Abstract
How an object is perceived depends on the temporal context in which it is encountered. Sensory signals in the brain also depend on temporal context, a phenomenon often referred to as adaptation. Traditional descriptions of adaptation effects emphasize various forms of response fatigue in single neurons, which grow in strength with exposure to a stimulus. Recent work on vision, and other sensory modalities, has shown that this description has substantial shortcomings. Here we review our emerging understanding of how adaptation alters the balance between excitatory and suppressive signals, how effects depend on adaptation duration, and how adaptation influences representations that are distributed within and across multiple brain structures. This work points to a sophisticated set of mechanisms for adjusting to recent sensory experience, and suggests new avenues for understanding their function.
Introduction
Adaptation affects how neurons respond to sensory stimuli, making them sensitive to the temporal context in which a stimulus is embedded. Adaptation thus adjusts brain processing to the current sensory environment, and it is generally thought that this improves performance in some way. Understanding how the brain adapts may therefore provide insight into its computational goals, and the constraints on its functional organization. Adaptation is also of interest because it is widely used in human functional imaging and perceptual studies to infer the selectivity of neurons and brain areas, and to deduce the computations involved in sensory processing. Proper inference in these domains requires a thorough understanding of how neurons and circuits adapt.
Early descriptions of adaptation effects emphasized fatigue of single neurons during the presentation of an effective stimulus. In mechanistic terms, these effects can be explained by the observation that adaptation shifts a neuron's state away from the threshold required for generation of action potentials [1–7]. This hyperpolarization is triggered by intrinsic mechanisms that are recruited during periods of high activity [2–4]. There are four central tenets that follow from this description and our understanding of the mechanisms responsible: first, it takes time for neurons to recover from periods of high activity, and neurons therefore show reduced responsiveness to subsequent stimuli; second, fatigue is more pronounced when an adaptor is presented for longer durations; third, the degree of fatigue depends on the effectiveness of the adaptor, so a stimulus that matches a neuron's preference will cause the strongest effects; and fourth, because it involves simple fatigue, adaptation will reduce sensitivity to all subsequent stimuli, not just those that resemble the adaptor.
The simple fatigue description fails to capture the stimulus specificity of adaptation effects: responses to stimuli that resemble the adaptor are reduced more than responses to stimuli that differ from it. When the adaptor falls on the flank of the tuning curve, this specificity causes the neuron's tuning to shift away from the adaptor [8–11]. Stimulus-specific adaptation effects can be accounted for by synaptic fatigue, which results in less synaptic input from neurons whose stimulus preference best matches the adaptor, either because these presynaptic cells are fatigued or because transmitter release from their terminals is depressed [12,13].
Stimulus-specific fatigue cannot explain all adaptation effects (see [14–20] for reviews). For instance, adaptation with dynamic stimulus sequences have revealed that neuronal input–output functions can adjust to encode the range of stimuli in the environment [21,22]. Nevertheless, the stimulus-specific fatigue description has provided a powerful and simple explanation for many physiological and perceptual observations [14–17,20], and is central to a number of functional proposals [9,23].
More recent work has revealed limitations of fatigue-based descriptions of adaptation effects, evident even in the simplest adapt-test paradigm. Our aim here is to highlight the emerging themes of this work. First, we will review evidence that adaptation can enhance responsivity, not just reduce it. Many of these facilitory effects of adaptation can be explained and predicted by invoking normalization, a widely observed component of sensory processing. Second, it is now clear that there is a complex relationship between adaptation duration and the effects it induces — and that one cannot assume that longer adaptation simply causes stronger effects. Third, recent work has begun to move beyond exploring effects in single neurons at discrete stages of processing, to studying how adaptation alters representations distributed within and across stages of processing. We will discuss how together this work reinvigorates, and informs, the search for a clear functional benefit of adaptation effects. We will focus on the visual cortex where much of this work has been performed, and draw parallels in other systems where available.
Normalization and Adaptation in Visual Cortex
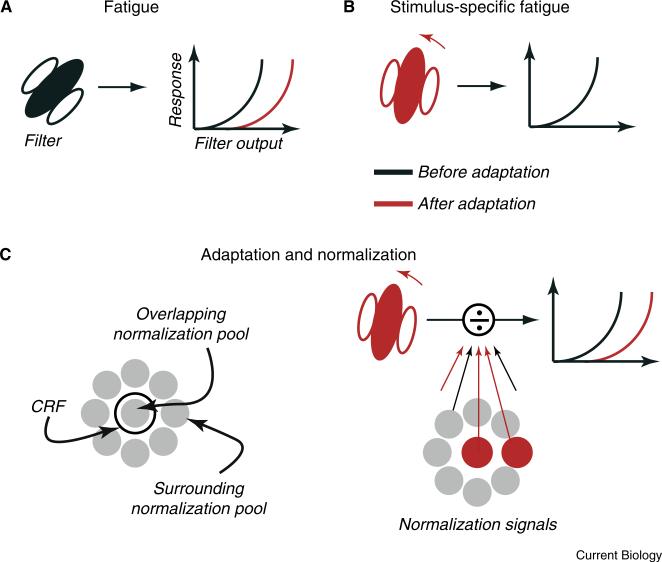
The receptive fields of sensory neurons consist of two distinct components. The first is the classical receptive field (CRF), which defines the stimuli that can directly drive spiking activity. For example, the CRF of a simple cell in primary visual cortex (V1) can be approximated by a linear filter, which determines selectivity for stimulus parameters such as position and orientation, and an output nonlinearity, which relates filter responses to spiking activity. The simple fatigue description of adaptation is captured by a change in the nonlinearity, thus affecting responses to all stimuli (Figure 1A); changes in tuning preference caused by stimulus-specific fatigue are captured by altering the shape of the filter (Figure 1B).
Figure 1. Frameworks of adaptation.
(A) Fatigue: simple models of sensory response include a linear stage, which captures the weighted summation of synaptic inputs and dictates the shape of tuning curves, and a non-linear stage that transforms this weighted sum into an output. Fatigue is captured by a change in the non-linear function that generates outputs, causing all subsequent responses to be reduced. Here and elsewhere the red indicates sites and effects after adaptation; black indicates before adaptation. (B) Stimulus-specific fatigue: fatigue is generated in a subset of synaptic inputs to the neuron under study and therefore deforms the linear filter of the CRF. The impact of adaptation is greatest for subsequent tests that resemble the adaptor, often generating “repulsive” shifts in tuning curves. (C) Normalization models interpose a gain control between the filter output and non-linearity. This gain control draws on a large pool of signals that cover the CRF and extend beyond it. Their suppressive impact is captured by a divisive interaction with the CRF. In this framework, adaptation can have independent impact on the CRF and normalization signals.
The second component of the receptive field is composed of ‘gain-controls’, or normalization signals (Figure 1C, left). These inputs have a divisive effect on the output of the CRF and thus suppress spiking activity. Normalization signals have been observed across sensory modalities, and are an integral component of modern functional models of sensory neurons [24]. Normalization signals are generally recruited by a broader range of stimuli than those that drive the CRF — a working description is that normalization arises from the activity of a pool of neurons, with a broad range of functional properties and receptive fields that can either spatially overlap the CRF of the target neuron or be offset from it. Normalization explains why there is sub-linear summation of responses to two sensory stimuli within the CRF, as in masking and contrast saturation. It also explains why large stimuli, which recruit normalization from the ‘surround’, evoke weaker responses than stimuli falling wholly within the CRF.
Recent work has shown that adaptation not only affects the CRF, but can also weaken normalization signals (Figure 1C, right). Because normalization is suppressive, weakening these signals is a form of disinhibition, which can enhance responses to subsequent stimuli. A clear example of such facilitation is evident when an adaptor is placed in the surround. Such adaptors elicit no response from the CRF, by definition, but do weaken normalization signals from the surround. As a result, responses to stimuli that cover both the CRF and surround are enhanced after adaptation of the surround (Figure 2A), in some cases by two- or three-fold [25,26].
