Abstract
Sepsis is a massive inflammatory response mediated by infection, characterized by oxidative stress, release of cytokines, and mitochondrial dysfunction. Melatonin accumulates in mitochondria, and both it and its metabolites have potent antioxidant and anti-inflammatory activities and may be useful in sepsis. We undertook a phase I dose escalation study in healthy volunteers to assess the tolerability and pharmacokinetics of 20, 30, 50, and 100 mg oral doses of melatonin. In addition, we developed an ex vivo whole blood model under conditions mimicking sepsis to determine the bioactivity of melatonin and the major metabolite 6-hydroxymelatonin at relevant concentrations. For the phase I trial, oral melatonin was given to five subjects in each dose cohort (n = 20). Blood and urine were collected for measurement of melatonin and 6-hydroxymelatonin, and symptoms and physiological measures were assessed. Validated sleep scales were completed. No adverse effects after oral melatonin, other than mild transient drowsiness with no effects on sleeping patterns, were seen, and no symptoms were reported. Melatonin was rapidly cleared at all doses with a median [range] elimination half-life of 51.7 [29.5–63.2] min across all doses. There was considerable variability in maximum melatonin levels within each dose cohort, but 6-hydoxymelatonin sulfate levels were less variable and remained stable for several hours. For the ex vivo study, blood from 20 volunteers was treated with lipopolysaccharide and peptidoglycan plus a range of concentrations of melatonin/6-hydroxymelatonin. Both melatonin and 6-hydroxymelatonin had beneficial effects on sepsis-induced mitochondrial dysfunction, oxidative stress, and cytokine responses at concentrations similar to those achieved in vivo.
Keywords: 6-hydroxymelatonin, cytokines, melatonin, phase I clinical trial, sepsis
Introduction
Around 37,000 people die from sepsis in the UK each year and as many as 8 million every year worldwide. Although the Surviving Sepsis Campaign, a performance improvement effort by hospitals across Europe, South America, and the United States, has improved outcomes, the mortality rate remains at 31% overall and >70% in those patients who go on to develop sepsis-induced multiple organ failure 1.
Oxidative stress in patients with sepsis has been consistently described over the last 20 yr 2. Mitochondrial dysfunction initiated by oxidative stress drives inflammation and is generally accepted as playing a major role in sepsis-induced organ failure 3. It has been recognized that exogenous antioxidants may be useful in sepsis 2, and more recently, the potential for antioxidants acting specifically in mitochondria has been highlighted 4,5. We showed previously that antioxidants targeted to mitochondria, including melatonin, reduced organ damage in a rat model of sepsis 6,7. Exogenous melatonin has potent antioxidant activity 8,9, and it accumulates throughout cells, particularly in mitochondria 10. Metabolites of melatonin also have antioxidant activity, and products from the reactions with oxidant species are also antioxidants 9,11,12.
In vitro models of sepsis show that melatonin and its major hydroxylated metabolite, 6-hydroxymelatonin, are both effective at reducing the levels of key inflammatory cytokines, oxidative stress, and mitochondrial dysfunction 8,9. In rat models of sepsis, melatonin reduces oxidative damage and organ dysfunction and also decreases mortality 7,13–15. The dose needed for antioxidant action is thought to be considerably higher than that given for modulation of the sleep–wake cycle, but the actual dose required in man is unclear, particularly because the major bioactive effects of oral melatonin in the context of inflammation are likely to be mediated primarily by metabolite levels.
We undertook a phase I dose escalation study in healthy volunteers using various doses of melatonin. We also developed an ex vivo whole blood/leukocyte model under conditions mimicking sepsis to determine the relative bioactivity of melatonin and its major hydroxylated metabolite, 6-hydroxymelatonin, at concentrations achieved in vivo after oral dosing.
Materials and methods
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich, Poole, Dorset, UK, and were of the highest grade available.
Clinical trial
The trial was registered at ClinicalTrials.gov (NCT01 724424) and ISCTRN (17088991).
