Abstract
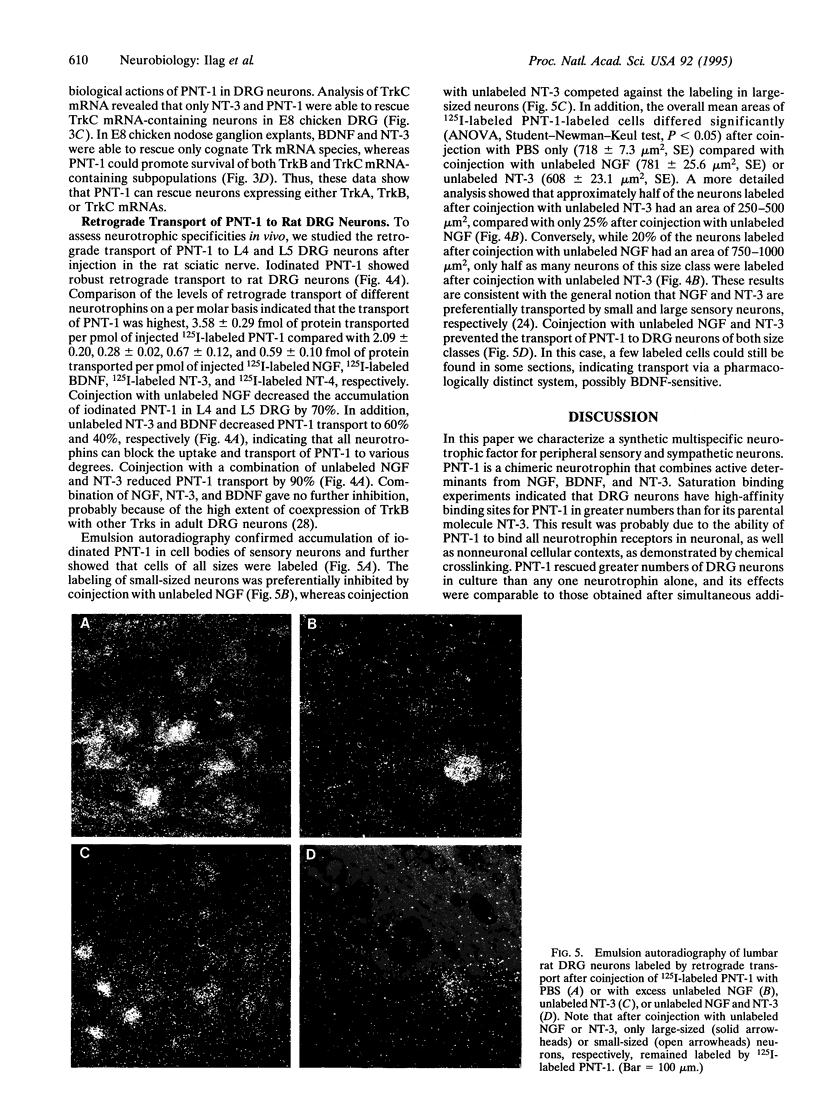
Pan-neurotrophin 1 (PNT-1) is a synthetic trophic factor engineered by combining active domains of the neurotrophins nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 (NT-3) into an NT-3 backbone. This molecule was produced in transiently transfected COS cells or in baculovirus-infected insect cells transfected COS cells or in baculovirus-infected insect cells and subsequently purified to homogeneity. Saturation binding in embryonic spinal sensory neurons demonstrated a greater number of high-affinity binding sites for PNT-1 than for its parental molecule NT-3. PNT-1 was shown to efficiently block the chemical crosslinking of NGF, BDNF, and NT-3 to their cognate Trk receptors and to the low-affintiy NGF receptor expressed on neuronal and nonneuronal cells. PNT-1 stimulated survival and proliferation of MG87 fibroblasts expressing either TrkA, TrkB, or TrkC. PNT-1 also promoted survival of a greater number of embryonic dorsal root ganglion neurons than any of the other neurotrophins alone, and its effects were equivalent to a combination of NGF, BDNF, and NT-3. Analysis of receptor-specific neurotrophic activities demonstrated that PNT-1 efficiently rescued TrkA mRNA-containing sympathetic neurons and TrkB and TrkC mRNA-containing sensory neurons from the dorsal root and nodose ganglia. Finally, PNT-1 showed robust retrograde transport to DRG neurons in vivo after injection into the sciatic nerve. Radiolabeled PNT-1 accumulated in small-, medium-, and large-sized neurons. Coinjection with different unlabeled neurotrophins inhibited PNT-1 transport in distinct subpopulations of neurons of different sizes, suggesting that this molecule affects sensory neurons of different modalities. These results indicate that PNT-1 is a potent and multispecific neurotrophic factor that may be useful in the treatment of peripheral neurophathies and nerve damage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson R. F., Alterman A. L., Barde Y. A., Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990 Sep;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Apfel S. C., Arezzo J. C., Brownlee M., Federoff H., Kessler J. A. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994 Jan 14;634(1):7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Arenas E., Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994 Jan 27;367(6461):368–371. doi: 10.1038/367368a0. [DOI] [PubMed] [Google Scholar]
- Crowley C., Spencer S. D., Nishimura M. C., Chen K. S., Pitts-Meek S., Armanini M. P., Ling L. H., McMahon S. B., Shelton D. L., Levinson A. D. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994 Mar 25;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Curtis R., Scherer S. S., Somogyi R., Adryan K. M., Ip N. Y., Zhu Y., Lindsay R. M., DiStefano P. S. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994 Jan;12(1):191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Davies A. M., Thoenen H., Barde Y. A. The response of chick sensory neurons to brain-derived neurotrophic factor. J Neurosci. 1986 Jul;6(7):1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G., Rodríguez-Tébar A., Kolbeck R., Barde Y. A. Specific high-affinity receptors for neurotrophin-3 on sympathetic neurons. J Neurosci. 1993 Jun;13(6):2610–2616. doi: 10.1523/JNEUROSCI.13-06-02610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano P. S., Friedman B., Radziejewski C., Alexander C., Boland P., Schick C. M., Lindsay R. M., Wiegand S. J. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992 May;8(5):983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee K. F., Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994 Mar 10;368(6467):147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee K. F., Kucera J., Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994 May 20;77(4):503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Tobkes N. J., McDonald N. Q., Hendrickson W. A., Ryan T. E., Nigam S., Acheson A., Cudny H., Panayotatos N. Characterization and crystallization of recombinant human neurotrophin-4. J Biol Chem. 1994 Jan 7;269(1):755–759. [PubMed] [Google Scholar]
