Abstract
Background
Next generation sequencing (NGS) allows the detection of minor variant HIV drug resistance mutations (DRMs). However data from new NGS platforms after Prevention-of-Mother-to Child-Transmission (PMTCT) regimen failure are limited.
Objective
To compare major and minor variant HIV DRMs with Illumina MiSeq and Life Technologies Ion Personal Genome machine (PGM) in infants infected despite a PMTCT regimen.
Study Design
We conducted a cross-sectional study of NGS for detecting DRMs in infants infected despite a zidovudine (AZT) and Nevirapine (NVP) regimen, before initiation of combination antiretroviral therapy. Sequencing was performed on PCR products from plasma samples on PGM and MiSeq platforms. Bioinformatic analyses were undertaken using a codon-aware version of the Smith-Waterman mapping algorithm and a mixture multinomial error filtering statistical model.
Results
Of 15 infants, tested at a median age of 3.4 months after birth, 2 (13%) had non-nucleoside reverse transcriptase inhibitor (NNRTI) DRMs (K103N and Y181C) by bulk sequencing, whereas PGM detected 4 (26%) and MiSeq 5 (30%). NGS enabled the detection of additional minor variant DRMs in the infant with K103N. Coverage and instrument quality scores were higher with MiSeq, increasing the confidence of minor variant calls.
Conclusions
NGS followed by bioinformatic analyses detected multiple minor variant DRMs in HIV-1 RT among infants where PMTCT failed. The high coverage of MiSeq and high read quality improved the confidence of identified DRMs and may make this platform ideal for minor variant detection.
Background
Between 2004 and 2010, a dual regimen of Zidovudine (AZT) and Nevirapine (NVP) was used to prevent mother to child transmission of HIV (PMTCT) in the Western Cape Province, South Africa [1]. During our study period (Oct 2006 to Oct 2009), mothers received AZT from 28 weeks of gestation and single dose NVP (sdNVP) intra-partum, while the neonate received sdNVP, AZT for one week and was formula fed. The HIV transmission rate was <10% during this period [1]. In 2010, the National guidelines replaced infant sdNVP with daily NVP for the first 6 weeks of life and the PMTCT-failure rate decreased to <3% [2]. Further, in 2013 the WHO option B plus, which recommends lifelong combination antiretroviral therapy (cART) for pregnant women regardless of CD4 count or disease stage was adopted in the Western Cape in order to decrease the PTMCT failure rate even further.
Children, infected despite prophylactic antiretrovirals, are at high risk of acquiring antiretroviral drug resistance mutations (DRMs)[3]. Even low frequency non-nucleoside reverse transcriptase inhibitor (NNRTI) DRMs could affect NNRTI-containing regimen outcomes [4–7]. In South Africa, however, all children, under the age of 3 years, receive a protease inhibitors (lopinavir/ritonavir [LPV/r]) in the first-line regimen [8,9]. The prevalence of minor variant DRMs to NVP, nevertheless, remains important where there is limited access to LPV/r infant formulations or where NNRTIs are required in second-line regimens.
Various investigations have employed allele-specific real-time PCR or oligonucleotide ligation assays (OLA) for detecting minor variant DRMs after NVP PMTCT exposure [6,10–12]. Despite the reported sensitivity, the utility of these methods is limited by mismatches in primer binding [13], and by a limit to the number of reactions that can be multiplexed. Next generation sequencing (NGS) offers an attractive alternative to potentially detect all DRMs across the HIV-1 reverse transcriptase (RT) coding region. The read lengths of modern NGS systems including Roche 454 (454 Life Sciences, Branford, CT, USA), Ion Torrent Personal Genome Machine (PGM) (Life Technologies, Carlsbad, CA, USA) (PGM) and MiSeq (Illumina, San Diego, CA, USA), also permit the study of linkage between some DRMs [14]. A recent study found good correlation of Roche 454 sequencing for K103N and Y181C, when screening PMTCT exposed children (less than 2 years of age) prior to cART initiation[15]. We conducted the first investigation, to our knowledge, comparing bulk sequencing to Ion PGM and MiSeq in investigating DRMs after PMTCT exposure.