After obtaining Clinical Trial Authorisation for a Clinical Trial of an Investigative Medical Product (CTIMP) from the Medicine and Healthcare Regulatory Authority (MHRA), plus Research Ethics and NHS Research and Development approval, healthy male volunteers aged 18–30 were invited to take part by advertising. Exclusion criteria were smoking, body weight above 100 kg, any regular medication or chronic health condition, or lack of consent. After obtaining written informed consent, the study visit took place in a side room of the Intensive Care Unit at Aberdeen Royal Infirmary, Aberdeen, UK. All subjects fasted for 12 hr before the study start at 8:00 hr and were requested to abstain from alcohol for 24 hr preceding the study visit. EKG electrodes, a noninvasive blood pressure cuff, a pulse oximeter, and an intravenous cannula were sited, and a basal blood sample was taken. Basal measurements of heart rate, oxygen saturation, EKG parameters, and blood pressure were recorded. Subjects then took melatonin (N-acetyl-5-methoxytryptamine or N-[2-(5-methoxy-1H-indol-3-yl)ethylacetamide) as 10 mg capsules (prepared by Nu-Pharm Laboratories Ltd., Deeside, Cheshire, UK, using melatonin manufactured and supplied by Flamma s.p.a., Bergamo, Italy).
A standard open-label dose escalation study design was used with sequential assignment of cohorts of five subjects each to doses of 20, 30, 50, and 100 mg. Venous blood samples were taken after 10, 30, 60, and 120 min and 3, 4, and 6 hr for melatonin and 6-hydroxymelatonin measurement, respectively. Routine biochemistry/hematology analyses were conducted at baseline and 6 hr, and a further blood sample for melatonin and 6-hydroxymelatonin was taken at 24 hr. Urine was also collected for 6 hr. Subjects ate a standardized sandwich lunch exactly 3 hr after the melatonin capsules, and water was allowed ad libitum. Subjects were questioned at 30-min intervals about any symptoms and whether they felt drowsy.
Subjects completed validated sleep scales about their sleep the day before the study visit, the study visit day itself, and 1 wk later. The Verran and Snyder-Halpern sleep scale uses 100-mm visual analogue scales to record three domains: sleep disturbance, sleep efficiency, and sleep supplementation 16.
A safety algorithm was designed which prospectively defined gradings for each biochemical and physiological parameter with clear stopping criteria. After each dose cohort, an independent Data Monitoring Committee (DMC) comprising a biochemist and two intensive care consultants, one local and one external, assessed the data and decided whether the investigators should continue to the next dose cohort.
Measurement of melatonin and 6-hydroxymelatonin
Blood samples were stored at 4°C until clotted and then immediately centrifuged, and the serum was frozen at −80°C until assay. Melatonin was measured using a Thermo Surveyor-TSQ Quantum liquid chromatography-tandem mass spectrometry (LC-MS/MS) system (Thermo Scientific, Hemel Hempstead, UK), which was set up and validated in-house. Sample aliquots (100 μL) were spiked with 10 μL of 60 ng/mL N-acetyltryptamine (internal standard), and plasma proteins were precipitated by the addition of 100 μL of 1.5% (w/v) sulfosalicylic acid. After vortex mixing, 500 μL of water was added and the samples were centrifuged at 4000 g for 10 min at 4°C. A 5-μL aliquot of the supernatant was injected onto the LC-MS/MS system equipped with a 150 × 2.1 mm, ACE 3 μ C18 column (Hichrom Ltd, Reading, UK) maintained at 50°C. Samples were maintained at 4°C prior to injection. Elution was achieved under isocratic conditions with a mobile phase of water/methanol (50/50) containing 0.1% (v/v) formic acid at a flow rate of 200 μL/min. Electrospray ionization was operated in positive ion mode with conditions optimized by flow injection analysis of the individual compounds: spray voltage, 4000 V; sheath gas pressure, 10 (arbitrary units); auxiliary gas pressure, 5 (arbitrary units); capillary temperature, 375°C; skimmer offset, −10V; collision pressure, 1.6 mTorr; and collision energy, 13 V. Tandem mass spectrometry was performed in single reaction monitoring (SRM) mode using the transition m/z 233–m/z 174 for melatonin and m/z 203–m/z 144 for N-acetyltryptamine. Under these conditions, melatonin eluted at 3.1 min and N-acetyltryptamine eluted at 3.5 min. Quantitation was based on an internal standard method using multilevel calibration curves (0.5–100 ng/mL melatonin) prepared in 5% bovine serum albumin with concentration plotted against the peak area ratio of the analyte to the internal standard. Weighted least squares regression analysis was carried out using Thermo Xcalibur software v.2.07 SP1 (Thermo Scientific, Hemel Hempstead, UK).
