Abstract
Since the first clinical application of analgesia following spinal anticholinesterase by 1940's, several clinical double-blind studies have been conducted to date, where intrathecal doses of neostigmine in humans ranged from 750 to 1 μg, due to side-effects. Conversely, epidural neostigmine has been evaluated in proportionally higher doses and represents an alternative, but still deserves more investigation concerning both acute and chronic pain, as it seems devoid of important side-effects.
Keywords: Epidural neostigmine, pain, review, spinal neostigmine
INTRODUCTION
The text focus on the natural evolution of neostigmine's applicability in pain relief. The story starts by anecdotal case reports of analgesia following spinal anticholinesterase by 1940's. 30 years late the interest in anti-nociception by anticholinesterase agents were restarted by Pleuvry and Tobias, in England, culminating with its demonstration of spinal analgesia in animals by 1980's. The search carried on in volunteers and finally with different sorts of clinical randomized double-blind studies, which started by 1990's. Firstly, the differences between patients and volunteers, subsequently the dose-dependent analgesia and side-effects of spinal neostigmine; and finally the surprise of analgesia after its epidural administration were described. When one analyze the past two decades, intrathecal (IT) doses of neostigmine in humans ranged from 750 to 1 μg. Due to side-effects the dose was substantially decreased. As a consequence of the small dose, neostigmine should be applied only as part of multimodal spinal analgesia and further clinical trials are still needed. Conversely, epidural neostigmine may be evaluated in proportionally higher doses and represents an alternative, but still deserves more investigation concerning both acute and chronic pain, as it seems devoid of important side-effects.
HISTORY OF THE CLINICAL APPLICATION OF ANTICHOLINESTERASE AGENTS AS ANALGESICS: FROM ANECDOTAL REPORTS OF PATIENTS, THROUGH ANIMAL DATA, TO A PROPER STUDY IN VOLUNTEERS (1933-1985)
Eighty years have elapsed since the first citation of the analgesic effects of anticholinesterase agents, when Pellandra[1] observed that intravenous administration of the anticholinesterase drug physostigmine produced analgesia in human beings. In 1942, Kremer described the use of cholinergic and anticholinesterase agents by the IT route to induce analgesia in hemiplegic patients.[2] By 1945, the systemic analgesic action of physostigmine and neostigmine and of the opioid morphine was evaluated in patients by Flodmark and Wrammer.[3]
In the seventies, the antinociceptive effect of cholinergic drugs and a link with opioid analgesia was emphasized.[4,5] Pleuvry and Tobias, in England, were the ones to restart the interest in the cholinergic mechanism of pain.[4] In 1981, autoradiography studies demonstrated the existence of muscarinic binding sites in the substantia gelatinosa, in laminae III and V of the dorsal gray matter of the medulla six, coinciding with the binding sites for opioids. By 1985, Yaksh et al. described the antinociception in rat and cat following spinal cholinomimetic drugs.[7] Petersson et al., impressed by the potential analgesic characteristics of anticholinesterase agents, confirmed that intravenous administration of physostigmine induced immediate, although short-lasting, post-operative analgesia in patients submitted to various types of surgical procedures.[8] In the nineties, a systematic approach to determine potential neurotoxicity of IT methyl sulfate neostigmine revealed safety after histological, physiologic and behavioral testing in rat, dog and sheep,[9,10] and neostigmine also did not influence the neurotoxicity of lidocaine in vitro.[11] Spinal cord histological examination in sheep and rat also revealed the safety of paraben-and glucose-containing IT neostigmine.[12] Subsequent clinical testing in human volunteers[13,14] and patients with terminal cancer refractory to conventional therapy[15,16] motivated further clinical trials, that will be detailed in succeeding sections.
SPINAL/EPIDURAL ANALGESIC ACTION OF NEOSTIGMINE
Pharmacokinetics
Neostigmine was introduced in 1931. It is a reversible inhibitor of the enzyme cholinesterase, which results in an increased concentration of the acetylcholine (Ach) neurotransmitter. However, due to its hydrophilic nature (presence of a functional quaternary ammonia), it does not cross the duramater, what justified the interest of its applicability as IT analgesic until early 1990's. After spinal administration of neostigmine, Ach concentration increased from <20 pmol/ml at baseline to >100 pmol/ml within 15 min, while plasma concentration was approximately 5 ng/ml. Concentration in cerebrospinal fluid could be measured for 24 h.[13] The pharmacokinetic of IT neostigmine was best described by a triexponential function with an absorption phase. Individual predicted concentrations varied 100-fold.[17] It was characterized by prolonged distribution (t1/2α = 23 min) and elimination (t1/2β = 260 min).[13] No study to date has evaluated the pharmacokinetics of epidural neostigmine.
Mechanisms of IT neostigmine analgesia
The analgesia resulting from spinal administration of neostigmine may be due to the increased concentration of Ach and the consequent binding to M1, M3, M2 and M4[18] muscarinic and to nicotinic receptors [Figure 1].[19,20,21,22,23]
Figure 1.
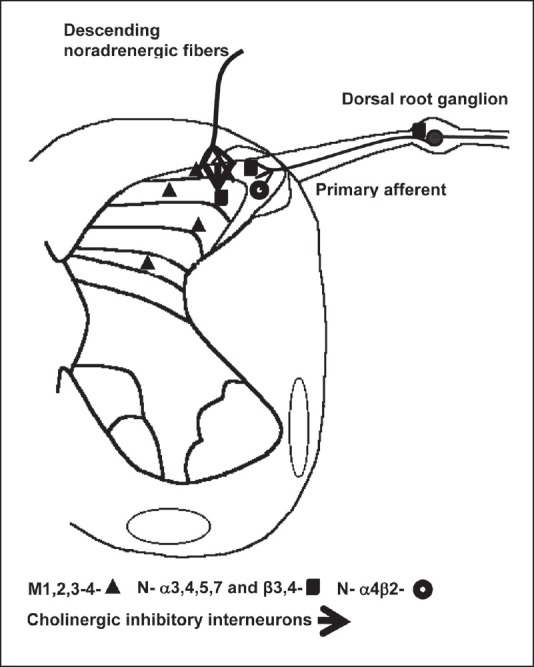
Cholinergic pathways at the dorsal horn
Muscarinic receptors are located in cholinergic interneurons of the dorsal horn of the spinal cord, in the substantia gelatinosa, in laminae III and V of the spinal cord;[6] while α3, α4, α5, α7, β2, β3 and β4 nicotinic subunits are expressed on primary afferent terminals, inhibitory interneurons, descending noradrenergic fibers[21] in the dorsal root ganglion[22] and in microglias.[23]
It was demonstrated that activation of spinal muscarinic type-2 receptors suppressed spinal gamma-amino butyric acid-B (GABA-B) receptor input[24] and that this disinhibiting mechanism ultimately lead to the release of adrenal catecholamines[25] and subsequent reduction in peripheral inflammation.[26] Spinal cord stimulation was also associated with the activation of the cholinergic system in the dorsal horn and mediated via muscarinic receptors, particularly M4, while nicotinic receptors appeared not to be involved.[27]
The existence of a difference in the predominance of different types of cholinergic receptors between males and females has been suggested,[28,29,30] although controversial.[31] While in males muscarinic receptors may be responsible for the antinociceptive effect, the participation of muscarinic and nicotinic receptors was demonstrated in females.[28]
The spinal cholinergic interneurons may be activated by serotoninergic and noradrenergic descending pathways that inhibit pain.[32] Gabaergic interneurons have muscarinic receptors in the terminal axons and in somatodendritic sites and activation of these receptors increase the excitability of inhibitory interneurons, enhancing the release of GABA in the substantia gelatinosa.[33] This inhibitory gabaergic system is also controlled by cholinergic neurons located deeply in the posterior horn of the spinal medulla.[34] The activation of muscarinic receptors inhibits spinal dorsal horn projection neurons, suggesting a partial role of GABA-B receptors in normal or in diabetic rats.[35] In addition spinal neostigmine administration resulted in reduced substance P,[36] and there appears to be unidirectional cross-tolerance between morphine and neostigmine.[37] In cats, spinal neostigmine showed antinociceptive effect and this inhibition was only partially mediated by cholinergic mechanism.[38]