Objective
To compare major and minor variant HIV DRMs with NGS via Illumina MiSeq and Life Technologies Ion Personal Genome machine (PGM) platforms in infants who failed a dual AZT and NVP PMTCT regimen.
Study Design
Patients
We conducted a retrospective study in 15 HIV-infected infants, born from Oct 2006 to Oct 2009, who became infected despite a regimen of maternal AZT from 28 weeks gestation, sdNVP, intrapartum, and neonatal sdNVP and 7 days of AZT.
Specimen processing, reverse transcription and cDNA quantification
Baseline plasma specimens, prior to cART, were collected and nucleic acids were extracted on the NucliSENS® Easymag® (BioMérieux, Craponne, France). Bulk sequencing was undertaken using in-house PCR and Sanger Sequencing[16]. For PGM and MiSeq deep sequencing, RNA was reverse transcribed with pentadecamers and SuperScript® III (Invitrogen, Carlsbad, CA, USA), cDNA was quantified with real-time PCR on the ABI 7900ht (Applied Biosystems, Carlsbad, USA) with a SYBR green mastermix and forward primer: RGCTCTMTTAGAYACAGGAGCAGAT (HXB2 position: 2315-2339) and reverse primer: ACTTTGATAAAACCTCCAATTCCYCC (HXB2: 2419-2394).
Library preparation and sequencing for NGS
Each cDNA sample was amplified in parallel through 14 pre- and 7 nested PCR’s using Expand High FidelityPlus (Roche, Basel, Switzerland). Outer primers were: TCAGAGCAGACCAGAGCCAACAGCCCCA (HBX2: 2136-2163) and CCTACTAACTTCTGTATGTCATTGACAGTCCAGCT (HBX2: 3334-3300); Inner primers were: CTCTCTTAGACACAGGAGCAGAT (HBX2: 2317-2340); CCATTTGTCAGGATGGAGTTCATA(HBX2: 3267-3243)
PGM
Amplicons were gel-separated and purified prior to enzymatic shearing, adapter and barcode ligation and size selection. The Ion Xpress Plus library preparation manual, rev. M was followed for library construction. In short, the amplicons were digested using Ion Shear Enzyme II followed by barcode ligation and size selection, for an average insert size of 200 bp, using the E-Gel system (Invitrogen). Template enrichment and sequencing was performed according to manufacturer recommendations with the Ion 200 Sequencing V1 kit.
MiSeq
The Nextera XT DNA sample preparation manual, rev. C was followed for library construction. In short, the digestion and adapter ligation was performed simultaneously followed by the addition of indexes via PCR. Size selection of 300–500bp fragments was performed using Ampure XP reagent (Beckman-Coulter). Sequencing was performed using the MiSeq Reagent Kit v2.
NGS bioinformatics
Reads were filtered using instrument quality scores and aligned to a subtype C reference sequence using a codon-aware version of the Smith-Waterman algorithm that corrects for homopolymer errors by considering both nucleotide and amino-acid homology, and directly penalizes for length miscall[17]. A Bayesian Dirichlet mixture of multinomials probabilistic model was used to distinguish sequencing error from true low-frequency variants (posterior probabilities of ≥99.99%)[18]. For each sample, we computed the mean of all pairwise Tamura-Nei 93 distances between reads with at least 100 overlapping base pairs to quantify nucleotide diversity[19]. We constructed a maximum likelihood tree (GTR + CAT model in FastTree), based on the first 630 nucleotides of reverse transcriptase (the region that was uniformly well-covered for all sequencing runs) using NGS majority consensus and Sanger bulk sequences.
