Abstract
Leukosialin (also known as Ly48, CD43, and sialophorin) is a major cell surface sialoglycoprotein found on a variety of hematopoietically derived cells. The precise function of this molecule is poorly understood but it has been implicated in cell proliferation and intercellular adhesion. We developed a transgenic mouse model to assess leukosialin's function in vivo. Our approach was to alter mouse CD43 (mCD43) expression in the B-cell lineage where it is tightly regulated, by expressing it in peripheral B cells where it is normally absent. To drive expression of leukosialin in mature B cells, the immunoglobulin heavy chain enhancer was fused to the mCD43 gene. mCD43-immunoglobulin heavy chain enhancer transgenic mice display splenomegaly due to increased numbers of B cells. Transgenic B cells show a striking increase in their ability to survive in vitro compared to B cells from nontransgenic control mice. This prolonged survival is reflected in a decreased susceptibility to apoptosis. These observations suggest that mCD43 plays an important role in the regulation of B-cell survival. The alteration of the temporal expression, or "disregulation," of a gene in transgenic mice provides a general strategy for elucidating the in vivo role of other molecules involved in cell signaling and adhesion.
Full text
PDF
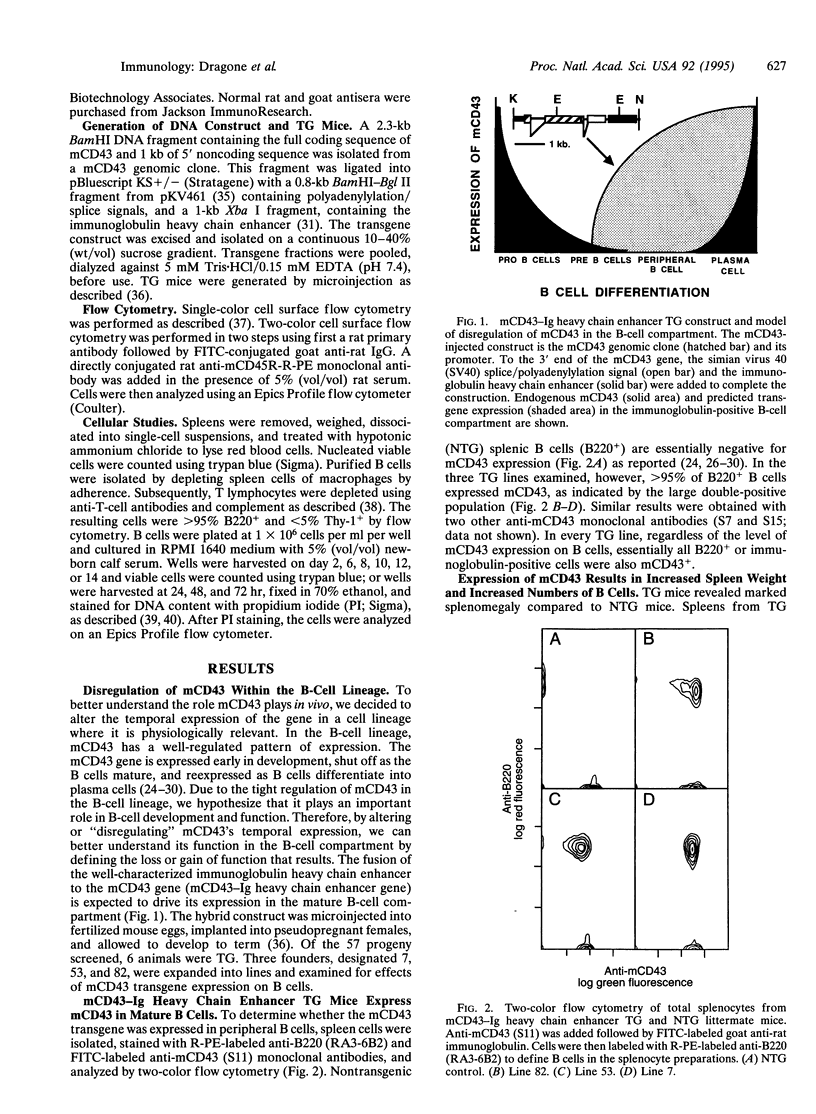
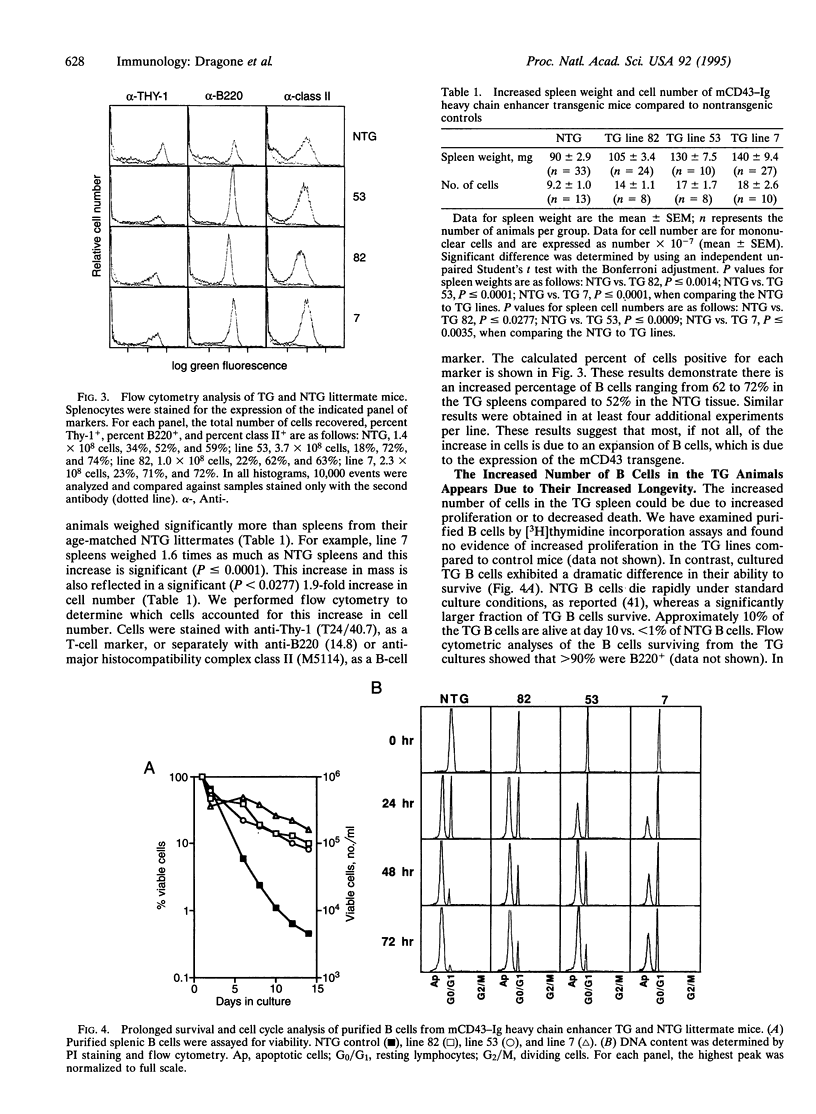


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Okada T., Matsumoto K., Ishida Y., Shiota J., Nishimura H., Hirose S., Sato H., Shirai T. The novel murine B cell differentiation antigen Lp-3. Int Immunol. 1989;1(6):576–581. doi: 10.1093/intimm/1.6.576. [DOI] [PubMed] [Google Scholar]
- Ardman B., Sikorski M. A., Settles M., Staunton D. E. Human immunodeficiency virus type 1-infected individuals make autoantibodies that bind to CD43 on normal thymic lymphocytes. J Exp Med. 1990 Oct 1;172(4):1151–1158. doi: 10.1084/jem.172.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardman B., Sikorski M. A., Staunton D. E. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Baecher-Allan C. M., Kemp J. D., Dorfman K. S., Barth R. K., Frelinger J. G. Differential epitope expression of Ly-48 (mouse leukosialin). Immunogenetics. 1993;37(3):183–192. doi: 10.1007/BF00191883. [DOI] [PubMed] [Google Scholar]
- Baecher C. M., Dorfman K. S., Mattei M. G., Frelinger J. G. cDNA cloning and localization of the mouse leukosialin gene (Ly48) to chromosome 7. Immunogenetics. 1990;31(5-6):307–314. doi: 10.1007/BF02115004. [DOI] [PubMed] [Google Scholar]
