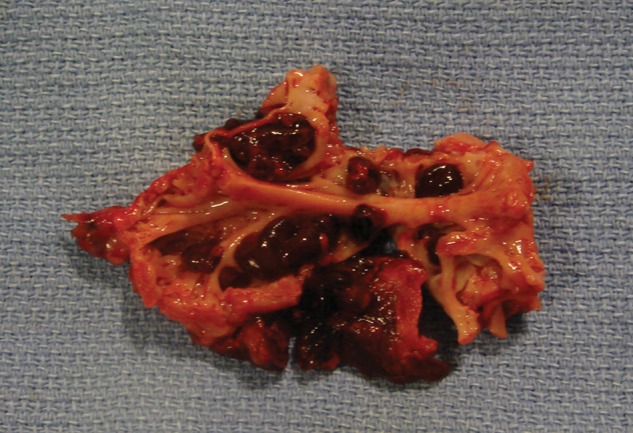
Right atrial appendage tissue resected during surgery, showing several thrombi attached to the wall.
Innovative surgical approaches to right EMF. Surgery is performed through right atriotomy, which exposes the dilated tricuspid valve, allowing wide access to the right ventricle, definition of the distribution of mural lesions, and evaluation of the extent of pathological involvement of the tricuspid valve. Endocardial resection starts near the tricuspid annulus, by retracting the leaflets of the valve or mobilizing them if fused. A cleavage plane is achieved by sharp dissection, and a combination of sharp and blunt dissection is used to excise the thick, fibrous endocardial lining, ensuring preservation and mobilization of the tricuspid valve chordae and papillary muscles; this exposes the fused muscular trabeculae (Fig. 4). These latter are carefully mobilized to recreate a cavity, taking care not to perforate the ventricular wall. Reconstruction of the tricuspid annulus is done with 2 bands of Gortex tubes.
Figure 4.
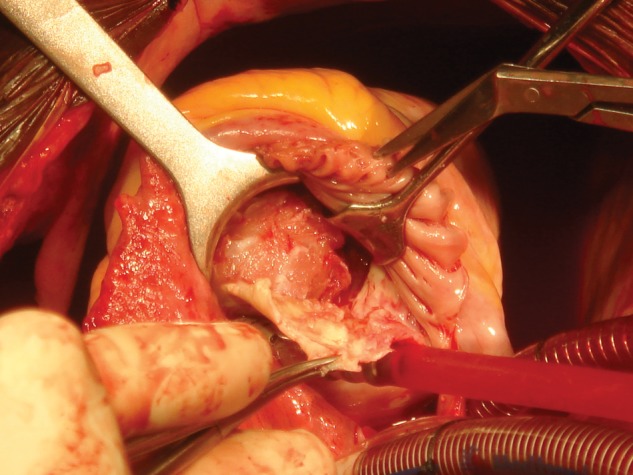
Image of endocardial resection, showing the removal of the fibrous plaque that releases the right ventricular myocardium.
By avoiding complete atrioventricular block, preserving the native atrioventricular valves, releasing the myocardium, and making use of viable myocardium in the obliterated area, this technique has improved the postoperative mortality and ameliorated the quality of life after surgery. Exclusion criteria for this surgery are severe fibrosis with notching of the right apex, severe ascites, and pulmonary arterial hypertension; this last is considered an important prognostic factor.36 Mild increases in pulmonary pressures or failure to achieve adequate right ventricular cavity size should stimulate the use of a Glenn procedure.49,50
The immediate postoperative follow-up shows clinical and hemodynamic improvement, as measured by echocardiography. However, there is a need to assess the rates of survival free of events and recurrence of fibrotic lesions in these patients.
Perspectives
Almost 7 decades after it was first described, EMF remains one of the most neglected cardiovascular diseases worldwide. Since it is confined to low-income settings, EMF has received little attention from the scientific community. Patients do not benefit from the modern management options currently available. Despite major developments in imaging techniques that have allowed better characterization of heart disease and its progression, there are many gaps in our knowledge, and the early phases of this condition have not been clearly defined.