Bulk sequencing was with an in-house genotyping method, with sequencing from nested PCR products, and spanning RT amino acid positions 1-262 [16]. As quality control for NGS, we performed clonal sequencing of PCR products (the same as for bulk Sanger sequencing) on the 3130xl Genetic Analyzer and 3730xl DNA Analyzer (Applied Biosystems), 250 clones each of A157, A158, A124 and 269 clones of A131, which all had NNRTI mutations variants > 1%, were sequenced. DRMs were identified using the Stanford University Drug Resistance Database HIValg Program (HIVdb Version 7.0) [20]. Summary statistics were performed and figures were drawn with R 3.1.0 [21] and R Commander (Rcmdr version 2.0–4) [22]
Results
Study population
Our study included 15 infants who became HIV-infected despite PMTCT with NVP and AZT. Patient information at the time of the investigation: age, CD4 count, HIV viral load and HIV-1 subtype, are provided in Table 1. The median age at sample collection was 3.4 months (interquartile range [IQR] 2.4–4.5), and 11 (73%) were female. The median viral load at sampling was 5.8 log10 HIV RNA copies/ml (IQR 5.1–6.2); median CD4 count was 1693 cells/μl(IQR: 650–2025); median CD4% of 26% (IQR 21%–35%).
Table 1.
Clinical data of patients at the time of Sanger-and next generation sequencing.
| Study No | Gender | Age (months) | CD4 0 | VL 0 | HIV-1 subtype | NNRTI resistance (by deep sequencing) |
|---|---|---|---|---|---|---|
| A124 | Female | 16 | 1208 | 2900000 | subtype C | Yes |
| A137 | Female | 4 | 4150 | 29000 | subtype C | No |
| A144 | Female | 2 | 2036 | 82000 | subtype C | No |
| A157 | Female | 2 | 540 | 660000 | subtype C | Yes |
| A158 | Female | 2 | 2913 | 650000 | subtype C | Yes |
| A202 | Female | 3 | 430 | NA | subtype C | No |
| A207 | Female | 2 | NA | NA | subtype C | No |
| A297 | Male | 4 | 2030 | 180000 | subtype C | No |
| A300 | Female | 4 | 1744 | 120000 | subtype C | Yes |
| A302 | Female | 2 | 801 | 500000 | subtype C | No |
| A312 | Male | 5 | 2009 | 3200 | subtype C | No |
| A313 | Female | 14 | 1886 | >1000000 | subtype C | Yes |
| A318 | Male | 7 | 518 | 2300000 | subtype C | No |
| A326 | Male | 4 | 1641 | >3000000 | subtype C | No |
| A364 | Female | 3 | 600 | 4300000 | subtype C | No |
Gender, CD4 counts, HIV viral loads and HIV-1 subtype are provided for patients at the time of Sanger and Deep Sequencing. There were no significant differences in baseline characteristics between the 5 patients with major NNRTI resistance mutations and the remainder without major NNRTI mutations (Mann-Whitney U and Fisher Exact Tests); however due to the small sample size there was insufficient power to compare baseline characteristics.”
Next generation sequencing
Similar to previous studies and considering read coverage (defined as the number of reads with adequate quality for RT amino acid positions 1–230), a threshold of 0.5% was used for reporting minor variants [14]. Median read-coverage was greater with MiSeq: 74 181 (IQR: 50 173–104 089) than with PGM 8 939 (IQR: 4 521–12 585) resulting in an overall higher coverage per template (Table 2). Overall, the median proportion of bases with q-scores of 30 or greater (error ≤0.1%) was 43.2% (40.5% – 46.0%) for PGM, and 89.1% (84.7 % – 93%) for MiSeq.
Table 2.
Comparison of mutations detected by bulk Sanger sequencing, PGM and MiSeq deep sequencing and respective coverage.