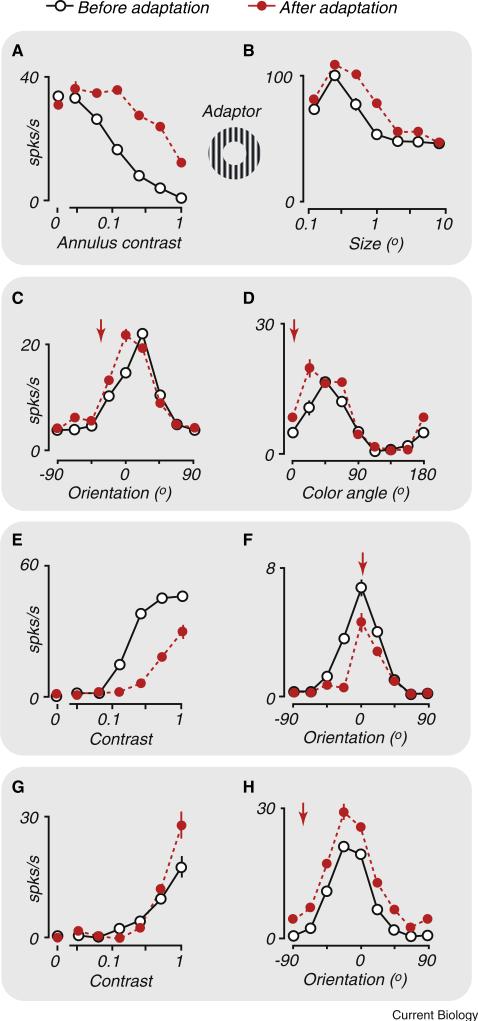
Figure 2. Impact of adaptation on visual sensory neurons.
Each panel compares response before (open symbols) and after (red filled symbols) prolonged adaptation to a high contrast stimulus. Arrows indicate the identity of the adapting stimulus when measuring tuning curves. (A,B) Adaptation to annular stimuli can enhance responsivity and change spatial summation. (A) Response of a V1 neuron to a stimulus within the CRF is suppressed by contrast in a surrounding annulus. Adaptation confined to the annular region weakens this suppression, facilitating responses to large stimuli. (B) Responses of an LGN neuron to stimuli of increasing size, illustrating a ‘suppressive surround’ that reduces response to large gratings. After adaptation to an annular grating, the sensitivity of the surround is reduced, increasing the effective summation area of the neuron. (C,D) Adaptation to a stimulus can attract tuning curves towards the adaptor. (C) Responses of V1 neurons to orientations that are similar to the adapting stimulus are facilitated, with little effect on response to other orientations. (D) Same as (C), but for color. (E,F) Adaptation effects include reduced responsivity. (E) Contrast-response function of a V1 neuron for a stimulus in its preferred orientation. Adaptation with the preferred stimulus shifts the contrast-response curve to the right. (F) Orientation tuning curve of a V1 neuron. Adaptation leads to weaker responses for most orientations. (G,H) Adaptation to a non-preferred stimulus can lead to response facilitation. Response of V1 neurons after adaptation to gratings orthogonal to the preferred orientation. (G) Adaptation can increase response to subsequent high contrast tests of the preferred orientation. (H) Response is increased across a wide range of stimuli, suggesting that adaptation has desensitised a broadly tuned normalization signal. (A) Redrawn from [25]. (B) Redrawn from [38]. (C) Redrawn from [26]. (D) Redrawn from [28]. (E) Redrawn from [31]. (F) Redrawn from [26]. (G) Redrawn from [31]. (H) Redrawn from [26].
Weakened normalization signals can also shape the effects of adaptation on neural tuning. Adaptation weakens normalization in a stimulus-specific way, yielding a tuned disinhibition, just as the specificity of its effects in the CRF results in tuned fatigue. Tuned disinhibition, in turn, results in maintained or enhanced responses to stimuli similar to the adaptor (see Box 1 for further discussion). Such effects have been observed in V1, where a large adaptor causes orientation tuning to be attracted towards the adapting orientation (Figure 2C) [11,26,27]. Adaptation can cause similar attractive shifts in V1 colour tuning (Figure 2D) [28], and direction tuning in visual cortical area MT [29,30].
Box 1 Adaptation in the context of normalization models.
The normalization model provides a functional description of neuronal responses. It captures the classical receptive field (CRF) as a weighted sum of driving inputs to a neuron. The impact of the CRF on firing rate is regulated by a gain control, or normalization signal, that captures the impact of other neural machinery [24]. Mathematically, this can be formulated as:
| Equation (1) |
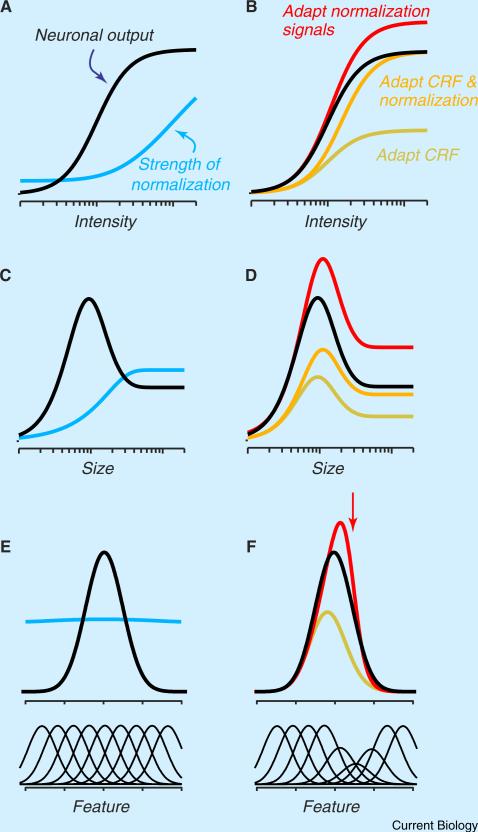
where R is the response of the neuron, i is the intensity of the stimulus, M is the maintained activity in absence of a patterned stimulus, Rmax the maximal response, and n an exponent that captures the impact of an output nonlinearity. Normalization is captured by this equation because stronger drive to the CRF – the numerator – results in stronger responses; stronger drive to the normalization pool, the denominator G, reduces activity, in a divisive manner. The normalization pool is typically considered to be the summed activity of many neurons, with different stimulus preferences, which are not explicitly defined here. The effective strength of the normalization signal is determined in part by the constant δ.
Panel A in the figure shows the intensity-response function of a typical sensory neuron: response grows with intensity, and then saturates. The blue line in panel A shows how the relative strength of the normalization signal is thought to increase with intensity [24]. Panel B in the figure shows predicted responses of a neuron when adaptation has an impact on the CRF (yellow line), normalization (red line), or both (orange line). If adaptation desensitises only the CRF, it affects responses at all intensities, as if there was a change in “response gain”. A weakening of the normalization signal enhances responses, particularly to stronger stimuli. Finally, if adaptation desensitises the CRF and normalization in a balanced way, it reduces response at low intensities but leaves responses to strong stimuli unaffected, as if there were a change in “contrast gain”. Note that joint adaptation of the CRF and normalization brings about a horizontal shift in the intensity-response function; this shift is a characteristic effect of adaptation and in previous work has been captured by changing s [31,33,34], which has the same impact in Equation (1).
A normalization model can also account for adaptation's effect on spatial tuning [24,38,57]. The black lines in figure panels C and D show the size-tuning function of a typical visual neuron: response first grows with size, reaches a plateau, and then declines. The initial rise primarily reflects summation within the CRF and the subsequent decline is because normalization from the surround is recruited when the stimulus extends beyond the CRF (blue line). Figure panel D shows predicted responses of a neuron when adaptation has an impact on the CRF (yellow line), the surround (red line), or both (orange line). If adaptation desensitisesonly theCRF, it reduces response to all sizes; if adaptation desensitises only surround normalization, response is increased primarily at larger sizes. Note that adaptation can have spatially specific effects in both the CRF and surround [38]; these can be capturedby supposingthat adaptation desensitises only those parts of each mechanism that are covered by the adaptor.
This simple model is also capable of explaining the diverse effects of adaptation on neural tuning along other stimulus dimensions [11,26,28]. Figure panel E illustrates the tuning of the CRF and normalization of sensory neurons when constructed by appropriately weighing tuned inputs, drawn below the panel. The normalization pooldrawsfrom a wider range ofinputsthanthe CRF.Whenanadaptor desensitises a subset of inputs to the CRF, it reduces response particularly around the adaptor and therefore shifts the preferred stimulus away from the adaptor (figure panel F, yellow); when it has an impact on normalization signals, adaptation can increase response, particularly for stimuli that resemble the adaptor (figure panel F, red).