- Baecher C. M., Infante A. J., Semcheski K. L., Frelinger J. G. Identification and characterization of a mouse cell surface antigen with alternative molecular forms. Immunogenetics. 1988;28(5):295–302. doi: 10.1007/BF00364226. [DOI] [PubMed] [Google Scholar]
- Bahler D. W., Cerosaletti K. M., Lord E. M., Frelinger J. G. Molecular analysis of deficient class I H-2 antigen expression by mouse lung carcinoma cells. J Immunol. 1988 Jun 1;140(11):4003–4012. [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Björck P., Axelsson B., Paulie S. Expression of CD40 and CD43 during activation of human B lymphocytes. Scand J Immunol. 1991 Feb;33(2):211–218. doi: 10.1111/j.1365-3083.1991.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. How B and T cells talk to each other. Nature. 1994 Feb 3;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Williams A. F. The importance of cross-linking in the homotypic aggregation of lymphocytes induced by anti-leukosialin (CD43) antibodies. Eur J Immunol. 1992 Oct;22(10):2565–2572. doi: 10.1002/eji.1830221015. [DOI] [PubMed] [Google Scholar]
- Deng G., Podack E. R. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Dorfman K. S., Litaker W., Baecher C. M., Frelinger J. G. The nucleotide sequence of Ly 48 (mouse leukosialin, sialophorin): the mouse homolog of CD43. Nucleic Acids Res. 1990 Aug 25;18(16):4932–4932. doi: 10.1093/nar/18.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991 Sep;1(4):347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown J., Molgaard H. V., Spurr N. K., Robertson D., Delia D., Sutherland D. R. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992;6 (Suppl 1):31–36. [PubMed] [Google Scholar]
- Gulley M. L., Ogata L. C., Thorson J. A., Dailey M. O., Kemp J. D. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988 Jun 1;140(11):3751–3757. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Kemp J. D., Hayakawa K. Analysis of lymphoid population in scid mice; detection of a potential B lymphocyte progenitor population present at normal levels in scid mice by three color flow cytometry with B220 and S7. Curr Top Microbiol Immunol. 1989;152:19–25. doi: 10.1007/978-3-642-74974-2_3. [DOI] [PubMed] [Google Scholar]
- Illera V. A., Perandones C. E., Stunz L. L., Mower D. A., Jr, Ashman R. F. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. J Immunol. 1993 Sep 15;151(6):2965–2973. [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. S., Hayakawa K., Hardy R. R. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993 Sep 1;178(3):951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Howell R. L., Smith H. C. Hypoxic stress induces reversible hypophosphorylation of pRB and reduction in cyclin A abundance independent of cell cycle progression. Oncogene. 1993 Feb;8(2):331–339. [PubMed] [Google Scholar]