| Patient number | Sanger DRM | PGM DRMs | PGM: Median Coverage: (per template) | MiSeq DRMs | MiSeq: Median Coverage: (per template) | Clonal Sequencing |
|---|---|---|---|---|---|---|
| A124 | none | K65R(1.4%), K103N(32.0%) | 5748 (0.9) | K65R(1.0%), K103N(15.7%) | 33037(5.2) | K65R(0.4%), K103N(11.2%) |
| A137 | none | K65R(1.2%) | 11 429(9.7) | K65R(1.0%) | 36 875(31.2) | NA |
| A144 | none | K65R(2.0%) | 17 020(6.2) | K65R(1.8%) | 23 753(8.7) | NA |
| A157 | K103N | K65R(1.4%), K103N(73.8%), V106M(1.4%), V106A(1.0%), Y181C(4.4%) | 4 210(3.8) | K65R(0.9%), K103N(51.6%), V106M(1.4%), V106A(1.0%), Y181C(4.4%) | 120 014(107.1) | K65R(0.4%) K103N(47.9%), V106M(0.4%), V106A(0.4%), Y181C(0.82%) |
| A158 | none | K65R(2.9%), Y181C(2.0%) | 4 190(1.3) | K65R(1.3%), Y181C(2.6%) | 122 232(37.4) | K65R(1.2%), Y181C(1.2%) |
| A202 | none | K65R(1.9%) | 13 178(0.3) | K65R(0.7%) | 127 778(2.9) | NA |
| A207 | none | K65R(1.2%) | 3 677(0.1) | K65R(1.1%) | 93 774(2.8) | NA |
| A297 | none | K65R(1.3%) | 9 781(4.6) | K65R(1.0%) | 86 060(40.5) | NA |
| A300 | none | K65R(2.5%) | 2 387(9.8) | K65R(1.1%), G190E(0.7%) | 73 929(304.2) | NA |
| A302 | none | K65R(1.5%) | 6 155(1.1) | K65R(1.5%) | 61 950(11.5) | NA |
| A312 | none | K65R(1.4%) | 6 925(8.9) | K65R(1.1%) | 103 222(132.8) | NA |
| A313 | Y181I | K65R(1.2%), Y181I(72.4%) | 8 545(1.1) | K65R(0.7%), Y181I(73.3%) | 64 063(8.5) | K65R(0.7%), Y181I(74.4%) |
| A318 | none | K65R(1.5%) | 5 940(2.5) | K65R(1.1%) | 118 474(50.5) | NA |
| A326 | none | K65R(1.2%) | 3 296(0.02) | K65R(1.1%) | 108 688(0.7) | NA |
| A364 | none | K65R(0.9%) | 11 218(NA) | K65R(0.9%) | 33 037(5.2) | NA |
Major variant drug resistance mutations were detected by genotyping with in-house PCR and Sanger sequencing (Sanger DRM). Major and minor variant drug resistance mutations detected with the Ion Personal Genome Machine (PGM DRMs) and Illumina MiSeq (MiSeq DRMs) and their respective frequencies, at a threshold of > 0.5% are shown in parenthesis. Median read coverage per template, shown for both NGS platforms is the ratio of median coverage to sampled cDNA. With reference to A364 cDNA quantity was not available (NA) as the real-time PCR failed.
K65R was detected in all samples; and is commonly detected by deep sequencing in HIV-1 subtype C.
Clonal sequencing confirmed the presence of DRMs > 1% and was performed for A124, A157, A158 (250 clones each) and A313 (269 clones). Agreement with clonal sequence frequency was better for MiSeq than Ion PGM.
Two infants (13%) had DRMs to NNRTI, detected by bulk sequencing; one had K103N and the other – Y181I. At 0.5%, Ion PGM sequencing detected NNRTI DRMs in 4 infants and MiSeq detected DRMs in 5 infants. For the infant with K103N, detected by bulk sequencing, both Ion PGM and MiSeq additionally detected V106A/M and Y181C DRMs. K65R was detected at a low frequency in all patients. (Table 2). The variant frequency detected by clonal sequencing had better agreement with MiSeq than Ion PGM (Table 2).