The effect of adaptors that provide drive to the CRF and normalization pool depends on the relative sensitivity of these two receptive field components to the adaptor and test. Responsivity will be reduced most when the adaptor provides strong drive to the CRF (Figures 2E,F), and weak drive to the normalization signals. Previous single neuron studies of adaptation have emphasized reduced responsivity because they usually tailored stimuli to match the CRF of the recorded neuron. In these cases, adaptation effects on the CRF overwhelm those on normalization signals. By contrast, an adaptor that strongly drives normalization signals, but is only moderately effective for the CRF, is likely to have facilita-tory effects. For instance, adaptation to a grating that is within the CRF but orthogonal to a neuron's preferred orientation will weaken normalization signals (Figure 2G,H; [31] but see [32]). If the tuning of the CRF is sufficiently narrow [33], orthogonal adaptors will then enhance responsivity [26,31]. Importantly, because normalization signals are more broadly tuned than the CRF, the effects of an adaptor on normalization signals will likely be evident in a larger population of neurons than are its effects on CRFs.
The normalization framework also explains how stimulus intensity can influence the effects of adaptation. The framework predicts that responses to weak test stimuli should be reduced particularly strongly after adaptation. These test stimuli do not recruit substantial normalization; thus, the impact of any adaptation-induced changes in normalization will be limited. Responses to high-intensity test stimuli, on the other hand, may be only slightly reduced by adaptation, or even enhanced, as recently reported (Figure 2G) [26,31,34]. This reasoning can also explain why repeated presentations of weak stimuli result in a greater loss of responsivity than repeated presentations of strong stimuli [30]. The relationship between stimulus intensity and normalization signals also predicts that the effects of adaptation on tuning should depend on test stimulus intensity: adaptation should repel tuning for low-intensity stimuli, but attract tuning for high-intensity ones. These points are discussed further in Box 1.
While the normalization framework can predict the relative strength of adaptation effects for different stimuli, the absolute strength of effects will also depend on the baseline against which the adapted state is compared. Frequently, the baseline involves stimuli presented in temporal isolation (that is, separated by long presentations of a blank screen). Such a state is highly unusual: in our everyday experience, stimuli appear in a steady stream [35,36]. Whether recent experience facilitates or suppresses neural responses under continuous sensory stimulation will depend on the change it induces in the ongoing adaptation state of both the CRF and normalization signals. For instance, even traditional descriptions would predict a relative enhancement of responsivity, if responses after an ineffective adaptor are compared to a baseline of greater fatigue [26].
In summary, recent work has shown that adaptation can have a diverse set of effects on neuronal tuning. Adaptation can sensitise or desensitise sensory neurons, deform tuning towards or away from the adapted stimulus, and also leave responsivity unaltered. These diverse effects can nevertheless be explained in a straightforward way: that is, by allowing normalization signals to be adaptable, and by understanding how an adaptor and subsequent test stimuli engage the CRF and normalization pool.
Adaptation-induced Disinhibition Outside Visual Cortex
We have so far focused on work in visual cortex, but research in other brain areas and sensory systems has revealed similar evidence of adaptation-induced disinhibition. Normalization may be a canonical computation [24], so these effects might also be explained within this framework. Nevertheless, because the concept of normalization has been applied less broadly in other systems, we will refer to these findings in more general terms — as examples of adaptation-induced changes in the balance between excitatory and suppressive signals.
In the retina and lateral geniculate nucleus (LGN), adaptation to a small stimulus that lies within the CRF can lead to a substantial reduction in responsivity [37–39]. Adaptation of suppressive signals that lie outside the CRF usually enhances responses to stimuli that cover the CRF and the surround, just as in visual cortex (Figure 2B) [38,40]. Unlike the effects of adaptation on the visual cortex, however, those in the retina and LGN have limited stimulus specificity [38,40], except for spatial location [38,40,41].
Retinal circuits can be systematically explored in vitro, allowing for detailed investigation of the mechanisms underlying adaptation effects. Recent in vitro work has revealed how adaptation alters excitatory and inhibitory signals in the retina. Adaptation usually reduces the excitatory synaptic input to retinal ganglion cells, in large part by reducing the sensitivity of bipolar cell synapses [13,42,43]. Adaptation can also depress the activity of inhibitory amacrine cells, particularly those that use GABA as a neurotransmitter [3,41,43,44]. As a result, adaptation can reduce the responsivity of some ganglion cells and increase that of others, depending on the complement of excitatory and inhibitory inputs each cell receives. Indeed, recent work suggests there are distinct functional classes of retinal ganglion cells: one whose responses are enhanced by adaptation with high contrast stimuli, and another whose responses are reduced [41,45].
Many recent findings in the whisker somatosensory (‘barrel’) system of rodents also bear a striking resemblance to those in the visual system. Repeated whisker deflections have long been known to reduce neuronal responsivity in barrel cortex (for example [46]), but recently it has been found that this adaptation is also capable of increasing responsivity [47]. This enhanced responsivity has been attributed to inhibition being weakened more than excitation by adaptation ([48], but see [49]). Adaptation also reduces cross-whisker suppression, akin to a reduction in suppressive signals from the surround in visual processing [50]. Finally, adaptation with weak stimuli reduces barrel neuron responsivity more than adaptation with strong stimuli [51], as predicted by the intensity-dependence of normalization signals.
Timescales of Adaptation
The term adaptation traditionally encompasses exposure periods ranging from tens of milliseconds to tens of minutes. Within this range, traditional descriptions suggest that prolonging adaptation will increase the magnitude and duration of effects, but not their qualitative nature — sometimes termed ‘duration scaling’ [52]. Duration scaling is consistent with much previous work: for example, changes in neuronal contrast sensitivity can be induced within 50 ms [53], but are stronger and longer-lasting after more prolonged adaptation [34]. Although duration scaling provides a reasonable first-order description, recent work has shown a more complex and sophisticated relationship between adaptation duration and induced effects.
One violation of duration scaling arises because adaptation effects are shaped by the time course of normalization signals, particularly those from the surround. These signals are often delayed relative to those from within the CRF [54–56]. The effects of brief adaptation can dissipate so rapidly that the CRF may recover before normalization signals arrive. It follows that when adapting stimuli have an impact on normalization signals, brief and prolonged adaptation can generate qualitatively different effects [11,27].
It is not certain that the CRF and normalization signals adapt at different rates, but there is evidence they might. For instance, receptive field size changes with presentation duration [57], suggesting effects on the CRF and surround are induced at different rates. Furthermore, the effects of high-intensity adaptors dissipate more rapidly than those of weak ones [51], perhaps because excitatory and inhibitory inputs recover at different rates [47]. Within the normalization pool, some elements may be more susceptible to adaptation than others [25,31,32]. Differences in induction or recovery rates may provide flexible time scales, the expression of which depends on both adaptation duration and how the adaptor recruits CRF and normalization signals. Because of this interaction, duration scaling is likely to provide a poor description of how effects depend on adaptation duration. However, models that incorporate distinct duration scaling rules for different sources of excitatory and suppressive signals may provide a straightforward explanation for these seemingly complex phenomena.
Duration scaling is associated with the idea that adaptation effects can be explained by a single fatigable mechanism, with a single time course. Recent work has revealed instead that cortical networks, and even individual neurons, can store multiple timescales of adaptation simultaneously [4,58–60]. Perceptual studies have revealed similarly sophisticated behaviours. Adaptation to an oriented pattern induces a robust tilt aftereffect, in which the perceived orientation of a test stimulus is repelled away from the adapted orientation [17]. If the adaptor is presented for four hours, a tilt aftereffect persists for tens of minutes. This aftereffect can be entirely reversed by 15 minutes exposure to natural images; however, this reversal dissipates quickly, and the aftereffect of the initial, prolonged adaptor, reappears [52]. This reappearance strongly suggests the simultaneous storage of the two aftereffects in a common neural substrate, each with a different degree of persistence (see also [61–63]).
In addition to the flexibility that is afforded by storing multiple timescales simultaneously, these timescales may themselves be modifiable. Organisms are sometimes confronted with environments in which statistics change slowly, but at other times the environment changes rapidly and frequently. A sensible strategy may be to yoke the time course of adaptation effects to the temporal constancy of the environment. Consistent with such a strategy, recordings from ganglion cells in mouse retina show that frequent switches between low and high contrast stimuli are associated with faster adaptive changes in response than those that occur with less frequent switches ([64], see also [21,60]). These observations parallel those in motor control, where the timescale of motor adjustment depends on whether errors arise from transient or more persistent disturbances [65].