The metabolite 6-hydroxymelatonin was measured in serum and urine as the sulfated form using commercially available enzyme immunoassays (Cusabio Biotech, Hubei Province, China, and US Biologicals, Salem, MA, USA, respectively).
Ex vivo study
After obtaining University of Aberdeen College of Life Sciences and Medicine Ethics Review Board approval, a second group of 20 healthy male volunteers was invited to take part by advertising. Exclusion criteria were again smoking, body weight above 100 kg, any regular medication or chronic health condition, or lack of consent.
A single venous blood sample was collected at 9:00 hr into lithium-heparin tube and used immediately for whole blood experiments or allowed to cool to room temperature before isolating leukocytes. A sample of blood was also collected into an EDTA tube for hemoglobin and full cell count determination. Leukocytes were isolated by sedimenting blood through 4% succinylated gelatine (‘Gelofusine’, B. Braun Medical Ltd., Sheffield, UK). Briefly, whole blood was centrifuged at 750 g for 10 min and the plasma removed and replaced by equal volumes of Gelofusine and RPMI 1640 medium (GE Healthcare, Chalfront St Giles, UK) supplemented with 10% fetal calf serum. The tube was then placed at 37°C for 30 min at a slight angle to allow erythrocytes to sediment. The leukocyte-rich upper layer was then aspirated and centrifuged at 500 g for 10 min, and the remaining erythrocytes were removed by hypotonic shock. The leukocyte pellet was then washed and resuspended in medium.
For experimentation, whole blood or isolated leukocytes were placed into 24- or 96-well plates (see experimental detail below) in the presence of 2 μg/mL lipopolysaccharide (LPS, from E. coli strain 055:B5) plus 20 μg/mL peptidoglycan (PepG, from S. aureus strain 6571), prepared as described previously 17. In addition, blood or cells were also exposed to solvent control or either melatonin or 6-hydroxymelatonin (Sigma-Aldrich Ltd) dissolved in ethanol (<1% v/v) at 0, 0.01, 0.1, 1, 10, 100, or 1000 ng/mL for 18 hr at 37°C in a humidified atmosphere of 95% air/5% CO2. The amount of LPS and PepG used in this study was determined based on optimal release of interleukin (IL)-6 in preliminary experiments (data not shown).
Cytokine and lipid hydroperoxide levels
Whole blood was treated as above in 24-well plates. Levels of IL-6 and IL-10 were measured in plasma using commercially available enzyme immunoassay kits (R&D Systems Europe Ltd, Oxford, UK). Plasma lipid hydroperoxide (LPO) levels were measured using a commercially available spectrophotometric kit (Cambridge Bioscience Ltd, Cambs, UK) after extracting LPO into chloroform. Precision data in our hands were as follows: intra-assay—IL-6 = 1.4%, IL-10 = 1.9%, LPO = 1.7%; interassay—IL-6 = 6.3%, IL-10 = 6.2%; LPO = 2.0%.
Respiratory burst
Whole blood (200 μL) was added to 800 μl phosphate-buffered saline (PBS, 1.37 m NaCl, 27 mm KCl, 95 mm NaH2PO4, 15 mm KH2PO4, pH 7.4) containing 5 mm glucose and treated immediately as described previously. Samples (100 μL) were transferred to 96 wells, and basal luminescence was measured before the addition of sodium luminol using an automated system (1.25 mm final concentration). Luminescence was measured every minute until a plateau was reached. Results were normalized to total monocyte/neutrophil number and hemoglobin content.
Mitochondrial superoxide production
Mitochondrial superoxide production was determined in intact leukocytes in 96-well plates following cell treatments as described previously 18. Briefly, cells were washed twice with PBS before being loaded with 5 μm MitoSox™ (Life Technologies, Paisley, UK) for 10 min at 37°C and then washed immediately with warm PBS containing 5 mm glucose. Fluorescence was measured without delay at an excitation wavelength of 485 nm and emission wavelength of 590 nm.