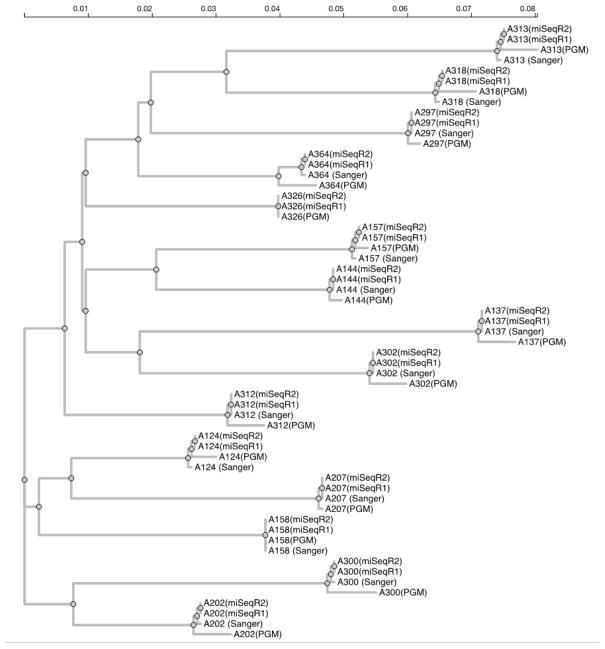
Maximum likelihood tree (see Figure 2)
Figure 2.
A maximum likelihood phylogenetic tree of NGS consensus and Sanger sequences (GTR+CAT model in FastTree). Note: MiSeqR1 and R2 indicate the forward and reverse reads, respectively. The scale is in expected substitutions per site per unit time. Each intra-host clade has bootstrap support >95%.
The NGS consensus sequences and Sanger sequences formed well-supported monophyletic groups. MiSeq consensus sequences were in much better agreement with Sanger bulk sequences than PGM consensus sequences. When resolving mixed bases in Sanger bulk sequencing to match the MiSeq consensus sequences, the bulk Sanger sequences and miSeq consensus sequences were identical; whereas the mean difference between PGM consensus and bulk Sanger sequences for a given patient were 2.2/630 nucleotides (IQR: 1–3, maximum 4).
Discussion
This is the first comparison, to our knowledge, of two modern NGS platforms for infants failing PMTCT. Of 15 infants exposed to AZT and NVP, only 2 (13%) had NNRTI mutations detected by bulk sequencing, 4 (26%) by Ion PGM and 5 (30%) by MiSeq. The additional detection of Y181C in the infant, where only K103N was detected by bulk sequencing, would reduce susceptibility to the second-line NNRTI, etravirine. No patients had minor or major variant AZT-associated DRMs.
The higher coverage, and higher read quality obtained in MiSeq data may have contributed to detecting an additional patient with a minor variant DRM (G190E, in patient A300) compared to PGM. MiSeq also had overall better agreement with clonal sequencing than PGM.
A recent South African study reported 56.8% of children having NNRTI resistance by bulk sequencing after PMTCT exposure; however, the majority of the infants received only NVP[23]. In a large randomized study, the addition of ZDV+ 3TC for 4 or 7 days to infants significantly reduced drug resistance in compared to sdNVP (NVP alone– 7/8 (88%), 4 days– 4/25 (17%) and 7 days–0/10 (0%))[24]. Data on the prevalence of minor DRMs after PMTCT remains limited, and most studies of minor variant DRMs after PMTCT used allele specific PCR only targeting a few important mutations [10,11].
A major limitation of 454 and PGM sequencing is homopolymer read error, which has been associated with false positive detection of K65R in HIV-1 subtype C using the 454 platform [25]. Interestingly, the frequency of K65R in our study was higher in PGM reads than MiSeq, suggesting that homopolymer read error contributed to K65R variant calling. Nevertheless, K65R was also present, albeit at lower frequency, in MiSeq and clonal sequences. Previous reports have indicated that using high fidelity PCR enzymes could limit the proportion of K65R minor variants [25], but despite our use of high fidelity PCR, we detected K65R > 0.5% in all infants, which might be unexpected in a tenofovir-naïve cohort[26]. Interestingly, NVP-containing regimens have been associated with an increased risk of K65R detection [27,28], but the mechanism has not yet been described. Overall, while HIV-1 subtype C has an increased risk of K65R under drug pressure[27,29], the extent to which these K65R mutations are from reverse transcription error in vitro or in vivo, is unclear.
Our study showed that deep NGS using PGM or MiSeq, combined with a bioinformatic ‘pipeline’ could enable the detection of minor variant reverse transcriptase DRMs after PMTCT.