Similarly, IT neostigmine may act in different ways on phase and tonic pain,[39] may interact synergistically with α2-agonists,[40,41] nitric oxide and muscimol,[42] may have an additive or synergistic analgesic effect when administered in combination with m agonists,[43] IT gabapentin[44] and nonsteroidal anti-inflammatory drugs.[45] Spinal administration of neostigmine to mice resulted in synergistic or supra-additive effects when the drug was used in combination with ketoprofen, paracetamol or diclofenac. However, only an additive analgesic effect occurred when neostigmine was combined with meloxicam and pyroxicam.[45]
Ach is a major neurotransmitter but also an important signaling molecule in neuron-glia interactions. Expression of Ach receptors has been reported in several glial cell populations, including oligodendrocytes,[46] which may be activated in chronic pan states. Recently, an electron microscopy analysis demonstrated that cholinergic boutons are presynaptic to the dorsal horn neurons as well as to the terminals of sensory primary afferents, suggesting that they are likely to modulate incoming somatosensory information.[47] The authors are suggested that this newly identified dorsal horn cholinergic system in monkeys was the source of the Ach involved in the analgesic effects of epidural neostigmine.[47]
Related to chronic pain, an increased expression of the α3 and α5 nicotinic subunits may contribute to the mechanical hypersensitivity observed following spinal nerve ligation,[21] and IT neostigmine reduced allodynia secondary to rib-retraction in rats, alleviating post-thoracotomy pain.[48] Neostigmine also stimulated the expression of HLA-DR (human leukocyte antigen-DR-microglial marker) and the production of tumor necrosis factor alpha (TNF-α) at dendritic cells while inhibiting the production of TNF-α and interleukin (IL)[13] triggered by long potential stimulation, supporting the existence of the loop through which Ach modulates the function of dendritic cells.[49]
Mechanisms of epidural neostigmine analgesia
Epidural neostigmine analgesia seems to be a result of central rather than peripheral action. In patients undergoing surgery, epidural neostigmine resulted in analgesia after the administration of a ten-fold lower dose (1 μg/kg), when compared to knee intraarticular administration, suggesting a central effect.[50] Epidural neostigmine acts on the enzymes acetylcholinesterase and butylcholinesterase expressed in the meninges that cover the spinal cord.[51,52] Another aspect to be considered is the possible direct action of neostigmine as a muscarinic agonist,[53] in addition to the indirect stimulation of the release of the second intracellular messenger, nitric oxide[54] and suppression of cFos expression.[55]
DOSE-RELATED EFFECTS OF NEOSTIGMINE
In 1995, Hood and Eisenach published the phase I safety assessment of IT neostigmine methylsulfate in humans, when 28 healthy volunteers received IT neostigmine (50-750 μg). The authors demonstrated a dose-dependent incidence of analgesia and adverse effects. Neostigmine (150 μg) caused mild nausea and 500-750 μg caused severe nausea and vomiting. Neostigmine (150-750 μg) produced subjective leg weakness, decreased deep tendon reflexes and sedation. The 750 μg dose was associated with anxiety, increased blood pressure and heart rate and decreased end-tidal carbon dioxide.[13]
At the time, it seemed reasonable to test spinal neostigmine doses ranging from 50 to 200 μg, based on volunteers data. In mid-1996, the first prospective double-blind scientific study evaluated 50, 100, and 200 μg IT neostigmine to patients submitted to standardized general anesthesia for gynecological procedures by the vaginal route.[54] This study confirmed data obtained with volunteers[13] demonstrating dose-dependent analgesia, with peculiar adverse effects such as nystagmus, salivation, mydriasis and bradycardia not responding to intravenous atropine.[56] In contrast, in volunteers who received only the administration of neostigmine by the IT route, the most prominent adverse effect was the occurrence of nausea and vomiting,[13] which was later seen as anguish-producing and stressful for patients submitted to anesthesia by the regional route.[57,58] The nausea and vomiting observed in volunteers depended on the dose used, on the baricity of the solution and on the method of administration. The cephalic ascension of the drug was apparently responsible for emesis.[13] However, since the doses tested were high (50-750 μg), the initial false impression was that emesis resulted from the high doses, whereas doses in the 100-200 μg range administered to volunteers resulted in analgesia devoid of adverse effects.[13] However, the differences observed at the time between volunteers and patients were intriguing. Specialists were aware of the fact that neostigmine caused analgesia and adverse effects, both of them dose dependent,[13] but the potencies differed between these two populations. Studies on volunteers did not demonstrate analgesia with the administration of doses lower than 100 μ,g,[13] whereas studies on patients demonstrated post-operative analgesia at lower doses.[56,59] A multicenter study demonstrated dose-dependent analgesia and adverse effects when 25, 50, and 100 μg neostigmine were administered intrathecally to patients submitted to hysterectomy by the vaginal route.[59] Similarly, 25, 50, and 75 μg IT neostigmine resulted in dose-dependent analgesia in patients submitted to orthopedic procedures under regional anesthesia.[60]
This difference agreed with results observed in animals that demonstrated greater potency of IT neostigmine when the drug was administered in the presence of a surgical stimulus compared with the absence of pain.[32] It was assumed that in the presence of a painful stimulus, the patients would have exacerbation of a cholinergic medullary pathway and therefore would not tolerate such high doses of neostigmine as volunteers not stimulated by a surgical act. Complementing this hypothesis, it was speculated that the ratio of the dose of IT neostigmine between patients and volunteers would be approximately 6 (ranging from 4 to 8) and it was inferred that 25-100 μg in patients would correspond to 150-750 μg in volunteers, being related to analgesia and adverse effects.[32] As an example of this theory, if only 750 μg caused analgesia in the hands of volunteers,[13] we may assume that 125 μg neostigmine by the IT route would result in the same benefit in patients submitted to surgery of the upper limb.[61]
CLINICAL TRIALS
Spinal neostigmine as part of multimodal analgesia
This section is divided in the clinical reports where neostigmine was administered alone or in combination with other drugs through the spinal or epidural space [Table 1].
Table 1.