Finally, we note that the simple fact that adaptation refers to an enormous range of timescales poses an inherent challenge to the duration scaling description. The broad range of timescales implies an ensemble of cellular and circuit mechanisms. For instance, measurements of rapid adaptation can involve exposures of brief stimuli in immediate succession. In these paradigms the adaptor and test may both fall within the integration time window of a neuron (~100 milliseconds; for example [66]). Effects on such brief timescales may thus reflect static properties of temporal integration rather than plastic changes in neural circuitry [67]. At the other extreme, some adaptation effects last for days [61], and these may be better framed as semi-permanent adjustments of cortical circuits, akin to long-term learning. For duration scaling to hold while recruiting such diverse mechanisms, they would all need to have similar consequences on neuronal responses. This seems unlikely.
In summary, recent work has falsified a central tenet of traditional descriptions: adaptation effects do not simply grow with adaptor duration. Rather, effects can be qualitatively different for brief and long periods of adaptation, and may depend on the relative drive provided to the CRF and normalization pool. In addition, multiple timescales of adaptation can be stored simultaneously, and these timescales may be plastic themselves.
Adaptation Effects Across Stages of Visual Processing
Much of the neurophysiological work on adaptation has focused on its effects on single neurons, at distinct stages of processing. Sensory processing, however, reflects the activity of neurons distributed both within and across brain areas. This distributed processing raises a number of critical questions. How does adaptation influence the coordination of population activity within a local network? Do neurons at multiple stages of processing each adapt to a sensory input, or are effects implemented at an early stage and then simply inherited by downstream networks? When effects are inherited, how does this influence the computations performed in the recipient network? In Box 2, we review evidence that adaptation alters population coordination, although findings remain too discrepant to draw conclusions about precisely how. Below we review progress in understanding how adaptation affects responses across stages of the visual hierarchy.
Box 2 Impact of adaptation on population activity.
It is well known that sensory processing requires populations of neurons. The information encoded by a population depends on the tuning of individual neurons, their response variability, and how activity is coordinated among neurons. The structure of pairwise ‘noise’ correlations, in particular, may strongly influence population performance [126–128].
Adaptation has been shown to alter correlations, but there is not yet consensus about its effects. In somatosensory cortex of anaesthetised rat, adaptation increases the magnitude of correlations and response variability, particularly for weak stimuli [46,129]. In anaesthetised cat V1, adaptation has little effect on correlations [99]; in awake monkey V1, adaptation may reduce correlations [130].
Other work has sought to understand how adaptation affects finer-temporal correlation, namely gamma-band activity or the synchronisation of spiking responses [131]. Adaptation increases high-frequency oscillatory activity in the locust olfactory system [132] and the visual cortex of primates [133,134]. Similar effects can be observed in MEG measurements from higher stages of human visual cortex [135]. However, other studies have found that adaptation reduces the synchronisation of spiking activity [136,137], and power in gamma and higher frequencies of the local field potential [75,77,138].
Some of the inconsistency across studies may reflect differences between species or sensory areas, or other experimental details. However, a tantalizing possibility is that the inconsistencies may be explained in part by variations in the recruitment of normalization signals. The magnitude of correlations depends strongly on network state and likely on the balance between excitation and inhibition [139]. Similarly high-frequency fluctuations like gamma are thought to reflect the temporal interaction of excitatory-inhibitory circuits, and are stronger for stimulus configurations that recruit normalization signals [138,140]. In this regard, it is worth noting that visual studies have measured adaptation effects on correlations with strong stimuli [99,130], whereas the increase in correlations in the barrel system is observed for weaker stimuli [46]. Thus, it may be important to characterise adaptation effects across a range of stimulus intensities [46], and to consider the behaviour of normalization signals at each intensity.
A final way in which adaptation may alter population coding is by affecting how those responses are ‘read out’ by downstream areas. In perceptual learning, another form of experience-based plasticity, there is some evidence that behavioural effects involve improved read out [141,142]. To date, adaptation work suggests little change in how adapted responses are interpreted by downstream networks, both because effects cascade through early stages of the visual system and because robust aftereffects suggest an inability of higher cortex to fully correct for perturbed sensory representations. Nevertheless, read out may be adjusted when an adaptation state is experienced repeatedly [143]. In addition, when adaptation weakens inhibition, it may alter the temporal window in which feedforward signals are summed by downstream networks [136,144], altering their sensitivity to coordinated input.
Neurons at nearly all stages of visual processing are affected by adaptation. In addition to the work in the retina, LGN, and V1 discussed above, recent studies have documented effects in higher areas such as V2 [33], V4 [68], MT [30,69–73], IT [74–77], and FEF [78], among others [16,20]. Are the effects observed in higher stages generated locally or inherited from earlier networks? One approach to answering this question is to record from earlier stages, and see whether adaptation effects there are similar in nature or magnitude. These comparisons can be surprisingly thorny, however; for example, adaptation-induced changes in contrast sensitivity were thought to arise in V1, because there was little evidence of altered sensitivity in the LGN [34,79]. Later studies revealed that adaptation can in fact change contrast sensitivity in many retinal and LGN neurons [38,40,80–82]. This discrepancy may be due to the existence of several pathways from retina to cortex, which are differently susceptible to adaptation [38,41].
Comparisons across areas are also hindered by the use of stimuli tailored to the preferences of individual neurons, which typically differ across stages of the hierarchy. This precludes a direct comparison of how different stages adapt to a particular stimulus. For example, previous work suggested that adaptation caused attractive shifts in MT direction tuning [29], but not in V1 [8–11]. Rather than reflecting a difference across areas, these findings can be explained by the use of large stimuli in MT (tailored to the large receptive fields of neurons there), and small stimuli in V1 (where receptive fields are smaller). Adaptation-induced shifts in tuning are in fact similar in V1 and MT, when measured with stimuli matched in size [30].
An alternative approach to testing for inheritance is to measure the spatial specificity of adaptation effects. The spatial size of receptive fields increases along the visual hierarchy. Thus, if an adapter confined to one sub-region of a receptive field does not influence responses to stimuli presented to another sub-region, this suggests that effects are induced at an earlier stage, where receptive fields are smaller. This approach has provided evidence for inherited adaptation effects in the retina ([37], but see [67]), and for some [71,83] but not all effects in MT [84].
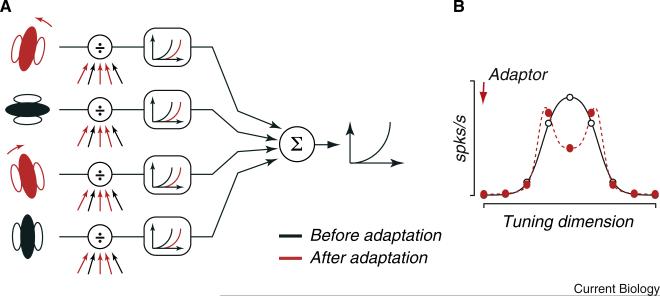
Understanding the relative contribution of inherited and locally-generated effects only begins to address the issue of cascading adaptation. Altering inputs to a network can generate a range of effects, which depend on the interaction between the pattern of adapted inputs and local recurrent circuitry [29,85]. The effects also depend on how adapted inputs are combined: in general, downstream networks are thought to produce new representations by precisely combining inputs from earlier areas and imposing non-linearities (Figure 3A; for example, [86,87]). When adaptation alters the pattern of inputs, it may disrupt the formation of new representations, unless there is appropriate compensation in the downstream network.
Figure 3. Cascading of adaptation in sensory pathways.
(A) Schematic of a generic, hierarchical sensory pathway. The outputs of neurons at earlier stages of processing are weighted and summed, and transformed into spiking activity. The higher order neuron is unaware of adaptation effects earlier in the pathway (red symbols). (B) Neural tuning of the higher-order neuron (open symbols), conferred by the weights it applied to neurons at earlier stages. Absent compensation for adaptation effects at earlier stages, tuning may be distorted by adaptation (red filled symbols), derailing the computations the higher-order neurons perform. Redrawn from [73].
Two recent studies [39,73] suggest that circuits do not compensate for inputs altered by adaptation. First, the deformation of spatial receptive fields in mouse V1 can be explained by weakened inputs from the LGN and unaltered pooling of these inputs by V1 neurons [39]. Second, adaptation disrupts a form of motion selectivity observed in primate MT, and this can be explained by assuming that adaptation desensitizes some inputs from V1, but does not change how MT neurons combine those inputs (Figure 3B) [73].
While these observations provide evidence for limited compensation in the targets of adapted neurons, recent work also offers a counter-example. McClelland et al. [88] showed that LGN neurons display robust responses at the offset of a prolonged presentation of a static stimulus. In V1, this after-response is much smaller in magnitude and shorter in duration [89]. This suggests that there are mechanisms capable of compensating for the adaptation state of inputs, so that downstream networks need not slavishly follow altered inputs.