- Manjunath N., Johnson R. S., Staunton D. E., Pasqualini R., Ardman B. Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J Immunol. 1993 Aug 1;151(3):1528–1534. [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Ding L., Veis D. J., Korsmeyer S. J., Nuñez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994 Feb 1;13(3):683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G., Kim N., Manoukian R., Phillips R. A., Rico-Vargas S. A., Jacobsen K. Dynamics and localization of early B-lymphocyte precursor cells (pro-B cells) in the bone marrow of scid mice. Blood. 1992 Apr 1;79(7):1695–1703. [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman R., Remold-O'Donnell E., Kenney D. M., Perrine S., Rosen F. S. Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet. 1981 Dec 19;2(8260-61):1387–1389. doi: 10.1016/s0140-6736(81)92802-6. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Orth K., Calame K. L. Binding in vitro of multiple cellular proteins to immunoglobulin heavy-chain enhancer DNA. Mol Cell Biol. 1986 Dec;6(12):4168–4178. doi: 10.1128/mcb.6.12.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W., Williams G., Barton S., Norris M., Neuberger M., Surani M. A. Provision of the immunoglobulin heavy chain enhancer downstream of a test gene is sufficient to confer lymphoid-specific expression in transgenic mice. Eur J Immunol. 1987 Apr;17(4):465–469. doi: 10.1002/eji.1830170405. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Haasner D., Nishikawa S., Melchers F. Changes in frequencies of clonable pre B cells during life in different lymphoid organs of mice. Blood. 1993 May 1;81(9):2290–2300. [PubMed] [Google Scholar]
- Roper R. L., Conrad D. H., Brown D. M., Warner G. L., Phipps R. P. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J Immunol. 1990 Oct 15;145(8):2644–2651. [PubMed] [Google Scholar]
- Rosenstein Y., Park J. K., Hahn W. C., Rosen F. S., Bierer B. E., Burakoff S. J. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991 Nov 21;354(6350):233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- Roxburgh A. E., Cooper I. A. Expression of leucocyte-common antigen and large sialoglycoprotein on leukemic cells in B-cell chronic lymphocytic leukemia and non-Hodgkin's lymphoma. Leuk Res. 1987;11(10):891–901. doi: 10.1016/0145-2126(87)90135-4. [DOI] [PubMed] [Google Scholar]