Analgesic effects of neostigmine
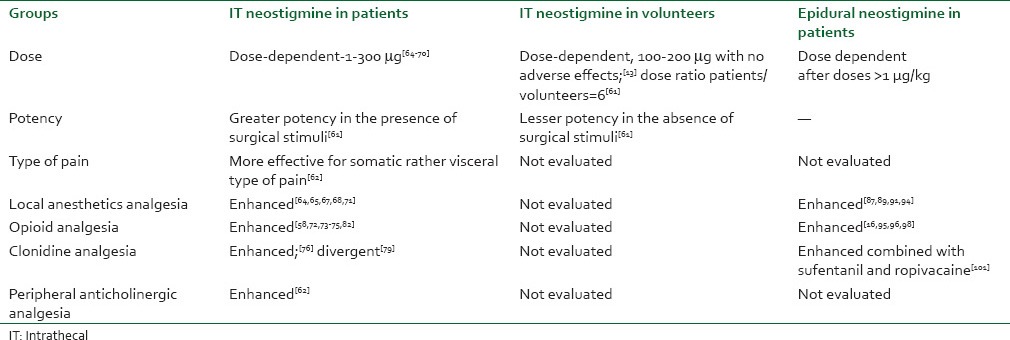
Spinal neostigmine alone
IT neostigmine (200, 100, and 50 μg) was initially evaluated in patients undergoing gynecologic surgeries under general anesthesia and demonstrated dose-dependent analgesia and adverse effects.[59] Afterward, a prospective double-blind study demonstrated that the analgesic effect of spinal neostigmine diluted in saline was not equally effective for the different types of pain or for the different intensities of surgical stimuli. An investigation involving the qualitative evaluation of the analgesic actions of neostigmine administered by the IT route demonstrated that the drug was more effective for pain of the somatic type compared with pain of the visceral type and that intravenous administration of the anticholinergic agent N-butyl scopolamine acted peripherally as an effective complement for visceral pain, suggesting an association between the central cholinergic system and the peripheral anticholinergic system.[61] The analgesic effect resulting from the peripheral anticholinergic drug was effective for visceral pain and might have reflected blockade of sympathetic ganglia through binding to nicotinic receptors, or a direct antispasmodic action on the viscera through an action on muscarinic receptors.[62]
Spinal neostigmine and local anesthetics
A double-blinded study reported 6-9 h analgesia after 100 and 50 μg IT neostigmine in inguinal herniorrhaphy, with a high incidence of nausea and vomiting.[63] In accordance, 50 and 25 μg neostigmine plus 10 mg hyperbaric bupivacaine for perianal surgery also demonstrate analgesia, emesis and prolonged motor blockade.[64] Another study described 7 h of analgesia after the IV low dose ketamine combined with 50 μg IT neostigmine and bupivacaine in gynecologic surgeries, associated with a high incidence of emesis.[65] In conformity, a study conducted on volunteers submitted to IT anesthesia with 7.5 mg bupivacaine in combination with 50, 12.5 or 6.25 μg neostigmine diluted in 5% glucose demonstrated that the incidence of nausea and vomiting was dose dependent and the duration of motor blockade was increased, limiting the use of this combination in ambulatory patients.[66]
Tan et al. evaluated IT 50 μg neostigmine compared to 300 μg morphine in patients submitted to knee arthrodesis. The study revealed the occurrence of 7 h of post-operative analgesia with the use of neostigmine, with greater patient satisfaction and a lower incidence of adverse effects.[67] Using an open-label, dose-ranging design, patients undergoing cesarean section received either IT placebo or neostigmine 100, 30 and 10 μg solution of 5% glucose in normal saline followed by 2% epidural lidocaine for cesarean section. Compared with the glucose control, neostigmine produced a dose-independent reduction in post-operative morphine use and hourly morphine use was significantly reduced in the neostigmine groups for 10 h post-operatively, without adverse fetal or maternal effects.[68] 5 μg IT neostigmine combined with bupivacaine resulted in a lower consumption of analgesics during the post-operative period and in 14 h of post-operative analgesia when combined with a skin patch with 5 mg nitroglycerin. No adverse effect was observed.[69]
In children, it was assessed analgesia of spinal 0.25, 0.5, 0.75, and 1 μ/kg neostigmine added to bupivacaine for lower abdominal and urogenital procedures. Neostigmine at a dose of 0.75 μ/kg added to bupivacaine significantly prolonged spinal anesthesia duration with reduced post-operative pain scores and rescue analgesic requirements in infants undergoing lower abdominal and urogenital procedures and no additional benefits were provided on increasing it to 1 μ/kg.[70]
Spinal neostigmine and opioids
A double-blinded study demonstrated that combined administration of 50 mg neostigmine and 50 μg morphine resulted in 23 h of effective analgesia in the study population.[58] In patients submitted to abdominal hysterectomy, the combination of 25 μg neostigmine with 25 μg fentanyl given intrathecally with 15 mg of hyperbaric bupivacaine delayed post-operative pain and lowered the number of rescue analgesics,[71] in accordance to others who recently evaluated 25 μg neostigmine with 25 μg fentanyl in the lower abdominal surgery.[72] A subsequent study assessed patients submitted to gynecologic surgeries by the abdominal route under spinal anesthesia with bupivacaine.[73] The patients received combined administration of morphine and low doses of neostigmine for pain of the visceral type. The results revealed that the control patients obtained 3 h of post-operative analgesia, with a higher consumption of analgesics over a period of 24 h. The group that received only bupivacaine and morphine had 4 h of post-operative analgesia. However, the combination of 1, 2.5 or 5 μg neostigmine and morphine resulted in 8 h of analgesia, demonstrating the enhancement of the analgesic effect of morphine, with no increase in the incidence of adverse effects.[72] In accordance, Jain et al. described enhanced analgesia after 1 μg IT neostigmine combined to 20 μg fentanyl in total knee replacement surgery.[74] The final analgesic effect may clinically reflect the importance of central cholinergic participation in the mediation of morphine nociception.[22]
Spinal neostigmine and clonidine
A prospective study in cesarean section demonstrated that the combination of 50 μg neostigmine and 150 μg clonidine resulted in a prolongation of post-operative analgesia, although with a higher incidence of nausea and vomiting and motor blockade.[75] The lower consumption of opioids would be explained by the exacerbation of the cholinergic tonus present during the painful stimulus in addition to the IT administration of neostigmine, with both events possibly resulting in increased concentrations of the neurotransmitter Ach in cerebrospinal fluid, present in medullary cholinergic interneurons, thus enhancing the antinociceptive effect of morphine.[76,77] However, another research group did not demonstrate a benefit of the addition of 10 μg IT neostigmine to the combination of IT bupivacaine, clonidine and sufentanil in spinal labor analgesia.[78]
Spinal neostigmine in obstetrics
Multimodal analgesia for the control of labor pain was also evaluated. One group of patients received 2.5 mg bupivacaine in combination with 25 μg IT fentanyl, while a second group additionally received 30 μg clonidine as a third drug and a third group received 10 μg neostigmine as the fourth drug. The addition of clonidine and neostigmine potentiated the analgesia of the first group, which however experienced a higher incidence of nausea.[79] An experimental study demonstrated that IT neostigmine resulted in a greater potentiation of the α2-agonist clonidine compared with dexmedetomidine.[41] The explanations proposed included the lower intrinsic potency of clonidine, which may be clinically enhanced by neostigmine, or the fact that the mechanism of action of dexmedetomidine may be less dependent on nitric oxide production.[41] Another study conducted on patients revealed that the addition of 10 μg IT neostigmine reduced the affective analgesic dose by 25% in 50% (ED 50%) of the patients, shifting the curve of the response to IT sufentanil to the left.[80]
Epidural neostigmine as part of multimodal analgesia
Selection of doses to be evaluated by the epidural route
Neostigmine is a hydrophilic molecule similar to morphine. It is known that only 10-20% of the morphine dose administered epidurally crosses the dura mater to reach the IT space, so that a 10 mg morphine dose administered epidurally is equivalent to 1 mg morphine administered intrathecally.[81] The extrapolation of these data to the definition of the neostigmine dose to be evaluated by the epidural route followed logical reasoning: As demonstrated in reports published up to that time, the literature suggested that IT doses of 5 μg or 10 μg[73] might be effective as part of multimodal analgesia and the administration of ten times the dose of IT neostigmine might result in post-operative analgesia.[80] Consequently, the IT dose of 10 mg neostigmine would be equivalent to 100 μg neostigmine by the epidural route (or 2 μg/kg in a patient weighing 50 kg) and the IT dose of 5 μg neostigmine would be equivalent to the epidural dose of 1 μg/kg.[82]
Epidural neostigmine alone
Two studies published on this subject are concerned to children[83] and adults.[84] The double-blinded study evaluated 120 children scheduled for surgical repair of hypospadias under general anesthesia. Children were divided into groups and received either no caudal block or neostigmine in doses of 10, 20, 30, 40 and 50 μg/kg respectively at the end of the surgery. Caudal neostigmine alone in the dose range of 20-50 μg/kg provided dose-dependent analgesia. However, dose exceeding 30 μg/kg was associated with a higher incidence of nausea and vomiting.[83] Never in the literature, a patient received such a high dose of epidural neostigmine. Such a dose would be equivalent to 700-3500 μg in a median 70-kg adult patient, more than ten-fold the safe tested dose used in adults, described by others.[85]
In the second study, epidural 4 or 8 μg/kg were infused epidurally diluted with saline under combined spinal anesthesia with lidocaine. The authors described that analgesia after the preemptive administration of epidural 8 μg/kg at 12 and 24 h compared with the other groups with no side-effects.[84]
Epidural neostigmine and local anesthetics
Studies on acute pain assessing the administration of neostigmine by the epidural route started in orthopedic surgery. Patients submitted to knee surgeries received saline or 1, 2 or 4 μg/kg neostigmine, diluted in 1% lidocaine, by the epidural route in a combined anesthetic technique involving spinal anesthesia/epidural analgesia.[82] The results demonstrated 8 h of post-operative analgesia regardless of the dose, with no adverse effects.[81] The local anesthetic lidocaine has been demonstrated to suppress different pain conditions when administered systemically, and part of the antinociceptive effect of systemic lidocaine appears to be mediated via muscarinic and nicotinic receptors.[86] Consequently, epidural lidocaine could also be partially systemically absorbed and enhance epidural neostigmine analgesia.