The read quality was best for MiSeq, probably as it is less prone to homopolymer error, and the higher coverage increased the confidence of minor variant calling. As NGS platforms become more affordable they may prove invaluable in the investigation of infants, when antiretroviral therapy for PMTCT failed to prevent transmission, for the following reasons: 1) NNRTI minor variant DRMs are associated with subsequent failure on a particular regimen [4,5], 2) PMTCT-failure of regimens relying on maternal cART may be associated with an increased risk of complex resistance patterns and 3) DRM detection using NGS, in contrast to allele specific PCR, allows for detection of all DRMs across a particular sequence.
Laboratories that process and PCR-enrich microbial or viral samples (such as HIV) do not require onsite NGS capability but could make use of commercial or academic core facilities that provide NGS on as a fee for service. Nevertheless it is critical that a specimen that is PCR enriched for NGS should be a representative sample of the source: in our case we quantified cDNA to confirm that the sample contained sufficient species to enable minor variant detection and we separated amplification in parallel PCRs to limit random PCR error. However of equal importance is the post-analytical processing of NGS data to correctly identify haplotypes (variants) from PCR and sequencing error. Although several published programs are freely available it often requires some programming capability and the integration across different bioinformatics platforms. Therefore a versatile and openly available bioinformatics ‘pipeline’ for processing of NGS sequences would be of great value. Our ‘pipeline’ combined the following processing and analysis functions: it exclusion of low quality reads, correction for homopolymer errors or random sequencing error and construction of consensus reads and frequent haplotypes (variants). The output of the analysis performed for this paper can be found at http://bit.ly/gvz-PMTCT-NGS. The authors are of opinion that online access to an open source service that performs quality control and trouble-shooting of next generation sequencing data would be very valuable in providing increased access to this technology. To our knowledge, there are no widely used open systems for HIV-specific NGS analyses. However, the bioinformatic pipeline, used in the present study is available in the public domain. We plan detailed instructions in a separate publication.
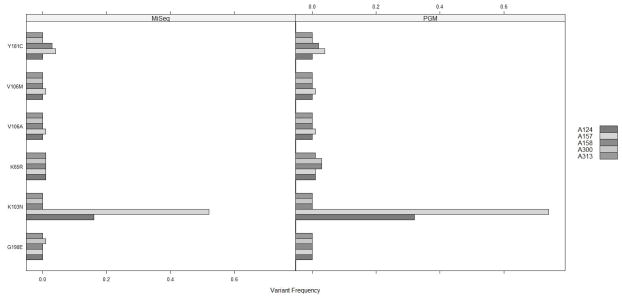
Figure 1. Frequencies of variants harboring mutations in 5 patients with major NNRTI mutations.
The mutation frequencies are shown at amino acid positions 65 (NRTI mutation), 103, 106,181,190 (NNRTI mutations) for patients who had variants making up > 0.5% of the total population. The variant frequencies were calculated after filtering for read error which meant that the mutation harboring variants and wild-type virus for each locus would sum to 100%. Note: in order to make the bars visible, bars were drawn below 0, and the frequency is represented by the bar height above 0.
Highlights.
Infants exposed to dual AZT and NVP regimen had a low prevalence of drug resistance
Next generation sequencing improves PMTCT-associated mutation detection
MiSeq had better sequence quality and read coverage than Ion PGM
Acknowledgments
Funding: This work was supported by University of California, San Diego, Center for AIDS Research sub award (PO# 10315768 – SUB); Polio Research Foundation; National Health Laboratory Research Trust (South Africa); The San Diego Veterans Affairs Healthcare System and grants from the National Institutes of Health: AI100665, MH097520, DA034978, MH083552, AI036214, GM093939, AI064086, AI069432 and by the UCSD Center for AIDS research (AI036214) Clinical Investigation and Biostatistics, Bioinformatics and Information Technology and Translational Virology Cores.