Data from India show that the incidence of the disease has decreased during the past decades, probably as a result of improvement in the living standards of people in areas where the disease was previously endemic, including declines in childhood malnutrition, infections, and parasite-associated eosinophilia.25 In contrast, EMF remains a public health problem in African countries such as Uganda and Mozambique, and sporadic cases have been reported in new areas of the continent, including Malawi, Zimbabwe, Tanzania, South Africa, Senegal, Kenya, Ghana, Zambia, Congo, and Ethiopia.51
Chronic inflammation, allergy, and thrombotic factors seem to be major determinants of the natural history of EMF. Understanding their roles in pathogenesis might allow the use of currently available drugs, with major impact on management and prognosis. This requires improvement in health systems and service provision, including access to surgery in areas where the disease is endemic, which may contribute to improving outcomes and fostering basic research into the mechanisms of this neglected condition.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: still a mystery after 60 years. PLoS Negl Trop Dis 2008;2(2):e97. doi:10.1371/journal.pntd.0000097. [DOI] [PMC free article] [PubMed]
- 2.Somers K. Restrictive cardiomyopathies. In Pongpanich B, Sueblinvong V, Vongprateep C, eds. Pediatric cardiology. International Congress Series 906. Amsterdam: Excerpta Medica, 1990.
- 3.Yacoub S, Mocumbi AO, Yacoub MH. Neglected tropical cardiomyopathies: I. Chagas disease. Heart 2008;94(2):244–248. [DOI] [PubMed]
- 4.Mocumbi A, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med 2008;359(1):43–49. [DOI] [PubMed]
- 5.Mocumbi AO, Yacoub S, Yacoub MH. Neglected tropical cardiomyopathies: II. Endomyocardial fibrosis. Heart 2008;94(3):384–390. [DOI] [PubMed]
- 6.Salemi VMC, Rochitte CE, Barbosa MM, Mady C. Clinical and echocardiographic dissociation in a patient with right ventricular endomyocardial fibrosis. Heart 2005;91(11):1399. [DOI] [PMC free article] [PubMed]
- 7.Olsen EGJ. Morphological overview and pathogenic mechanism in EMF associated with eosinophilia. In: Olsen EGJ, Sekiguchi M, eds. Cardiomyopathy update 3. Tokyo: University of Tokyo Press, 1990:21.
- 8.Parry EHO, Abrahams DG. The natural history of endomyocardial fibrosis. Q J Med 1965;34(4):383–408. [PubMed]
- 9.Fernández Vázquez E, Lacárcel Bautista C, Alcázar Navarrete B, Casado Moreno I, Espejo Guerrero A, Ruiz Carazo E. Chronic thromboembolic pulmonary hypertension associated with endomyocardial fibrosis of the right ventricle. Arch Bronconeumol 2003;39(8):370–372. [DOI] [PubMed]
- 10.Ashok PP, Sapru RP, Radhakrishnan VV, Virmani V. Skeletal muscle involvement in tropical endomyocardial fibrosis. J Neurol Sci 1982;54(1):1–12. [DOI] [PubMed]
- 11.Connor DH, Somers K, Hutt MS, Manion WC, D’Arbela PG. Endomyocardial fibrosis in Uganda (Davies’ disease). Part I: an epidemiologic, clinical, and pathologic study. Am Heart J 1967;74(5):687–709. [DOI] [PubMed]
- 12.Mocumbi AO, Yacoub MH, Yokohama H, Ferreira MB. Right ventricular endomyocardial fibrosis. Cardiovasc Pathol 2009;18(1):64–65. [DOI] [PubMed]
- 13.Farrer-Brown G, Tarbit MH, Somers K, Hutt MSR. Microvascular study of hearts with endomyocardial fibrosis. Br Heart J 1972;34(12):1250–1262. [DOI] [PMC free article] [PubMed]
- 14.Andrade ZA, Teixeira ARL. Changes in the vasculature in endomyocardial fibrosis and their possible significance. Am Heart J 1973;86(2):152–158. [DOI] [PubMed]
- 15.Mocumbi AO, Carrilho C, Burke MM, Wright G, Yacoub MH. Emergency surgical treatment of advanced endomyocardial fibrosis in Mozambique. Nat Clin Pract Cardiovasc Med 2009;6(3):210–214. [DOI] [PubMed]
- 16.Connor DH, Somers K, Hutt NSR, Manion WC, D’Arbela P. Endomyocardial fibrosis in Uganda (Davies’ disease). Part II: an epidemiologic, clinical, and pathologic study. Am Heart J 1968;75(1):107–124. [DOI] [PubMed]
- 17.Chopra P, Narula J, Talwar KK, Kumar V, Bhatia ML. Histomorphologic characteristics of endomyocardial fibrosis: an endomyocardial biopsy study. Hum Pathol 1990;21(6):613–616. [DOI] [PubMed]
- 18.Seth S, Thatai D, Sharma S, Chopra P, Talwar KK. Clinico-pathological evaluation of restrictive cardiomyopathy (endomyocardial fibrosis and idiopathic restrictive cardiomyopathy) in India. Eur J Heart Fail 2004;6(6):723–729. [DOI] [PubMed]
- 19.Jaiyesimi F, Falase AO. Extracardiac manifestations of endomyocardial fibrosis and their psychosocial complications. Trop Cardiol 1976;2(5):5–11.