A different study evaluated epidural lidocaine alone or combined with epidural neostigmine (100 and 200 μg). The addition of neostigmine resulted in significant longer duration of analgesia (dose independent) and sedation (dose dependent). Sensory and motor blockade were identical in all three groups. The authors considered useful analgesia and desirable sedation.[87]
Genitourinary procedures in children demonstrated no efficacy of 1 μg/kg caudal neostigmine mixed with bupivacaine.[88] However, a single caudal injection of 2 μg/kg neostigmine added to ropivacaine offered an advantage over ropivacaine alone for pain relief in children undergoing genitourinary surgery.[89] Another study demonstrated that caudal neostigmine (2, 3 and 4 μg/kg) with bupivacaine produced a dose-independent analgesic effect (16-17 h) and a reduction in post-operative rescue analgesic consumption without increasing the incidence of adverse effects.[90] Another research group evaluated the post-operative analgesic action of high doses of epidural neostigmine and reported that 10 μg/kg, but not 5 μg/kg, resulted in 6 h of analgesia in patients submitted to hysterectomy. The patients did not experience emesis and were submitted to general anesthesia in combination with epidural blockade,[91] nevertheless, sedation was not evaluated.
A total of 80 patients undergoing elective cesarean were given combined spinal-epidural anesthesia with 8 mg hyperbaric bupivacaine plus 10 μg fentanyl. Patients were randomized to receive either saline or 75, 150, or 300 μg neostigmine in 10 ml saline after cord clamping. Global pain assessment for the first 24 h was reduced from 5.4 in the saline group to 3.5 in the neostigmine groups. Nausea and morphine consumption were similar among groups. Intraoperative shivering and sedation were increased in the 300 μg neostigmine group only and post-operative sedation was increased by neostigmine in a dose-independent fashion suggesting a limited role for single bolus-administration epidural neostigmine for analgesia after cesarean delivery.[85] Finally, 60 patients undergoing below umbilical surgeries under epidural anesthesia received 20 ml of 0.5% bupivacaine with either 1 ml of normal saline, 100 μg of neostigmine or 50 mg of ketamine. Both neostigmine and ketamine demonstrated better hemodynamic stability with lesser incidence of hypotension and better analgesia.[92]
Epidural neostigmine and opioids
The combined administration of low doses of morphine and neostigmine by the epidural route was subsequently evaluated.[93] 60 μg neostigmine and 0.6 mg morphine, both administered epidurally, resulted in 11 h of post-operative analgesia in orthopedic surgeries, with no adverse effects.[93] In accordance, a prospective double-blind study concerning the administration of epidural neostigmine in cancer pain described enhancement of epidural morphine analgesia, devoid of adverse effects.[16] Similarly, caudal 2 μg/kg neostigmine was demonstrated to enhance caudal sufentanil perioperative analgesia.[94]
Epidural neostigmine in obstetrics
Labor, a model of acute pain involving both somatic and visceral pain types revealed no benefit (but no side-effects) off adding 4 μg/kg epidural neostigmine to the mixture 100 mg ropivacaine/10 μg sufentanil,[95] however similar duration of analgesia following 6-7 μg/kg epidural neostigmine combined with 10 mg sufentanil compared to epidural 20 μg sufentanil[96] and further demonstrated that the inclusion of 60 mg lidocaine with epinephrine epidural test dose would affect ambulation in earlier labor.[97]
Prior to 2009, studies examined only single epidural neostigmine bolus administration and did not assess the efficacy of continuous epidural infusion or several aspects of maternal and fetal safety. After then, new data was available concerning epidural neostigmine efficacy and safety for both fetus and mother. A total of 12 healthy women scheduled for elective cesarean delivery were assigned to receive epidural neostigmine 40 or 80 μg as a single bolus, with fetal heart rate and uterine contractions monitored for 20 min. In a subsequent experiment, 40 healthy laboring women were randomized to receive bupivacaine 1.25 mg/mL alone or with neostigmine 4 μg/mL by patient-controlled epidural analgesia. The results revealed that epidural neostigmine bolus did not alter fetal heart rate, nor induce contractions, neither produce nausea. Epidural neostigmine infusion reduced bupivacaine requirement by 19% in all patients and 25% in those with >4 h of treatment but might have contributed to the incidence of mild sedation. Mode of delivery, incidence of maternal nausea, and fetal heart rate abnormality were similar between groups.[98]
In a different study, 70 laboring patients received spinal analgesia with ropivacaine and sufentanil. At 15 min after spinal injection, 10 mL of plain saline or neostigmine 500 μg combined with clonidine 75 μg. Epidural clonidine and neostigmine significantly prolonged initial analgesia and reduced hourly ropivacaine consumption. More patients in the experimental group delivered before the first request for additional analgesia (9 vs. 2). As a conclusion, administration of neostigmine 500 μg and clonidine 75 μg, following the IT injection of ropivacaine and sufentanil, prolongs analgesia, reduced hourly ropivacaine consumption[99] and apparently improved time of delivery.
Prophylactic analgesic action of epidural neostigmine
The authors reported that opioid consumption over a period of 48 h was lower in the group that received 500 μg neostigmine compared to the control group and to the group that received 25 mg of bupivacaine and 50 mg epidural ketamine.[100] The observation of such long-lasting prophylactic effect by neostigmine[100] may reflect the transiently suppression of the stress response demonstrated.[101] The preincisional epidural neostigmine resulted in lower plasma levels of cortisol, while IL-6 was not affected during lower open abdominal.[85] Continuous thoracic epidural neostigmine was evaluated in thoracotomy. The epidural dose of 500 μg bolus followed by 125 μg/h provided preemptive analgesia and an analgesic-sparing effect that improved post-operative analgesia for these patients without increasing the incidence of adverse effects.[102]
ADVERSE EFFECTS
Adverse effects are summarized in Table 2.
Table 2.