Abbreviations
- DRMs
Drug resistance mutations
- NGS
Next generation sequencing
- PGM
Ion Personal Genome Machine
- MiSeq
Illumina MiSeq Sequencer
- PMTCT
Prevention of HIV mother to child transmission
- cART
combination antiretroviral therapy
- LPV/r
lopinavir/ritonavir co-formulation
- sdNVP
Single dose nevirapine
Footnotes
Author contributions: GvZ, RH and DS planned the study. CE recruited patients with MC providing clinical oversight. RF extracted nucleic acids and PCR-enriched templates and quantified cDNA RS prepared sequencing libraries for PGM and MiSeq sequencing, and performed PGM sequencing. BK performed MiSeq sequencing. SKP and BM conducted bioinformatic analysis. GvZ, RF, SKP and DS wrote the draft manuscript. All authors approved the final manuscript.
Ethical approval: The study was approved by the Stellenbosch University Committee for Human Research: N06/05/081.
Potential competing interests: RH received honoraria or consultant fees from BMS, Gilead Sciences, Janssen and Merck (unrelated to this work) and research support (to UCSD) from Abbott, GlaxoSmithKline, Pfizer and Merck. MC received research support (SU) from BMS and Gilead Sciences. GvZ received honoraria (unrelated to this work) from Abbvie. BM, BK, CE, DS, RF, RS and SKP report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Draper B, Abdullah F. A review of the prevention of mother-to-child transmission programme of the Western Cape provincial government, 2003 – 2004. S Afr Med J. 2008;98:431–4. [PubMed] [Google Scholar]
- 2.Barron P, Pillay Y, Doherty T, Sherman G, Jackson D, Bhardwaj S, et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ. 2013;91:70–4. doi: 10.2471/BLT.12.106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ton Q, Frenkel L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr HIV Res. 2013;11:126–36. doi: 10.2174/1570162x11311020005. [DOI] [PubMed] [Google Scholar]
- 4.Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Kozal MJ, Hullsiek KH, et al. Relationship between minority nonnucleoside reverse transcriptase inhibitor resistance mutations, adherence, and the risk of virologic failure. AIDS. 2012;26:185–92. doi: 10.1097/QAD.0b013e32834e9d7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes R, Lalama CM, Ribaudo HJ, Schackman BR, Shikuma C, Giguel F, et al. Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J Infect Dis. 2010;201:662–71. doi: 10.1086/650543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jourdain G, Wagner TA, Ngo-Giang-Huong N, Sirirungsi W, Klinbuayaem V, Fregonese F, et al. Association between detection of HIV-1 DNA resistance mutations by a sensitive assay at initiation of antiretroviral therapy and virologic failure. Clin Infect Dis. 2010;50:1397–404. doi: 10.1086/652148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JZ, Paredes R, Ribaudo HJ, Kozal MJ, Svarovskaia ES, Johnson JA, et al. Impact of minority nonnucleoside reverse transcriptase inhibitor resistance mutations on resistance genotype after virologic failure. J Infect Dis. 2013;207:893–7. doi: 10.1093/infdis/jis925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowley CF, Boutwell CL, Lee EJ, MacLeod IJ, Ribaudo HJ, Essex M, et al. Ultrasensitive detection of minor drug-resistant variants for HIV after nevirapine exposure using allele-specific PCR: clinical significance. AIDS Res Hum Retroviruses. 2010;26:293–300. doi: 10.1089/aid.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon DK, Zheng L, Hitti J, Chan ES, Halvas EK, Hong F, et al. Greater suppression of nevirapine resistance with 21- vs 7-day antiretroviral regimens after intrapartum single-dose nevirapine for prevention of mother-to-child transmission of HIV. Clin Infect Dis. 2013;56:1044–51. doi: 10.1093/cid/cis1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer S, Boltz VF, Chow JY, Martinson NA, McIntyre JA, Gray GE, et al. Short-course Combivir after single-dose nevirapine reduces but does not eliminate the emergence of nevirapine resistance in women. Antivir Ther. 2012;17:327–36. doi: 10.3851/IMP1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowley CF, Boutwell CL, Lockman S, Essex M. Improvement in allele-specific PCR assay with the use of polymorphism-specific primers for the analysis of minor variant drug resistance in HIV-1 subtype C. J Virol Methods. 