In parallel with this neurophysiological work, perceptual experiments have also provided evidence that many adaptation effects cascade. Most generally, the existence of maladaptive aftereffects — the tilt or motion aftereffects [17,90] — suggests that higher sensory representations cannot easily divine the adaptation state of earlier representations. Perceptual work has also shown that adaptation to a simple visual feature can disrupt the representation of more complex stimuli, perhaps because effects in early stages derail downstream computations. For instance, adaptation to line curvature — which presumably affects the early visual system — can alter the perception of global form [91] and the inferred emotion of cartoon faces [92,93]. Similarly, adaptation to low-level features such as luminance and contrast can give rise to percepts of illusory motion [94]. Indeed, many aftereffects of motion adaptation can be explained by a model in which adapted non-directional units cascade onto directional units [95].
In summary, we now understand that adaptation effects cascade downstream, and can disrupt computations performed at later stages of processing. These findings raise challenges for understanding the function of adaptation-induced plasticity and for making appropriate inferences about sensory processing in imaging and perceptual studies. Yet many of these observations may be explained by relatively simple models in which downstream networks are ‘unaware’ of adaptation-induced changes in their inputs [96].
Functions of Adaptation
It is incongruous that we have learnt so much about the impact of adaptation on sensory systems, but still know little of the purpose of these effects. It is widely believed that adaptation effects are beneficial, but for many effects it is not clear how. An answer is critical for understanding adaptation, but it may also shed light on key questions in sensory processing. Knowledge of how sensory systems adjust to different environments seems fundamental to understanding the strategies of sensory processing and the tradeoffs they entail. The recent empirical progress reviewed above raises questions for some existing proposals (of the many put forth [14–20]), and offers new possibilities.
One long-standing proposal is that adaptation sharpens acuity (discriminability) either around the adaptor or for offset stimuli. This proposal has been contentious, and perceptual evidence for improved acuity has been difficult to obtain [16,20]. Measurements in visual cortex suggest that adaptation can improve the acuity of single neurons [8,9], but it is not yet clear how this translates to population performance (Box 2).
A second hypothesis is that adaptation reduces the redundancy of sensory representations. An efficient neural representation should utilise the full range of activity patterns that it can produce. When some stimuli are more common than others, a subset of possible activity patterns is overrep-resented, and the encoding capacity is underutilised. Barlow [23] suggested that stimulus-specific fatigue alters neuronal tuning so that the full range of a network's activity patterns are used to encode the environment. It is important to note that this strategy is optimal only under the assumption that sensory inputs are noiseless, and that noise in the system is the same before and after adaptation; more realistic assumptions of noise give rise to distinct adaptation strategies [97,98].
A recent study in cat visual cortex offers initial experimental support for the Barlow proposal [99]. Population responses to rapid sequences of oriented patterns were measured; the distribution of orientations over time was either uniform, or biased such that one orientation was more common than the others. The efficient coding hypothesis predicts that neuronal tuning should adjust to reduce the response correlations caused by some stimuli being more common than others, and this is the case for a range of stimulus biases.
Achieving an efficient representation is unlikely to be the sole goal of adaptation. As discussed above, when adaptation reduces normalization signals it can enhance responsivity and attract tuning curves towards the adaptor. This should preserve or enhance the representation of an adapter and stimuli like it, opposite to the effects that motivated Barlow's proposal. What potential alternative functions are suggested by considering adaptable normalization signals? One possibility is that the weakening of surround normalization signals by adaptation enhances spatial integration [50]. Another is that pathways with ‘sensitizing’ adaptation effects — due, for instance, to weakened inhibition — allow the system to ‘hedge its bets’ [45,51,100]. Reducing neuronal responsivity may be a good strategy in the face of strong inputs, but it leaves the system vulnerable: if the environment suddenly changes, weak signals will be undetected. By having some neurons that sensitize and others that fatigue, the system can function in both environments.
A final, intriguing possibility is that adaptation modulates stimulus salience. This possibility arises in part from the effects of adaptation on normalization. Normalization signals from the surround are thought to be important for salience [101,102]. Because adaptation can weaken these signals, the salience of objects will depend on their temporal context. Consistent with this suggestion, psychophysical studies have shown that adaptation improves performance in visual search tasks, by modulating the salience of objects [103,104]. Recent physiological studies have also provided intriguing examples of adaptation influencing stimulus salience, by highlighting stimuli that are novel. Retinal ganglion cells, for instance, generate strong responses when an expected stimulus is omitted from an established sequence [105,106]. Direction-selective neurons in fly show sensitized responses when peripheral regions of the CRF experience motion opposite to that recently encountered [107]. Finally, in primate superior colliculus, weak stimuli have been shown to generate surprisingly strong responses when these stimuli are unexpected [108].
These findings are consistent with the presence of mechanisms that boost responses to unexpected events. Novel events are also naturally highlighted by fatigue-related adaptation effects: stimulus-specific fatigue reduces responses to unchanging features of the environment, emphasizing novel stimuli [109]. Together these processes can be thought of as an alternative form of redundancy reduction, or of predictive coding: persistent or recurring inputs are discounted to highlight new ones. In audition, a role in novelty detection has been ascribed to both fatigue-based and more active mechanisms. Specifically, the auditory evoked potential is larger when a sound is presented rarely, or embedded in a sequence of different sounds, than when it is presented frequently — an enhanced response called the ‘mismatch negativity’ [110]. Many instantiations of the mismatch negativity can be explained by stimulus-specific fatigue [111,112], but other work suggests the mismatch negativity reflects a more sophisticated predictive scheme [113–115]. At the perceptual level, unexpected stimuli that are associated with a mismatch negativity are also detected more easily [115,116].
Lastly, modelling work suggests that, in fatigue-based predictive coding, persistent stimuli can be ‘explained away’ using strengthened normalization [23,117,118]. While there is limited empirical support for stimulus-specific fatigue arising from strengthened normalization at a mechanistic level [119,120], the impact on neuronal tuning might indeed be captured by the normalization framework we have proposed for understanding disinhibitory effects of adaptation. This raises the possibility that stimulus-specific fatigue (strengthened normalization) and sensitization (weakened normalization) may reflect complementary strategies, the former emphasizing ‘explaining away’ and the latter emphasizing ‘predicting’ [41].
Conclusions
Our understanding of sensory adaptation has been greatly enriched by work over the last decade. We have learned that adaptation has an effect on both excitatory and suppressive signals, and its effects do not simply grow with adaptation duration. Furthermore, adaptation alters population coordination and its effects cascade through the stages of processing, influencing downstream networks in sometimes unexpected ways.
Adaptation effects are thus substantially more complex than suggested by traditional fatigue-based descriptions. Fortunately, much of this complexity may be explained by simple models of brain circuits that incorporate a normalization framework, and invoke fixed integration by downstream networks. In any case, it is now clear that understanding adaptation effects will require them to be interpreted in the context of modern functional models of sensory processing, rather than as occurring in isolated individual neurons.
This new knowledge means that existing experimental approaches may need to be re-evaluated. For instance, perceptual and human brain imaging work often assume that repeated presentations of a stimulus reduce responsivity in the relevant neurons [121]. The cascading of adaptation effects, the dependence of those effects on adaptation duration, and the possibility of facilitation due to weakened normalization signals all raise significant concerns about inferences based on this assumption.
The empirical progress we have reviewed also calls for more theoretical work. We need theoretical frameworks that explain the impact of adaptation on population responses including normalization signals, consider how these effects influence downstream processing, and generate predictions for how adaptation can improve performance. We must keep in mind that the functional benefit of a representational change at one stage may be offset by the disruption that it imposes on subsequent processing.
These new observations also offer exciting directions for future work. First, the interaction between normalization and adaptation suggests a role in modulating salience and in predictive coding. Second, the normalization framework of adaptation may also offer a way to explore its role in other cortical functions. This is because normalization underlies a broad range of computations [122], and has been invoked to explain aspects of cognition like attention [123,124] and decision-making [125]. Finally, determining how adaptation affects population representations — distributed both within and across stages of sensory processing — is likely to offer powerful tools for dissecting the functional architecture of sensory processing.
Acknowledgements
We thank N. Dhruv, C. Henry, J. Larsson, I. Mareschal and O. Schwartz for comments on previous versions of this review. This work was supported by project grants from the Australian National Health and Medical Research Council (S.G.S), the Marie-Curie scheme from the European Commission (S.G.S), and the NIH (EY016774 and EY021371, A.K.).