- 20.Somers K. A history of endomyocardial fibrosis in Uganda. In: Valiathan MS, Somers K, Kartha CC, eds. Endomyocardial fibrosis. Delhi: Oxford University Press, 1993:3–9.
- 21.Bolarin DM, Andy J. Clinical feminization and serum testosterone levels in male patients with chronic African endomyocardial fibrosis. Trop Geogr Med 1982;34(4):309–312. [PubMed]
- 22.Abrahams DG. Endomyocardial fibrosis of the right ventricle. Q J Med 1962;31(1):1–20. [PubMed]
- 23.Jaiyesimi F. Controversies and advances in endomyocardial fibrosis: a review. Afr J Med Med Sci 1982;11(2):37–46. [PubMed]
- 24.Mocumbi AO. Endomyocardial fibrosis. In: da Cruz EM, Ivy D, Jaggers J, eds. Pediatric and congenital cardiology, cardiac surgery and intensive care. Vol. 4. London: Springer, 2014:2421–2438. doi:10.1007/978-1-4471-4619-3_10.
- 25.Vijayaraghavan G, Sivasankaran S. Tropical endomyocardial fibrosis in India: a vanishing disease! Indian J Med Res 2012;136(5):729–738. [PMC free article] [PubMed]
- 26.Ribeiro PA, Muthusamy R, Duran CM. Right-sided endomyocardial fibrosis with recurrent pulmonary emboli leading to irreversible pulmonary hypertension. Br Heart J 1992;68(3):326–329. [DOI] [PMC free article] [PubMed]
- 27.Mocumbi AO, Sidi D, Vouhe P, Yacoub M. An innovative technique for the relief of right ventricular trabecular cavity obliteration in endomyocardial fibrosis. J Thorac Cardiovasc Surg 2007;134(4):1070–1072. [DOI] [PubMed]
- 28.Brockington IF, Olsen EGJ, Goodwin JF. Endomyocardial fibrosis in Europeans resident in Africa. Lancet 1967;289(7490):583–588. [DOI] [PubMed]
- 29.Beck W, Schrire V. Endomyocardial fibrosis in Caucasians previously resident in tropical Africa. Br Heart J 1972;34(9):915–918. [DOI] [PMC free article] [PubMed]
- 30.Rashwan MA, Ayman M, Ashour S, Hassanin MM, Zeina AA. Endomyocardial fibrosis in Egypt: an illustrated review. Br Heart J 1995;73(3):284–289. [DOI] [PMC free article] [PubMed]
- 31.Victor EG, Lira V, Arruda A, Monteiro I, Lima R. Granulomas cardiacos a ovos de schistosoma e fibrose endomiocardica. Arq Bras Cardiol 1996;67(4):259–261. [PubMed]
- 32.Carneiro RC, Santos AL, Brant LC, Rabelo FT, Ligeiro CM, Barcelos IP, Silva V, Silva VS, Nunes MC. Endomyocardial fibrosis associated with mansoni schistosomiasis. Rev Soc Bras Trop 2011;44(5):644–645. [DOI] [PubMed]
- 33.Gran F, Albert DC, Moreno A. Schistosomiasis and tropical endomyocardial fibrosis with pulmonary hypertension. Rev Esp Cardiol 2011;64(8):713. [DOI] [PubMed]
- 34.Barbosa MM, Lamounier JA, Oliveira EC, Souza MV, Marques DS, Lambertucci JR. Endomyocardial fibrosis and cardiomyopathy in an area endemic for schistosomiasis. Am J Trop Med Hyg 1998;58(1):26–27. [DOI] [PubMed]
- 35.Heath D, Shaba J, Williams A, Smith P, Kombe A. A pulmonary hypertension-producing plant from Tanzania. Thorax 1975;30(4):399–404. [DOI] [PMC free article] [PubMed]
- 36.Mocumbi AO. Epidemiology, pathogenesis and management of endomyocardial fibrosis [PhD thesis]. Imperial College London, 2008.