Adverse effects of IT and epidural neostigmine
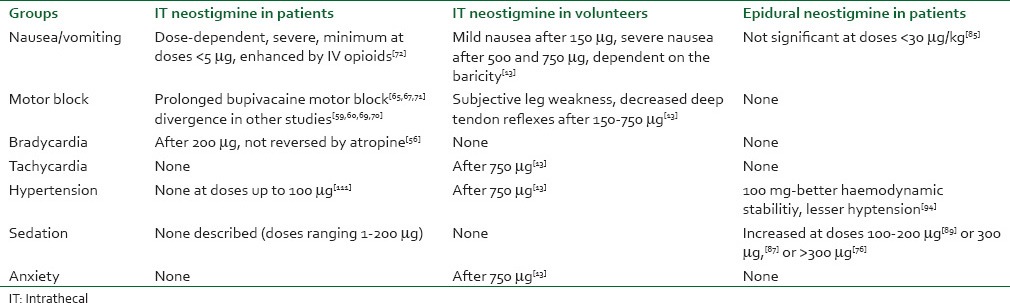
Spinal neostigmine
IT neostigmine increased incidence of nausea and vomiting, bradycardia requiring intravenous atropine, anxiety, agitation, or restlessness. It did not affect the duration of motor blockade or the total amount of ephedrine required.[103] The nausea and vomiting observed in volunteers after spinal neostigmine were depended on the dose used, on the baricity of the solution and on the method of administration and the cephalic ascension of the drug was apparently responsible for emesis.[13] Emesis secondary to IT neostigmine used to be difficult to treat in awake or lightly sedated patients and exacerbated by the combination of opioids injected intravenously, but not when they were injected intrathecally.[71]
Other studies that were conducted to assess the efficacy of different antiemetic drugs in the control of nausea and vomiting characterized emesis as being more intense after manipulation of intra-abdominal viscera[57] compared to orthopedic procedures (somatic type of pain),[58] but not responsive to intravenous droperidol (500 mg), metoclopramide (10 mg),[57,58] ondansetron (4 mg),[65] or dexamethasone (10 mg).[106] The only effective drug seems to be intravenous propofol (2-4 mg/kg/h), but only during the period of infusion,[57,58] secondary to the intrinsic antiemetic property of propofol at sub-hypnotic doses.[105] A possible sedative effect may have reduced the sensation of nausea.[106]
Epidural neostigmine
Administration of neostigmine by the epidural route would be mainly characterized by the action of enzymes located in the meninges,[51,52] with low participation at spinal sites.[13] Until date, epidural doses exceeding 30 μg/kg were associated with a higher incidence of nausea and vomiting[84] and post-operative sedation was increased after 300 μg epidural neostigmine following cesarean delivery.[75]
Anti-hypotensive action of spinal neostigmine
Continued efforts were made in order to assess another possible property of spinal neostigmine, i.e., the ability of the drug to antagonize the hypotensive action secondary to IT anesthesia. In 1994, a study on sheep suggested that hypotension secondary to the administration of a α2-agonist may be prevented by the stimulation of M2 spinal muscarinic cholinergic receptors and by nitric oxide synthesis.[107] It would be extremely interesting and clinically applicable if the drug could minimize the hypotension resulting from regional blockade with a local anesthetic, as demonstrated in rats,[108] in addition to providing post-operative analgesia.
Unfortunately, because of emesis, the doses that could be used in patients were limited and did not reduce the occurrence of hypotension in patients submitted to regional anesthesia with bupivacaine.[109] Studies on sheep[110] clarified that the difference observed between the results obtained with rats and with patients were probably due to the larger size and consequent lower exposure of the spinal cord of the patients proportionally compared with the size of the spinal cord of small animals.[108,110]
CONCLUSIONS
When one analyze the past two decades, IT doses of neostigmine in humans ranged from 750 to 1 μg. Due to side-effects the dose was substantially decreased. Because of the small doses, neostigmine should be applied only as part of multimodal spinal analgesia, and further clinical trials are still needed. Conversely, epidural neostigmine may be evaluated in proportionally higher doses and represents an alternative, but still deserves more investigation concerning both acute and chronic pain, as it seems devoid of important side-effects. Future studies may also include formulations containing liposomes using technology of gradual neostigmine release.[111]
Based on the present data, IT neostigmine dose related efficacy and safety is better approached in patients with spinal doses less than 10 μg, while epidural neostigmine can afford to trials with different doses due to the apparent lack of side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pellandra CL. The morphine geneserine adjuvants of general anesthesia. Lyon Med. 1933;157:653–7. [Google Scholar]
- 2.Kremer M. Action of intrathecally injected prostigmine acetylcholine, and eserine on the central nervous system in man. J Exp Physiol. 1942;31:337–57. [Google Scholar]
- 3.Flodmark S, Wrammer T. The analgesic action of morphine, eserine and prostigmine studied by a modified Handy-Wolff-Goodel method. Acta Physiol Scand. 1994;9:88–96. [Google Scholar]
- 4.Pleuvry BJ, Tobias MA. Comparison of the antinociceptive activities of physostigmine, oxotremorine and morphine in the mouse. Br J Pharmacol. 1971;43:706–14. doi: 10.1111/j.1476-5381.1971.tb07205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nistri A, Pepeu G, Cammelli E, Spina L, De Bellis AM. Effects of morphine on brain and spinal acetylcholine levels and nociceptive threshold in the frog. Brain Res. 1974;80:199–209. doi: 10.1016/0006-8993(74)90684-2. [DOI] [PubMed] [Google Scholar]
- 6.Wamsley JK, Lewis MS, Young WS, 3rd, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. 1981;1:176–91. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985;117:81–8. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- 8.Peterson I, Gordh TE, Hartvig P, Wiklund L. A double-blind trial of the analgesic properties of physostigmine in postoperative patients. Acta Anaesthesiol Scand. 1986;30:283–8. doi: 10.1111/j.1399-6576.1986.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 9.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–27. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Hood DD, Eisenach JC, Tong C, Tommasi E, Yaksh TL. Cardiorespiratory and spinal cord blood flow effects of intrathecal neostigmine methylsulfate, clonidine, and their combination in sheep. Anesthesiology. 1995;82:428–35. doi: 10.1097/00000542-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Werdehausen R, Braun S, Hermanns H, Kremer D, Küry P, Hollmann MW, et al. The influence of adjuvants used in regional anesthesia on lidocaine-induced neurotoxicity in vitro. Reg Anesth Pain Med. 2011;36:436–43. doi: 10.1097/AAP.0b013e318226ba62. [DOI] [PubMed] [Google Scholar]
- 12.Gurun MS, Leinbach R, Moore L, Lee CS, Owen MD, Eisenach JC. Studies on the safety of glucose and paraben-containing neostigmine for intrathecal administration. Anesth Analg. 1997;85:317–23. doi: 10.1097/00000539-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hood DD, Mallak KA, Eisenach JC, Tong C. Interaction between intrathecal neostigmine and epidural clonidine in human volunteers. Anesthesiology. 1996;85:315–25. doi: 10.1097/00000542-199608000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Klamt JG, Dos Reis MP, Barbieri Neto J, Prado WA. Analgesic effect of subarachnoid neostigmine in two patients with cancer pain. Pain. 1996;66:389–91. doi: 10.1016/0304-3959(96)03045-x. [DOI] [PubMed] [Google Scholar]