References
- 1.Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J. Neurosci. 2000;20:4267–4285. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 4.Pozzorini C, Naud R, Mensi S, Gerstner W. Temporal whitening by power-law adaptation in neocortical neurons. Nat. Neurosci. 2013;16:942–948. doi: 10.1038/nn.3431. [DOI] [PubMed] [Google Scholar]
- 5.Descalzo VF, Nowak LG, Brumberg JC, McCormick DA, Sanchez-Vives MV. Slow adaptation in fast-spiking neurons of visual cortex. J. Neurophysiol. 2005;93:1111–1118. doi: 10.1152/jn.00658.2004. [DOI] [PubMed] [Google Scholar]
- 6.Harris RA, O'Carroll DC, Laughlin SB. Contrast gain reduction in fly motion adaptation. Neuron. 2000;28:595–606. doi: 10.1016/s0896-6273(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 7.Priebe NJ, Lampl I, Ferster D. Mechanisms of direction selectivity in cat primary visual cortex as revealed by visual adaptation. J. Neurophysiol. 2010;104:2615–2623. doi: 10.1152/jn.00241.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- 9.Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat. Neurosci. 2002;5:883–891. doi: 10.1038/nn900. [DOI] [PubMed] [Google Scholar]
- 10.Felsen G, Shen YS, Yao H, Spor G, Li C, Dan Y. Dynamic modification of cortical orientation tuning mediated by recurrent connections. Neuron. 2002;36:945–954. doi: 10.1016/s0896-6273(02)01011-5. [DOI] [PubMed] [Google Scholar]
- 11.Patterson CA, Wissig SC, Kohn A. Distinct effects of brief and prolonged adaptation on orientation tuning in primary visual cortex. J. Neurosci. 2013;33:532–543. doi: 10.1523/JNEUROSCI.3345-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 13.Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Clifford CWG, Rhodes G. Fitting the Mind to the World. Oxford University Press; Oxford: 2005. [Google Scholar]
- 15.Clifford CW, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O. Visual adaptation: neural, psychological and computational aspects. Vision Res. 2007;47:3125–3131. doi: 10.1016/j.visres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J. Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nat. Rev. Neurosci. 2007;8:522–535. doi: 10.1038/nrn2155. [DOI] [PubMed] [Google Scholar]
- 18.Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr. Opin. Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieke F, Rudd ME. The challenges natural images pose for visual adaptation. Neuron. 2009;64:605–616. doi: 10.1016/j.neuron.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Webster MA. Adaptation and visual coding. J. Vis. 2011;11:3. doi: 10.1167/11.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairhall AL, Lewen GD, Bialek W, de Ruyter Van Steveninck RR. Efficiency and ambiguity in an adaptive neural code. Nature. 2001;412:787–792. doi: 10.1038/35090500. [DOI] [PubMed] [Google Scholar]
- 22.Borst A, Flanagin VL, Sompolinsky H. Adaptation without parameter change: Dynamic gain control in motion detection. Proc. Natl. Acad. Sci. USA. 2005;102:6172–6176. doi: 10.1073/pnas.0500491102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlow HB. A theory about the functional role and synaptic mechanisms of visual after-effects. In: Blakemore C, editor. Vision: Coding and Efficiency. Cambridge University Press; New York: 1990. pp. 363–375. [Google Scholar]
- 24.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2011;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. J. Neurosci. 2005;25:11666–11675. doi: 10.1523/JNEUROSCI.3414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wissig SC, Kohn A. The influence of surround suppression on adaptation effects in primary visual cortex. J. Neurophysiol. 2012;107:3370–3384. doi: 10.1152/jn.00739.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghisovan N, Nemri A, Shumikhina S, Molotchnikoff S. Long adaptation reveals mostly attractive shifts of orientation tuning in cat primary visual cortex. Neuroscience. 2009;164:1274–1283. doi: 10.1016/j.neuroscience.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Tailby C, Solomon SG, Dhruv NT, Lennie P. Habituation reveals fundamental chromatic mechanisms in striate cortex of macaque. J. Neurosci. 2008;28:1131–1139. doi: 10.1523/JNEUROSCI.4682-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat. Neurosci. 2004;7:764–772. doi: 10.1038/nn1267. [DOI] [PubMed] [Google Scholar]
- 30.Patterson CA, Duijnhouwer J, Wissig SC, Krekelberg B, Kohn A. Similar adaptation effects in primary visual cortex and area MT of the macaque monkey under matched stimulus conditions. J. Neurophysiol. 2014;111:1203–1213. doi: 10.1152/jn.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhruv NT, Tailby C, Sokol SH, Lennie P. Multiple adaptable mechanisms early in the primate visual pathway. J. Neurosci. 2011;31:15016–15025. doi: 10.1523/JNEUROSCI.0890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman TC, Durand S, Kiper DC, Carandini M. Suppression without inhibition in visual cortex. Neuron. 2002;35:759–771. doi: 10.1016/s0896-6273(02)00819-x. [DOI] [PubMed] [Google Scholar]
- 33.Crowder NA, Price NS, Hietanen MA, Dreher B, Clifford CW, Ibbotson MR. Relationship between contrast adaptation and orientation tuning in V1 and V2 of cat visual cortex. J. Neurophysiol. 2006;95:271–283. doi: 10.1152/jn.00871.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat's visual system. J. Neurophysiol. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- 35.Bex P, Solomon SG, Dakin SC. Contrast sensitivity in natural scenes depends on edge as well as spatial frequency structure. J. Vis. 2009;9:1.1–1.19. doi: 10.1167/9.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfson SS, Graham N. Two contrast adaptation processes: contrast normalization and shifting, rectifying contrast comparison. J. Vis. 2009;9:30.1–30.23. doi: 10.1167/9.4.30. [DOI] [PubMed] [Google Scholar]
- 37.Brown SP, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat. Neurosci. 2001;4:44–51. doi: 10.1038/82888. [DOI] [PubMed] [Google Scholar]
- 38.Camp AJ, Tailby C, Solomon SG. Adaptable mechanisms that regulate the contrast response of neurons in the primate lateral geniculate nucleus. J. Neurosci. 2009;29:5009–5021. doi: 10.1523/JNEUROSCI.0219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhruv NT, Carandini M. Cascaded effects of spatial adaptation in the early visual system. Neuron. 2014;81:529–535. doi: 10.1016/j.neuron.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron. 2004;42:155–162. doi: 10.1016/s0896-6273(04)00178-3. [DOI] [PubMed] [Google Scholar]
- 41.Kastner DB, Baccus SA. Spatial segregation of adaptation and predictive sensitization in retinal ganglion cells. Neuron. 2013;79:541–554. doi: 10.1016/j.neuron.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, Singer JH. A synaptic mechanism for retinal adaptation to luminance and contrast. J. Neurosci. 2011;31:11003–11015. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Marco SD, Protti DA, Solomon SG. Excitatory and inhibitory contributions to receptive fields of alpha-like retinal ganglion cells in mouse. J. Neurophysiol. 2013;110:1426–1440. doi: 10.1152/jn.01097.2012. [DOI] [PubMed] [Google Scholar]
- 44.Nikolaev A, Leung KM, Odermatt B, Lagnado L. Synaptic mechanisms of adaptation and sensitization in the retina. Nat. Neurosci. 2013;16:934–941. doi: 10.1038/nn.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kastner DB, Baccus SA. Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat. Neurosci. 2011;14:1317–1322. doi: 10.1038/nn.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adibi M, McDonald JS, Clifford CW, Arabzadeh E. Adaptation improves neural coding efficiency despite increasing correlations in variability. J. Neurosci. 2013;33:2108–2120. doi: 10.1523/JNEUROSCI.3449-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen-Kashi Malina K, Jubran M, Katz Y, Lampl I. Imbalance between excitation and inhibition in the somatosensory cortex produces postadaptation facilitation. J. Neurosci. 2013;33:8463–8471. doi: 10.1523/JNEUROSCI.4845-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heiss JE, Katz Y, Ganmor E, Lampl I. Shift in the balance between excitation and inhibition during sensory adaptation of S1 neurons. J. Neurosci. 2008;28:13320–13330. doi: 10.1523/JNEUROSCI.2646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higley MJ, Contreras D. Frequency adaptation modulates spatial integration of sensory responses in the rat whisker system. J. Neurophysiol. 2007;97:3819–3824. doi: 10.1152/jn.00098.2007. [DOI] [PubMed] [Google Scholar]