- 37.Mocumbi AO, Falase AO. Recent advances in the epidemiology, diagnosis and treatment of endomyocardial fibrosis in Africa. Heart 2013;99(20):1481–1487. [DOI] [PubMed]
- 38.Ikeme AC. The diagnosis of endomyocardial fibrosis. Afr J Med Sci 1972;3(4):327–333. [PubMed]
- 39.Vijayaraghavan G, Davies J, Sadanandan S, Spry CJF, Gibson DG, Goodwin JF. Echocardiographic features of tropical endomyocardial fibrosis in South India. Br Heart J 1983;50(5):450–459. [DOI] [PMC free article] [PubMed]
- 40.Okereke OUJ, Chikwendu VC, Ihenacho HNC, Ikeh VO. Non-invasive diagnosis of endomyocardial fibrosis in Nigeria using two-dimensional echocardiography. Trop Cardiol 1991;17(67):97–103.
- 41.Berensztein CS, Piñeiro G, Marcotegui M, Brunoldi R, Vázquez Blanco M, Lerman J. Usefulness of echocardiography and Doppler echocardiographiy in endomyocardial fibrosis. J Am Soc Echocardiogr 2000;13(5):385–392. [DOI] [PubMed]
- 42.Irwin RB, Luckie RB, Luckie M, Khattar RS. Tricuspid regurgitation: contemporary management of a neglected valvular lesion. Postgrad Med J 2010;86(1021):648–655. [DOI] [PubMed]
- 43.Smedema JP, Winckels SKG, Snoep G, Vainer J, Bekkers SCAM, Crijns HJGM. Tropical endomyocardial fibrosis (Davies’ disease): case report demonstrating the role of magnetic resonance imaging. Int J Cardiovasc Imaging 2004;20(6):517–522. [DOI] [PubMed]
- 44.Estornell J, López MP, Dicenta F, Igual B, Martínez V, Sonlleva A. Usefulness of magnetic resonance imaging in the assessment of endomyocardial fibrosis. Rev Esp Cardiol 2003;56(3):321–324. [DOI] [PubMed]
- 45.Cury RC, Abbara S, Sandoval LJ, Houser S, Brady TJ, Palacios IF. Visualization of EMF by delayed-enhancement magnetic resonance imaging. Circulation 2005;111(9):e115–e117. [DOI] [PubMed]
- 46.Graham JM, Lawrie GM, Feteih NM, Debakey ME. Management of endomyocardial fibrosis: successful surgical treatment of biventricular involvement and consideration of the superiority of operative intervention. Am Heart J 1981;102(4):771–775. [DOI] [PubMed]
- 47.Hassan W, Fawzy ME, Al Helaly S, Hegazy H, Malik S. Pitfalls in diagnosis and clinical, echocardiographic, and hemodynamic findings in endomyocardial fibrosis: a 25-year experience. Chest 2005;128(6):3985–3992. [DOI] [PubMed]
- 48.Moraes CR, Buffolo E, Moraes Neto F, Rodrigues JV, Gomes CA, Branco JN, Aguiar L. Recurrence of fibrosis after endomyocardial fibrosis surgery [in Portuguese]. Arq Bras Cardiol 1996;67(4):297–299. [PubMed]
- 49.Yie K, Sung S, Kim D, Woo J. Bidirectional cavopulmonary shunt as a rescue procedure for right ventricular endomyocardial fibrosis. Interact Cardiovasc Thorac Surg 2004;3(1):86–88. [DOI] [PubMed]
- 50.Anbarasu M, Krishna Manobar SR, Titus T, Neelakandha KS. One-and-a-half ventricle repair for right ventricular endomyocardial fibrosis. Asian Cardiovasc Thorac Ann 2004;12(4):363–365. [DOI] [PubMed]
- 51.Mocumbi AO. Recent trends in the epidemiology of endomyocardial fibrosis in Africa. Pediatr Int Child Health 2012;32(2):63–64. [DOI] [PubMed]