- 16.Lauretti GR, Gomes JM, Reis MP, Pereira NL. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J Clin Anesth. 1999;11:663–8. doi: 10.1016/s0952-8180(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 17.Shafer SL, Eisenach JC, Hood DD, Tong C. Cerebrospinal fluid pharmacokinetics and pharmacodynamics of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1998;89:1074–88. doi: 10.1097/00000542-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Honda K, Harada A, Takano Y, Kamiya H. Involvement of M3 muscarinic receptors of the spinal cord in formalin-induced nociception in mice. Brain Res. 2000;859:38–44. doi: 10.1016/s0006-8993(99)02456-7. [DOI] [PubMed] [Google Scholar]
- 19.Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, et al. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002;62:1084–93. doi: 10.1124/mol.62.5.1084. [DOI] [PubMed] [Google Scholar]
- 20.Chen SR, Pan HL. Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology. 2001;95:525–30. doi: 10.1097/00000542-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 21.Vincler M, Eisenach JC. Plasticity of spinal nicotinic acetylcholine receptors following spinal nerve ligation. Neurosci Res. 2004;48:139–45. doi: 10.1016/j.neures.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–82. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen MS, Mikkelsen JD. The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Umeda E, Aramaki Y, Mori T, Kazama T. Muscarinic receptor subtypes modulate the release of [3H]-noradrenaline in rat spinal cord slices. Brain Res Bull. 2006;70:99–102. doi: 10.1016/j.brainresbull.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Rashid MH, Furue H, Yoshimura M, Ueda H. Tonic inhibitory role of alpha4 beta2 subtype of nicotinic acetylcholine receptors on nociceptive transmission in the spinal cord in mice. Pain. 2006;125:125–35. doi: 10.1016/j.pain.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SY, Kwon YB, Kim HW, Roh DH, Seo HS, Han HJ, et al. A spinal muscarinic M2 receptor-GABAergic disinhibition pathway that modulates peripheral inflammation in mice. Neuropharmacology. 2007;53:677–86. doi: 10.1016/j.neuropharm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136–45. doi: 10.1016/j.pain.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Chiari A, Tobin JR, Pan HL, Hood DD, Eisenach JC. Sex differences in cholinergic analgesia I: A supplemental nicotinic mechanism in normal females. Anesthesiology. 1999;91:1447–54. doi: 10.1097/00000542-199911000-00038. [DOI] [PubMed] [Google Scholar]
- 29.Lavand’homme PM, Eisenach JC. Sex differences in cholinergic analgesia II: Differing mechanisms in two models of allodynia. Anesthesiology. 1999;91:1455–61. doi: 10.1097/00000542-199911000-00039. [DOI] [PubMed] [Google Scholar]
- 30.Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296:132–40. [PubMed] [Google Scholar]
- 31.Kroin JS, Buvanendran A, Nagalla SK, Tuman KJ. Postoperative pain and analgesic responses are similar in male and female Sprague-Dawley rats. Can J Anaesth. 2003;50:904–8. doi: 10.1007/BF03018737. [DOI] [PubMed] [Google Scholar]
- 32.Bouaziz H, Tong C, Eisenach JC. Postoperative analgesia from intrathecal neostigmine in sheep. Anesth Analg. 1995;80:1–5. doi: 10.1097/00000539-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Baba H, Kohno T, Okamoto M, Goldstein PA, Shimoji K, Yoshimura M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol. 1998;508:83–93. doi: 10.1111/j.1469-7793.1998.083br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SR, Pan HL. Activation of muscarinic receptors inhibits spinal dorsal horn projection neurons: Role of GABAB receptors. Neuroscience. 2004;125:141–8. doi: 10.1016/j.neuroscience.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res. 2003;965:67–74. doi: 10.1016/s0006-8993(02)04123-9. [DOI] [PubMed] [Google Scholar]
- 36.Smith MD, Yang XH, Nha JY, Buccafusco JJ. Antinociceptive effect of spinal cholinergic stimulation: Interaction with substance P. Life Sci. 1989;45:1255–61. doi: 10.1016/0024-3205(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 37.Dunbar SA, Karamian IG. Cross-tolerance between spinal neostigmine and morphine in the rat. Br J Anaesth. 2003;91:427–9. doi: 10.1093/bja/aeg187. [DOI] [PubMed] [Google Scholar]
- 38.Saeki S, Kakishita M, Nakamura T, Kobayashi T, Ogawa S. The effects of intrathecally administered neostigmine on somato-sympathetic reflex potentials. Masui. 2002;51:1322–30. [PubMed] [Google Scholar]
- 39.Prado WA, Gonçalves AS. Antinociceptive effect of intrathecal neostigmine evaluated in rats by two different pain models. Braz J Med Biol Res. 1997;30:1225–31. doi: 10.1590/s0100-879x1997001000014. [DOI] [PubMed] [Google Scholar]
- 40.Detweiler DJ, Eisenach JC, Tong C, Jackson C. A cholinergic interaction in alpha 2 adrenoceptor-mediated antinociception in sheep. J Pharmacol Exp Ther. 1993;265:536–42. [PubMed] [Google Scholar]
- 41.Bouaziz H, Hewitt C, Eisenach JC. Subarachnoid neostigmine potentiation of alpha 2-adrenergic agonist analgesia. Dexmedetomidine versus clonidine. Reg Anesth. 1995;20:121–7. [PubMed] [Google Scholar]
- 42.Hwang JH, Hwang KS, Kim JU, Choi IC, Park PH, Han SM. The interaction between intrathecal neostigmine and GABA receptor agonists in rats with nerve ligation injury. Anesth Analg. 2001;93:1297–303. doi: 10.1097/00000539-200111000-00054. [DOI] [PubMed] [Google Scholar]
- 43.Winne RP, Abram SE. Intrathecal morphine and neostigmine produce synergistic analgesia to noxious thermal stimuli in rats. Reg Anesth. 1994;19:2:6. [Google Scholar]
- 44.Yoon MH, Choi JI, Kwak SH. Characteristic of interactions between intrathecal gabapentin and either clonidine or neostigmine in the formalin test. Anesth Analg. 2004;98:1374–9. doi: 10.1213/01.ane.0000107937.00902.fc. [DOI] [PubMed] [Google Scholar]
- 45.Miranda HF, Sierralta F, Pinardi G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br J Pharmacol. 2002;135:1591–7. doi: 10.1038/sj.bjp.0704599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol. 2012;72:713–28. doi: 10.1002/dneu.20976. [DOI] [PubMed] [Google Scholar]
- 47.Pawlowski SA, Gaillard S, Ghorayeb I, Ribeiro-da-Silva A, Schlichter R, Cordero-Erausquin M. A novel population of cholinergic neurons in the macaque spinal dorsal horn of potential clinical relevance for pain therapy. J Neurosci. 2013;33:3727–37. doi: 10.1523/JNEUROSCI.3954-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buvanendran A, Kroin JS, Kerns JM, Nagalla SN, Tuman KJ. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg. 2004;99:1453–60. doi: 10.1213/01.ANE.0000134806.61887.0D. [DOI] [PubMed] [Google Scholar]
- 49.Salamone G, Lombardi G, Gori S, Nahmod K, Jancic C, Amaral MM, et al. Cholinergic modulation of dendritic cell function. J Neuroimmunol. 2011;236:47–56. doi: 10.1016/j.jneuroim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Lauretti GR, de Oliveira R, Perez MV, Paccola CA. Postoperative analgesia by intraarticular and epidural neostigmine following knee surgery. J Clin Anesth. 2000;12:444–8. doi: 10.1016/s0952-8180(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 51.Ummenhofer WC, Brown SM, Bernards CM. Acetylcholinesterase and butyrylcholinesterase are expressed in the spinal meninges of monkeys and pigs. Anesthesiology. 1998;88:1259–65. doi: 10.1097/00000542-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–20. doi: 10.1002/jemt.1085. [DOI] [PubMed] [Google Scholar]