- 51.Ganmor E, Katz Y, Lampl I. Intensity-dependent adaptation of cortical and thalamic neurons is controlled by brainstem circuits of the sensory pathway. Neuron. 2010;66:273–286. doi: 10.1016/j.neuron.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Bao M, Engel SA. Distinct mechanism for long-term contrast adaptation. Proc. Natl. Acad. Sci. USA. 2012;109:5898–5903. doi: 10.1073/pnas.1113503109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonds AB. Temporal dynamics of contrast gain in single cells of the striate cortex. Visual Neurosci. 1991;6:239–255. doi: 10.1017/s0952523800006258. [DOI] [PubMed] [Google Scholar]
- 54.Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J. Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MA, Bair W, Movshon JA. Dynamics of suppression in macaque primary visual cortex. J. Neurosci. 2006;26:4826–4834. doi: 10.1523/JNEUROSCI.5542-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry CA, Joshi S, Xing D, Shapley RM, Hawken MJ. Functional characterization of the extraclassical receptive field in macaque V1: contrast, orientation, and temporal dynamics. J. Neurosci. 2013;33:6230–6242. doi: 10.1523/JNEUROSCI.4155-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J. Neurophysiol. 2002;88:2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- 58.Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. J. Neurosci. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drew PJ, Abbott LF. Models and properties of power-law adaptation in neural systems. J. Neurophysiol. 2006;96:826–833. doi: 10.1152/jn.00134.2006. [DOI] [PubMed] [Google Scholar]
- 60.Lundstrom BN, Higgs MH, Spain WJ, Fairhall AL. Fractional differentiation by neocortical pyramidal neurons. Nat. Neurosci. 2008;11:1335–1342. doi: 10.1038/nn.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vul E, Krizay E, MacLeod DI. The McCollough effect reflects permanent and transient adaptation in early visual cortex. J. Vis. 2008;8:4.1–4.12. doi: 10.1167/8.12.4. [DOI] [PubMed] [Google Scholar]
- 62.Mesik J, Bao M, Engel SA. Spontaneous recovery of motion and face aftereffects. Vision Res. 2013;89:72–78. doi: 10.1016/j.visres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Chopin A, Mamassian P. Predictive properties of visual adaptation. Curr. Biol. 2012;22:622–626. doi: 10.1016/j.cub.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Wark B, Fairhall A, Rieke F. Timescales of inference in visual adaptation. Neuron. 2009;61:750–761. doi: 10.1016/j.neuron.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat. Neurosci. 2007;10:779–786. doi: 10.1038/nn1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bair W, Movshon JA. Adaptive temporal integration of motion in direction-selective neurons in macaque visual cortex. J. Neurosci. 2004;24:7305–7323. doi: 10.1523/JNEUROSCI.0554-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garvert MM, Gollisch T. Local and global contrast adaptation in retinal ganglion cells. Neuron. 2013;77:915–928. doi: 10.1016/j.neuron.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 68.Tolias AS, Keliris GA, Smirnakis SM, Logothetis NK. Neurons in macaque area V4 acquire directional tuning after adaptation to motion stimuli. Nat. Neurosci. 2005;8:591–593. doi: 10.1038/nn1446. [DOI] [PubMed] [Google Scholar]
- 69.Schlack A, Krekelberg B, Albright TD. Recent history of stimulus speeds affects the speed tuning of neurons in area MT. J. Neurosci. 2007;27:11009–11018. doi: 10.1523/JNEUROSCI.3165-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Lisberger SG. Relationship between adapted neural population responses in MT and motion adaptation in speed and direction of smooth-pursuit eye movements. J. Neurophysiol. 2009;101:2693–2707. doi: 10.1152/jn.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasser DM, Tsui JM, Pack CC, Tadin D. Perceptual and neural consequences of rapid motion adaptation. Proc. Natl. Acad. Sci. USA. 2011;108:1080–1088. doi: 10.1073/pnas.1101141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Price NS, Born RT. Adaptation to speed in macaque middle temporal and medial superior temporal areas. J. Neurosci. 2013;33:4359–4368. doi: 10.1523/JNEUROSCI.3165-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patterson CA, Wissig SC, Kohn A. Adaptation disrupts motion integration in the primate dorsal stream. Neuron. 2014;81:674–686. doi: 10.1016/j.neuron.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Murray SO, Jagadeesh B. Time course and stimulus dependence of repetition-inducd response suppression in inferotemporal cortex. J. Neurophysiol. 2009;101:418–436. doi: 10.1152/jn.90960.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaliukhovich DA, Vogels R. Stimulus repetition affects both strength and synchrony of macaque inferior temporal cortical activity. J. Neurophysiol. 2012;107:3509–3527. doi: 10.1152/jn.00059.2012. [DOI] [PubMed] [Google Scholar]
- 76.Verhoef BE, Kayaert G, Franko E, Vangeneugden J, Vogels R. Stimulus similarity-contingent neural adaptation can be time and cortical area dependent. J. Neurosci. 2008;28:10631–10640. doi: 10.1523/JNEUROSCI.3333-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cerebral Cortex. 2010;20:2145–2165. doi: 10.1093/cercor/bhp277. [DOI] [PubMed] [Google Scholar]
- 78.Mayo JP, Sommer MA. Neuronal adaptation caused by sequential visual stimulation in the frontal eye field. J. Neurophysiol. 2008;100:1923–1935. doi: 10.1152/jn.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;278:850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- 80.Demb JB. Functional circuitry of visual adaptation in the retina. J. Physiol. 2008;586:4377–4384. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J. Neurosci. 2008;28:4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shou T, Li X, Zhou Y, Hu B. Adaptation of visually evoked responses of relay cells in the dorsal lateral geniculate nucleus of the cat following prolonged exposure to drifting gratings. Vis. Neurosci. 1996;13:605–613. doi: 10.1017/s0952523800008518. [DOI] [PubMed] [Google Scholar]
- 83.Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron. 2003;39:681–691. doi: 10.1016/s0896-6273(03)00438-0. [DOI] [PubMed] [Google Scholar]
- 84.Priebe NJ, Churchland MM, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. I. the role of input and intrinsic mechanisms. J. Neurophysiol. 2002;88:354–369. doi: 10.1152/.00852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Compte A, Wang XJ. Tuning curve shift by attention modulation in cortical neurons: a computational study of its mechanisms. Cerebral Cortex. 2006;16:761–778. doi: 10.1093/cercor/bhj021. [DOI] [PubMed] [Google Scholar]
- 86.Rust NC, Mante V, Simoncelli EP, Movshon JA. How MT cells analyze the motion of visual patterns. Nat. Neurosci. 2006;9:1421–1431. doi: 10.1038/nn1786. [DOI] [PubMed] [Google Scholar]
- 87.Cadieu C, Kouh M, Pasupathy A, Connor CE, Riesenhuber M, Poggio T. A model of V4 shape selectivity and invariance. J. Neurophysiol. 2007;98:1733–1750. doi: 10.1152/jn.01265.2006. [DOI] [PubMed] [Google Scholar]
- 88.McLelland D, Ahmed B, Bair W. Responses to static visual images in macaque lateral geniculate nucleus: implications for adaptation, negative afterimages, and visual fading. J. Neurosci. 2009;29:8996–9001. doi: 10.1523/JNEUROSCI.0467-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McLelland D, Baker PM, Ahmed B, Bair W. Neuronal responses during and after the presentation of static visual stimuli in macaque primary visual cortex. J. Neurosci. 2010;30:12619–12631. doi: 10.1523/JNEUROSCI.0815-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mather G, Verstraten F, Anstis S. The Motion Aftereffect: a Modern Perspective. MIT Press; Cambridge, MA: 1998. [DOI] [PubMed] [Google Scholar]
- 91.Dickinson JE, Almeida RA, Bell J, Badcock DR. Global shape aftereffects have a local substrate: a tilt aftereffect field. J. Vis. 2010;10:5.1–5.12. doi: 10.1167/10.13.5. [DOI] [PubMed] [Google Scholar]
- 92.Xu H, Dayan P, Lipkin RM, Qian N. Adaptation across the cortical hierarchy: low-level curve adaptation affects high-level facial-expression judgments. J. Neurosci. 2008;28:3374–3383. doi: 10.1523/JNEUROSCI.0182-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu H, Liu P, Dayan P, Qian N. Multi-level visual adaptation: dissociating curvature and facial-expression aftereffects produced by the same adapting stimuli. Vision Res. 2012;72:42–53. doi: 10.1016/j.visres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Backus BT, Oruç I. Illusory motion from change over time in the response to contrast and luminance. J. Vis. 2005;5:1055–1069. doi: 10.1167/5.11.10. [DOI] [PubMed] [Google Scholar]