- Friedman W. J., Ibáez C. F., Hallbök F., Persson H., Cain L. D., Dreyfus C. F., Black I. B. Differential actions of neurotrophins in the locus coeruleus and basal forebrain. Exp Neurol. 1993 Jan;119(1):72–78. doi: 10.1006/exnr.1993.1007. [DOI] [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos P. A., Suri C., Glass D. J., Goldfarb M. P., Yancopoulos G. D. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron. 1994 Jul;13(1):187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Camu W., Mettling C., Gouin A., Poulsen K., Karihaloo M., Rullamas J., Evans T., McMahon S. B., Armanini M. P. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993 May 20;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Hory-Lee F., Russell M., Lindsay R. M., Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C., Juhasz M., Jackson C., Wright P., Ip N. Y., Lindsay R. M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994 Jan;14(1):335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáez C. F., Ernfors P., Timmusk T., Ip N. Y., Arenas E., Yancopoulos G. D., Persson H. Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development. 1993 Apr;117(4):1345–1353. doi: 10.1242/dev.117.4.1345. [DOI] [PubMed] [Google Scholar]
- Ibáez C. F., Ilag L. L., Murray-Rust J., Persson H. An extended surface of binding to Trk tyrosine kinase receptors in NGF and BDNF allows the engineering of a multifunctional pan-neurotrophin. EMBO J. 1993 Jun;12(6):2281–2293. doi: 10.1002/j.1460-2075.1993.tb05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilag L. L., Lönnerberg P., Persson H., Ibáez C. F. Role of variable beta-hairpin loop in determining biological specificities in neurotrophin family. J Biol Chem. 1994 Aug 5;269(31):19941–19946. [PubMed] [Google Scholar]
- Ip N. Y., Ibáez C. F., Nye S. H., McClain J., Jones P. F., Gies D. R., Belluscio L., Le Beau M. M., Espinosa R., 3rd, Squinto S. P. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., Stitt T. N., Tapley P., Klein R., Glass D. J., Fandl J., Greene L. A., Barbacid M., Yancopoulos G. D. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993 Feb;10(2):137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Lapatto R., Murray-Rust J., Gunning J., Wlodawer A., Blundell T. L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991 Dec 5;354(6352):411–414. doi: 10.1038/354411a0. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Armanini M. P., Ling L. H., Phillips H. S. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994 May;12(5):1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Shooter E. M. The nerve growth factor family of receptors. Trends Neurosci. 1992 Sep;15(9):323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Ruit K. G., Elliott J. L., Osborne P. A., Yan Q., Snider W. D. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992 Mar;8(3):573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Verdi J. M., Birren S. J., Ibáez C. F., Persson H., Kaplan D. R., Benedetti M., Chao M. V., Anderson D. J. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994 Apr;12(4):733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]