- 53.Craig HJ. Anaesthetic gases. In: Dundee JW, Clarke RS, McCaughey W, editors. Clinical Anesthetic Pharmacology. New York: Churchill Livingstone; 1990. pp. 127–36. [Google Scholar]
- 54.Xu Z, Tong C, Eisenach JC. Acetylcholine stimulates the release of nitric oxide from rat spinal cord. Anesthesiology. 1996;85:107–11. doi: 10.1097/00000542-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Lin X, Wang Q, Huang W, Zhu L, Yang B. Study on the mechanism of antinociception of intrathecal neostigmine in the formalin test in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:698–700. [PubMed] [Google Scholar]
- 56.Lauretti GR, Reis MP, Prado WA, Klamt JG. Dose-response study of intrathecal morphine versus intrathecal neostigmine, their combination, or placebo for postoperative analgesia in patients undergoing anterior and posterior vaginoplasty. Anesth Analg. 1996;82:1182–7. doi: 10.1097/00000539-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Lauretti GR, Mattos AL, Gomes JM, Pereira NL. Postoperative analgesia and antiemetic efficacy after intrathecal neostigmine in patients undergoing abdominal hysterectomy during spinal anesthesia. Reg Anesth. 1997;22:527–33. [PubMed] [Google Scholar]
- 58.Lauretti GR, Reis MP. Postoperative analgesia and antiemetic efficacy after subarachnoid neostigmine in orthopedic surgery. Reg Anesth. 1997;22:337–42. doi: 10.1016/s1098-7339(97)80008-9. [DOI] [PubMed] [Google Scholar]
- 59.Lauretti GR, Hood DD, Eisenach JC, Pfeifer BL. A multi-center study of intrathecal neostigmine for analgesia following vaginal hysterectomy. Anesthesiology. 1998;89:913–8. doi: 10.1097/00000542-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Lauretti GR, Mattos AL, Reis MP, Prado WA. Intrathecal neostigmine for postoperative analgesia after orthopedic surgery. J Clin Anesth. 1997;9:473–7. doi: 10.1016/s0952-8180(97)00103-7. [DOI] [PubMed] [Google Scholar]
- 61.Lauretti GR. The clinical use of intrathecal neostigmine in regional anesthesia. LASRA Journal. 1997;2:2–3. [Google Scholar]
- 62.Lauretti GR, Lima IC. The effects of intrathecal neostigmine on somatic and visceral pain: Improvement by association with a peripheral anticholinergic. Anesth Analg. 1996;82:617–20. doi: 10.1097/00000539-199603000-00033. [DOI] [PubMed] [Google Scholar]
- 63.Tan P-H, Kuo J-H, Liu K, Hung C-C, Tsai TC, Deng TY. Efficacy of intrathecal neostigmine for the relief of postinguinal hemiorrhaphy pain. Acta Anaesthesiol Scand. 2000;44:1056–60. doi: 10.1034/j.1399-6576.2000.440904.x. [DOI] [PubMed] [Google Scholar]
- 64.Yegin A, Yilmaz M, Karsli B, Erman M. Analgesic effects of intrathecal neostigmine in perianal surgery. Eur J Anaesthesiol. 2003;20:404–8. doi: 10.1017/s0265021503000620. [DOI] [PubMed] [Google Scholar]
- 65.Lauretti GR, Azevedo VM. Intravenous ketamine or fentanyl prolongs postoperative analgesia after intrathecal neostigmine. Anesth Analg. 1996;83:766–70. doi: 10.1097/00000539-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 66.Liu SS, Hodgson PS, Moore JM, Trautman WJ, Burkhead DL. Dose-response effects of spinal neostigmine added to bupivacaine spinal anesthesia in volunteers. Anesthesiology. 1999;90:710–7. doi: 10.1097/00000542-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Tan PH, Chia YY, Lo Y, Liu K, Yang LC, Lee TH. Intrathecal bupivacaine with morphine or neostigmine for postoperative analgesia after total knee replacement surgery. Can J Anaesth. 2001;48:551–6. doi: 10.1007/BF03016831. [DOI] [PubMed] [Google Scholar]
- 68.Krukowski JA, Hood DD, Eisenach JC, Mallak KA, Parker RL. Intrathecal neostigmine for post-cesarean section analgesia: Dose response. Anesth Analg. 1997;84:1269–75. doi: 10.1097/00000539-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Lauretti GR, Oliveira AP, Julião MC, Reis MP, Pereira NL. Transdermal nitroglycerine enhances spinal neostigmine postoperative analgesia following gynecological surgery. Anesthesiology. 2000;93:943–6. doi: 10.1097/00000542-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Batra YK, Rajeev S, Panda NB, Lokesh VC, Rao KL. Intrathecal neostigmine with bupivacaine for infants undergoing lower abdominal and urogenital procedures: Dose response. Acta Anaesthesiol Scand. 2009;53:470–5. doi: 10.1111/j.1399-6576.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 71.Lauretti GR, Mattos AL, Reis MP, Pereira NL. Combined intrathecal fentanyl and neostigmine: Therapy for postoperative abdominal hysterectomy pain relief. J Clin Anesth. 1998;10:291–6. doi: 10.1016/s0952-8180(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 72.Akinwale MO, Sotunmbi PT, Akinyemi OA. Analgesic effect of intrathecal neostigmine combined with bupivacaine and fentanyl. Afr J Med Med Sci. 2012;41:231–7. [PubMed] [Google Scholar]
- 73.Almeida RA, Lauretti GR, Mattos AL. Antinociceptive effect of low-dose intrathecal neostigmine combined with intrathecal morphine following gynecologic surgery. Anesthesiology. 2003;98:495–8. doi: 10.1097/00000542-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 74.Jain A, Jain K, Bhardawaj N. Analgesic efficacy of low-dose intrathecal neostigmine in combination with fentanyl and bupivacaine for total knee replacement surgery. J Anaesthesiol Clin Pharmacol. 2012;28:486–90. doi: 10.4103/0970-9185.101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan PM, Huang CT, Wei TT, Mok MS. Enhancement of analgesic effect of intrathecal neostigmine and clonidine on bupivacaine spinal anesthesia. Reg Anesth Pain Med. 1998;23:49–56. doi: 10.1016/s1098-7339(98)90110-9. [DOI] [PubMed] [Google Scholar]
- 76.Dirksen R, Nijhuis GM. The relevance of cholinergic transmission at the spinal level to opiate effectiveness. Eur J Pharmacol. 1983;91:215–21. doi: 10.1016/0014-2999(83)90467-3. [DOI] [PubMed] [Google Scholar]
- 77.Hood DD, Mallak KA, James RL, Tuttle R, Eisenach JC. Enhancement of analgesia from systemic opioid in humans by spinal cholinesterase inhibition. J Pharmacol Exp Ther. 1997;282:86–92. [PubMed] [Google Scholar]
- 78.D’Angelo R, Dean LS, Meister GC, Nelson KE. Neostigmine combined with bupivacaine, clonidine, and sufentanil for spinal labor analgesia. Anesth Analg. 2001;93:1560–4. doi: 10.1097/00000539-200112000-00048. [DOI] [PubMed] [Google Scholar]
- 79.Owen MD, Ozsaraç O, Sahin S, Uçkunkaya N, Kaplan N, Magunaci I. Low-dose clonidine and neostigmine prolong the duration of intrathecal bupivacaine-fentanyl for labor analgesia. Anesthesiology. 2000;92:361–6. doi: 10.1097/00000542-200002000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Nelson KE, D’Angelo R, Foss ML, Meister GC, Hood DD, Eisenach JC. Intrathecal neostigmine and sufentanil for early labor analgesia. Anesthesiology. 1999;91:1293–8. doi: 10.1097/00000542-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 81.Watson PJ, Moore RA, McQuay HJ. Plasma morphine concentrations and analgesic effects of lumbar extradural morphine and heroin. Anesth Analg. 1984;63:629–34. [PubMed] [Google Scholar]