- Sawada R., Tsuboi S., Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1994 Jan 14;269(2):1425–1431. [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Davis A. E., 3rd, Bruns G. A., Rosen F. S., Carroll M. C., Whitehead A. S. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2819–2823. doi: 10.1073/pnas.86.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Rosen F. S., Whitehead A. S. Structure of the human sialophorin (CD43) gene. Identification of features atypical of genes encoding integral membrane proteins. Biochem J. 1990 Sep 15;270(3):569–576. doi: 10.1042/bj2700569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota J., Nishimura H., Okamoto H., Yu B., Hattori S., Abe M., Okada T., Nozawa S., Tsurui H., Hirose S. A unique murine CD43 epitope Lp-3: distinct distribution from another CD43 epitope S7. Cell Immunol. 1994 May;155(2):402–413. doi: 10.1006/cimm.1994.1133. [DOI] [PubMed] [Google Scholar]
- Sowden M., Harrison S., Ashfield R., Kingsman A. J., Kingsman S. M. Multiple cooperative interactions constrain BPV-1 E2 dependent activation of transcription. Nucleic Acids Res. 1989 Apr 25;17(8):2959–2972. doi: 10.1093/nar/17.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Shotton D. M. Effects of capping on the non-ionic detergent solubility of rat thymocyte glycoproteins. Eur J Cell Biol. 1989 Dec;50(2):324–332. [PubMed] [Google Scholar]
- Wikén M., Björck P., Axelsson B., Perlmann P. Induction of CD43 expression during activation and terminal differentiation of human B cells. Scand J Immunol. 1988 Oct;28(4):457–464. doi: 10.1111/j.1365-3083.1988.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Wikén M., Robertsson E. S., Axelsson B., Perlmann P. Functional characterization of human B cells carrying the lymphocyte large sialoglycoprotein gp150. Scand J Immunol. 1987 Nov;26(5):477–485. doi: 10.1111/j.1365-3083.1987.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Cellular interactions. Out of equilibrium. Nature. 1991 Aug 8;352(6335):473–474. doi: 10.1038/352473a0. [DOI] [PubMed] [Google Scholar]
- Yonemura S., Nagafuchi A., Sato N., Tsukita S. Concentration of an integral membrane protein, CD43 (leukosialin, sialophorin), in the cleavage furrow through the interaction of its cytoplasmic domain with actin-based cytoskeletons. J Cell Biol. 1993 Jan;120(2):437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S. Lectin-binding and spontaneous capping characteristics of the thymocyte glycophorin-like glycoprotein. Exp Cell Res. 1984 Jun;152(2):510–519. doi: 10.1016/0014-4827(84)90653-0. [DOI] [PubMed] [Google Scholar]
- de Petris S. Nonuniform distribution of concanavalin-A receptors and surface antigens on uropod-forming thymocytes. J Cell Biol. 1978 Oct;79(1):235–251. doi: 10.1083/jcb.79.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]