2008;149:69–75. doi: 10.1016/j.jviromet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kijak GH, Sanders-Buell E, Harbolick EA, Pham P, Chenine AL, Eller LA, et al. Targeted deep sequencing of HIV-1 using the IonTorrentPGM platform. J Virol Methods. 2014;205C:7–16. doi: 10.1016/j.jviromet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt GM, Morris L, Moorthy A, Coovadia A, Abrams EJ, Strehlau R, et al. Concordance between allele-specific PCR and ultra-deep pyrosequencing for the detection of HIV-1 non-nucleoside reverse transcriptase inhibitor resistance mutations. J Virol Methods. 2014;207C:182–7. doi: 10.1016/j.jviromet.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claassen M, van Zyl GU. The improvement, validation and quality assurance of in-houses HIV-1 antiretroviral resistance tests for practical use in resistance monitoring of patients receiving antiretroviral combination theray at Tygerberg Hospital Family Clinic. STellenbosch University; SUNScholar: 2008. [Google Scholar]
- 17.Gianella S, Delport W, Pacold ME, Young JA, Choi JY, Little SJ, et al. Detection of minority resistance during early HIV-1 infection: natural variation and spurious detection rather than transmission and evolution of multiple viral variants. J Virol. 2011;85:8359–67. doi: 10.1128/JVI.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murrell B, Moola S, Mabona A, Weighill T, Sheward D, Kosakovsky Pond SL, et al. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol. 2013;30:1196–205. doi: 10.1093/molbev/mst030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheim JO, Leigh Brown AJ, Hepler NL, Mehta SR, Richman DD, Smith DM, et al. The global transmission network of HIV-1. J Infect Dis. 2014;209:304–13. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanford University. HIV Drug Resistance Database: HIVAlg Program (HIVdb Version 7.0) n.d [Google Scholar]
- 21.R Core Team. R. A Language and Environment for Statistical Computing. 2014. [Google Scholar]
- 22.Fox J. The R Commander: A Basic Statistics Graphical User Interface to R. J Stat Softw. 2005;14:1–42. [Google Scholar]
- 23.Kuhn L, Hunt G, Technau K-G, Coovadia A, Ledwaba J, Pickerill S, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014 doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre JA, Hopley M, Moodley D, Eklund M, Gray GE, Hall DB, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 2009;6:e1000172. doi: 10.1371/journal.pmed.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese V, Wang E, Babrzadeh F, Bachmann MH, Shahriar R, Liu T, et al. Nucleic acid template and the risk of a PCR-Induced HIV-1 drug resistance mutation. PLoS One. 2010;5:e10992. doi: 10.1371/journal.pone.0010992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recordon-Pinson P, Papuchon J, Reigadas S, Deshpande A, Fleury H. K65R in subtype C HIV-1 isolates from patients failing on a first-line regimen including d4T or AZT: comparison of Sanger and UDP sequencing data. PLoS One. 2012;7:e36549. doi: 10.1371/journal.pone.0036549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner BG, Coutsinos D. The K65R mutation in HIV-1 reverse transcriptase: genetic barriers, resistance profile and clinical implications. HIV Ther. 2009;3:583–94. doi: 10.2217/hiv.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang MW, Rhee S-Y, Bertagnolio S, Ford N, Holmes S, Sigaloff KC, et al. Nucleoside reverse transcriptase inhibitor resistance mutations associated with first-line stavudine-containing antiretroviral therapy: programmatic implications for countries phasing out stavudine. J Infect Dis. 2013;207 (Suppl ):S70–7. doi: 10.1093/infdis/jit114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutsinos D, Invernizzi CF, Moisi D, Oliveira M, Martinez-Cajas JL, Brenner BG, et al. A template-dependent dislocation mechanism potentiates K65R reverse transcriptase mutation development in subtype C variants of HIV-1. PLoS One. 2011;6:e20208. doi: 10.1371/journal.pone.0020208. [DOI] [PMC free article] [PubMed] [Google Scholar]