- 95.Stocker AA, Simoncelli EP. Visual motion aftereffects arise from a cascade of two isomorphic adaptation mechanisms. J. Vis. 2009;9:9.1–9.14. doi: 10.1167/9.9.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seriès P, Stocker AA, Simoncelli EP. Is the homunculus “aware” of sensory adaptation? Neural Comput. 2009;21:3271–3304. doi: 10.1162/neco.2009.09-08-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wainwright MJ. Visual adaptation as optimal information transmission. Vision Res. 1999;39:3960–3974. doi: 10.1016/s0042-6989(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 98.Dayan P, Sahani M, Deback G. Adaptation and unsupervised learning. In: Becker S, Thrun S, Obermayer K, editors. Advances in Neural Information Processing Systems. Vol. 15. MIT Press; Cambridge MA: 2002. pp. 237–244. [Google Scholar]
- 99.Benucci A, Saleem AB, Carandini M. Adaptation maintains population homeostasis in primary visual cortex. Nat. Neurosci. 2013;16:724–729. doi: 10.1038/nn.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohar B, Katz Y, Lampl I. Opposite adaptive processing of stimulus intensity in two major nuclei of the somatosensory brainstem. J. Neurosci. 2013;33:15394–15400. doi: 10.1523/JNEUROSCI.1886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z. Contextual influences in V1 as basis for pop out and asymmetry in visual search. Proc. Natl. Acad. Sci. USA. 1999;96:10530–10535. doi: 10.1073/pnas.96.18.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res. 2000;40:1489–1506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 103.McDermott KC, Malkoc G, Mulligan JB, Webster MA. Adaptation and visual salience. J. Vis. 2010;10:17.1–17.32. doi: 10.1167/10.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wissig SC, Patterson CA, Kohn A. Adaptation improves performance on a visual search task. J. Vis. 2013;13:6.1–6.15. doi: 10.1167/13.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz G, Harris R, Shrom D, Berry MJ. Detection and prediction of periodic patterns by the retina. Nat. Neurosci. 2007;10:552–554. doi: 10.1038/nn1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwartz G, Berry MJ. Sophisticated temporal pattern recognition in retinal ganglion cells. J. Neurophysiol. 2008;99:1787–1798. doi: 10.1152/jn.01025.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neri P, Laughlin SB. Global versus local adaptation in fly motion-sensitive neurons. Proc. Biol. Sci. 2005;272:2243–2249. doi: 10.1098/rspb.2005.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boehnke SE, Berg DJ, Marino RA, Baldi PF, Itti L, Munoz DP. Visual adaptation and novelty response in the superior colliculus. Eur. J. Neurosci. 2011;34:766–779. doi: 10.1111/j.1460-9568.2011.07805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- 110.Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends Neurosci. 2001;24:283–288. doi: 10.1016/s0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- 111.Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat. Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- 112.Fishman YI, Steinschneider M. Searching for the mismatch negativity in primary auditory cortex of the awake monkey: deviance detection or stimulus specific adaptation? J. Neurosci. 2012;32:15747–15758. doi: 10.1523/JNEUROSCI.2835-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sussman ES, Chen S, Sussman-Fort J, Dinces E. The five myths of MMN: Redefining how to use MMN in basic and clinical research. Brain Topogr. 2013;27:553–564. doi: 10.1007/s10548-013-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yaron A, Hershenhoren I, Nelken I. Sensitivity to complex statistical regularities in rat auditory cortex. Neuron. 2012;76:603–615. doi: 10.1016/j.neuron.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 115.Garrido MI, Sahani M, Dolan RJ. Outlier responses reflect sensitivity to statistical structure in the human brain. PLoS. Comput. Biol. 2013;9:e1002999. doi: 10.1371/journal.pcbi.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tiitinen H, May P, Reinikainen K, Näätänen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- 117.Lochmann T, Ernst UA, Denève S. Perceptual inference predicts contextual modulations of sensory responses. J. Neurosci. 2012;32:4179–4195. doi: 10.1523/JNEUROSCI.0817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wainwright MJ, Schwartz O, Simoncelli EP. Natural image statistics and divisive normalization: Modeling nonlinearity and adaptation in cortical neurons. In: Rao R, Olshausen B, Lewicki M, editors. Probabilistic Models of the Brain: Perception and Neural Function. MIT Press; Cambridge, MA: 2002. pp. 203–222. [Google Scholar]
- 119.Carandini M, Movshon JA, Ferster D. Pattern adaptation and cross-orientation interactions in the primary visual cortex. Neuropharmacology. 1998;37:501–511. doi: 10.1016/s0028-3908(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 120.Stange A, Myoga MH, Lingner A, Ford MC, Alexandrova O, Felmy F, Pecka M, Siveke I, Grothe B. Adaptation in sound localization: from GABA(B) receptor-mediated synaptic modulation to perception. Nat. Neurosci. 2013;16:1840–1847. doi: 10.1038/nn.3548. [DOI] [PubMed] [Google Scholar]
- 121.Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 122.Beck JM, Latham PE, Pouget A. Marginalization in neural circuits with divisive normalization. J. Neurosci. 2011;31:15310–15319. doi: 10.1523/JNEUROSCI.1706-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee J, Maunsell JH. A normalization model of attentional modulation of single unit responses. PLoS One. 2009;4:e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Louie K, Khaw MW, Glimcher PW. Normalization is a general neural mechanism for context-dependent decision making. Proc. Natl. Acad. Sci. USA. 2013;110:6139–6144. doi: 10.1073/pnas.1217854110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat. Rev. Neurosci. 2006;7:358–366. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- 127.Cortes JM, Marinazzo D, Series P, Oram MW, Sejnowski TJ, van Rossum MC. The effect of neural adaptation on population coding accuracy. J Comput. Neurosci. 2012;32:387–402. doi: 10.1007/s10827-011-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeanne JM, Sharpee TO, Gentner TQ. Associative learning enhances population coding by inverting interneuronal correlation patterns. Neuron. 2013;78:352–363. doi: 10.1016/j.neuron.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adibi M, Clifford CW, Arabzadeh E. Informational basis of sensory adaptation: entropy and single-spike efficiency in rat barrel cortex. J. Neurosci. 2013;33:14921–14926. doi: 10.1523/JNEUROSCI.1313-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gutnisky DA, Dragoi V. Adaptive coding of visual information in neural populations. Nature. 2008;452:220–224. doi: 10.1038/nature06563. [DOI] [PubMed] [Google Scholar]
- 131.Gotts SJ, Chow CC, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn. Neurosci. 2012;3:227–237. doi: 10.1080/17588928.2012.670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G. Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron. 2005;46:483–492. doi: 10.1016/j.neuron.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hansen BJ, Dragoi V. Adaptation-induced synchronisation in laminar cortical cicuits. Proc. Natl. Acad. Sci. USA. 2011;108:10720–10725. doi: 10.1073/pnas.1102017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brunet NM, Bosman CA, Vinck M, Roberts M, Oostenveld R, Desimone R, De Weerd P, Fries P. Stimulus repetition modulates gamma-band synchronisation in primate visual cortex. Proc. Natl. Acad. Sci. USA. 2014;111:3626–3631. doi: 10.1073/pnas.1309714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gilbert JR, Gotts SJ, Carver FW, Martin A. Object repetition leads to local increases in the temporal coordination of neural responses. Front. Human Neurosci. 2010;4:30. doi: 10.3389/fnhum.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Q, Webber RM, Stanley GB. Thalamic synchrony and the adaptive gating of information flow to cortex. Nat. Neurosci. 2010;13:1534–1541. doi: 10.1038/nn.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Temereanca S, Brown EN, Simons DJ. Rapid changes in thalamic firing synchrony during repetitive whisker stimulation. J. Neurosci. 2008;28:11153–11164. doi: 10.1523/JNEUROSCI.1586-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jia X, Smith MA, Kohn A. Stimulus selectivity and spatial coherence of gamma components of the local field potential. J. Neuroscience. 2011;31:9390–9403. doi: 10.1523/JNEUROSCI.0645-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat. Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bejjanki VR, Beck JM, Lu ZL, Pouget A. Perceptual learning as improved probabilistic inference in early sensory areas. Nat. Neurosci. 2011;14:642–648. doi: 10.1038/nn.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGovern DP, Roach NW, Webb BS. Perceptual learning reconfigures the effects of visual adaptation. J. Neurosci. 2012;32:13621–13629. doi: 10.1523/JNEUROSCI.1363-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]