- 82.Lauretti GR, de Oliveira R, Reis MP, Juliâo MC, Pereira NL. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology. 1999;90:1534–8. doi: 10.1097/00000542-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Batra YK, Arya VK, Mahajan R, Chari P. Dose response study of caudal neostigmine for postoperative analgesia in paediatric patients undergoing genitourinary surgery. Paediatr Anaesth. 2003;13:515–21. doi: 10.1046/j.1460-9592.2003.01066.x. [DOI] [PubMed] [Google Scholar]
- 84.Taspinar V, Pala Y, Diker S, Ornek HD, Ozdogan L, Akcay M, et al. Pre-emptive analgesic and haemodynamic efficacy of combined spinal-epidural neostigmine delivery. J Coll Physicians Surg Pak. 2012;22:201–6. [PubMed] [Google Scholar]
- 85.Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–5. doi: 10.1097/00000542-200402000-00030. [DOI] [PubMed] [Google Scholar]
- 86.Abelson KS, Höglund AU. Intravenously administered lidocaine in therapeutic doses increases the intraspinal release of acetylcholine in rats. Neurosci Lett. 2002;317:93–6. doi: 10.1016/s0304-3940(01)02440-5. [DOI] [PubMed] [Google Scholar]
- 87.Harjai M, Chandra G, Bhatia VK, Singh D, Bhaskar P. A comparative study of two different doses of epidural neostigmine coadministered with lignocaine for post operative analgesia and sedation. J Anaesthesiol Clin Pharmacol. 2010;26:461–4. [PMC free article] [PubMed] [Google Scholar]
- 88.Memiş D, Turan A, Karamanlioğlu B, Kaya G, Süt N, Pamukçu Z. Caudal neostigmine for postoperative analgesia in paediatric surgery. Paediatr Anaesth. 2003;13:324–8. doi: 10.1046/j.1460-9592.2003.01020.x. [DOI] [PubMed] [Google Scholar]
- 89.Turan A, Memiş D, Başaran UN, Karamanlioğlu B, Süt N. Caudal ropivacaine and neostigmine in pediatric surgery. Anesthesiology. 2003;98:719–22. doi: 10.1097/00000542-200303000-00021. [DOI] [PubMed] [Google Scholar]
- 90.Mahajan R, Grover VK, Chari P. Caudal neostigmine with bupivacaine produces a dose-independent analgesic effect in children. Can J Anaesth. 2004;51:702–6. doi: 10.1007/BF03018429. [DOI] [PubMed] [Google Scholar]
- 91.Nakayama M, Ichinose H, Nakabayashi K, Satoh O, Yamamoto S, Namiki A. Analgesic effect of epidural neostigmine after abdominal hysterectomy. J Clin Anesth. 2001;13:86–9. doi: 10.1016/s0952-8180(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 92.Dadu S, Mishra LS, Agrawal M, Chandola HC. Comparative clinical study of effect of neostigmine and ketamine for postoperative analgesia. J Indian Med Assoc. 2011;109:308–11. [PubMed] [Google Scholar]
- 93.Omais M, Lauretti GR, Paccola CA. Epidural morphine and neostigmine for postoperative analgesia after orthopedic surgery. Anesth Analg. 2002;95:1698–701. doi: 10.1097/00000539-200212000-00042. [DOI] [PubMed] [Google Scholar]
- 94.Lauretti GR, Azevedo VM, Lopes BC, Mattos AL. Comparison between the intravenous and caudal route of sufentanil in children undergoing orchidopexy. Further evaluation of the association of caudal adrenaline and neostigmine. Saudi J Anaesth. 2014;8:345–50. doi: 10.4103/1658-354X.136430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roelants F, Rizzo M, Lavand’homme P. The effect of epidural neostigmine combined with ropivacaine and sufentanil on neuraxial analgesia during labor. Anesth Analg. 2003;96:1161–6. doi: 10.1213/01.ANE.0000050480.73209.9C. [DOI] [PubMed] [Google Scholar]
- 96.Roelants F, Lavand’homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–44. doi: 10.1097/00000542-200408000-00025. [DOI] [PubMed] [Google Scholar]
- 97.Roelants F, Mercier-Fuzier V, Lavand’homme PM. The effect of a lidocaine test dose on analgesia and mobility after an epidural combination of neostigmine and sufentanil in early labor. Anesth Analg. 2006;103:1534–9. doi: 10.1213/01.ane.0000244595.03322.52. [DOI] [PubMed] [Google Scholar]
- 98.Ross VH, Pan PH, Owen MD, Seid MH, Harris L, Clyne B, et al. Neostigmine decreases bupivacaine use by patient-controlled epidural analgesia during labor: A randomized controlled study. Anesth Analg. 2009;109:524–31. doi: 10.1213/ane.0b013e31819518e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van de Velde M, Berends N, Kumar A, Devroe S, Devlieger R, Vandermeersch E, et al. Effects of epidural clonidine and neostigmine following intrathecal labour analgesia: A randomised, double-blind, placebo-controlled trial. Int J Obstet Anesth. 2009;18:207–14. doi: 10.1016/j.ijoa.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Kirdemir P, Ozkoçak I, Demir T, Gögüş N. Comparison of postoperative analgesic effects of preemptively used epidural ketamine and neostigmine. J Clin Anesth. 2000;12:543–8. doi: 10.1016/s0952-8180(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 101.Masaki E, Saito H, Shoji K, Matsushima M. Postoperative analgesic effect of epidural neostigmine and plasma cortisol and IL-6 responses. J Clin Anesth. 2004;16:488–92. doi: 10.1016/j.jclinane.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Chia YY, Chang TH, Liu K, Chang HC, Ko NH, Wang YM. The efficacy of thoracic epidural neostigmine infusion after thoracotomy. Anesth Analg. 2006;102:201–8. doi: 10.1213/01.ane.0000184812.94185.b3. [DOI] [PubMed] [Google Scholar]
- 103.Ho KM, Ismail H, Lee KC, Branch R. Use of intrathecal neostigmine as an adjunct to other spinal medications in perioperative and peripartum analgesia: A meta-analysis. Anaesth Intensive Care. 2005;33:41–53. doi: 10.1177/0310057X0503300107. [DOI] [PubMed] [Google Scholar]
- 104.Tan PH, Liu K, Peng CH, Yang LC, Lin CR, Lu CY. The effect of dexamethasone on postoperative pain and emesis after intrathecal neostigmine. Anesth Analg. 2001;92:228–32. doi: 10.1097/00000539-200101000-00044. [DOI] [PubMed] [Google Scholar]
- 105.Borgeat A, Wilder-Smith OH, Saiah M, Rifat K. Subhypnotic doses of propofol possess direct antiemetic properties. Anesth Analg. 1992;74:539–41. doi: 10.1213/00000539-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 106.Hvarfner A, Hammas B, Thörn SE, Wattwil M. The influence of propofol on vomiting induced by apomorphine. Anesth Analg. 1995;80:967–9. doi: 10.1097/00000539-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 107.Lothe A, Li P, Tong C, Yoon Y, Bouaziz H, Detweiler DJ, et al. Spinal cholinergic alpha-2 adrenergic interactions in analgesia and hemodynamic control: Role of muscarinic receptor subtypes and nitric oxide. J Pharmacol Exp Ther. 1994;270:1301–6. [PubMed] [Google Scholar]
- 108.Carp H, Jayaram A, Morrow D. Intrathecal cholinergic agonists lessen bupivacaine spinal-block-induced hypotension in rats. Anesth Analg. 1994;79:112–6. [PubMed] [Google Scholar]
- 109.Lauretti GR, Reis MP. Subarachnoid neostigmine does not affect blood pressure or heart rate during bupivacaine spinal anesthesia. Reg Anesth. 1996;21:586–91. [PubMed] [Google Scholar]
- 110.Rose G, Xu Z, Tong C, Eisenach JC. Spinal neostigmine diminishes, but does not abolish, hypotension from spinal bupivacaine in sheep. Anesth Analg. 1996;83:1041–5. doi: 10.1097/00000539-199611000-00024. [DOI] [PubMed] [Google Scholar]
- 111.Grant GJ, Piskoun B, Bansinath M. Intrathecal administration of liposomal neostigmine prolongs analgesia in mice. Acta Anaesthesiol Scand. 2002;46:90–4. doi: 10.1034/j.1399-6576.2002.460116.x. [DOI] [PubMed] [Google Scholar]