Intracellular total radical production
Total radical production was measured in intact leukocytes in 96-well plates following cell treatment as above. Briefly, leukocytes were washed with PBS before being loaded with 50 μm oxidation-sensitive 5-(6)-carboxy-2,7′-dichlorodihydrofluorescein diacetate (CDHFD) or 50 μm oxidation-insensitive 5,(6)-carboxy-2,7-dichlorofluorescein (CDF) for 60 min in the dark at 37°C. Cells were then washed twice with PBS, and the rate of total radical formation was determined by subtracting CDF fluorescence from CDHFD fluorescence by continuous recording until saturation was reached, at excitation/emission wavelengths of 485/530 nm 19.
Mitochondrial membrane potential
Mitochondrial membrane potential was analyzed in isolated leukocytes in 96-well plates using the fluorescent probe 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazol-carbo-cyanine iodide (JC-1). Briefly, following cell treatments, leukocytes were washed twice with PBS and then incubated for 30 min with 10 μg/ml JC-1 in PBS containing 5 mm glucose at 37°C in the dark. Following incubation, cells were washed twice with PBS, and the red and green fluorescence was measured immediately (excitation, 490 nm; emission, 590 or 520 nm). In intact mitochondria, ‘J aggregates’ form and JC-1 fluoresces red. When the mitochondrial membrane potential decreases, the JC-1 assumes a monomeric form and fluoresces green; results are expressed as the ratio of red to green fluorescence 20.
Oxygen consumption
Leukocyte oxygen consumption was measured after cell treatments using a Clark-type oxygen electrode. Briefly, 1 × 106 cells were transferred to a 1-mL oxygen electrode chamber maintained at 37°C containing RPMI medium supplemented with 10% v/v FCS. Oxygen consumption was measured continuously for 15 min 21.
Phagocytosis
Neutrophils were isolated from whole blood using Polymorphprep™ (Axis-Shield, Oslo, Norway), diluted in RPMI 1640 containing 10% autologous serum, and used within 1 hr of isolation (viability >97%). E. coli stain 8959 (Public Health England, Porton Down, UK) were grown overnight in nutrient broth at 37°C with rotation at 250 rpm. Freshly prepared 2 × 106 neutrophils were added to 2 × 107 cfu E. coli plus 1 or 100 pg/mL melatonin or 6-hydroxymelatonin or solvent control. Identical control tubes were prepared without neutrophils to determine the starting number of bacteria and growth over the time course of the experiment. Tubes were rotated end-over-end at 37°C for 30, 60, and 90 min, and samples were removed into ice-cold PBS and centrifuged at 4°C to pellet the neutrophils. The supernatant containing extracellular bacteria was removed, and the neutrophil pellet was resuspended in water at pH 11.0 and incubated at room temperature for 5 min and then vortexed thoroughly for 5 s to release intracellular bacteria. Each sample was diluted and spread on an agar plate and incubated overnight at 37°C, and colonies were then counted.
Statistics
Phase I dose escalation trial
Multilevel linear modeling was used to analyze oxygen saturation, blood pressure, heart rate, and ECG measures. Generalized linear models were used to analyze variables with two measures at baseline and 6 hr. The models all evaluated the effect of increasing melatonin doses on each outcome variable and were adjusted for age, weight, and height. Assumptions were checked, and where necessary, transformed data were used. The analyses were carried out using SPSS version 21 (IBM, Portsmouth, UK) or SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) using two-tailed tests with P < 0.05 used to indicate statistical significance. The visual analogue scores for the sleep scales were measured independently by two observers and entered into an Excel spreadsheet for summation of the total domain score. Data were analyzed nonparametrically. Each domain total from the three sleep scales was compared using the Friedman test for repeated measures, with Wilcoxon signed-rank post hoc test if P < 0.05. Domain totals between each dose cohort were compared using Kruskal–Wallis followed by Mann–Whitney U post hoc test if appropriate. Multiple comparisons were adjusted using Bonferroni correction where appropriate.
Ex vivo study
Data were not normally distributed and so are presented as median and interquartile range. Data were analyzed using Mann–Whitney U-test or Friedman's test, with either post hoc testing using Dunn's test for multiple comparisons or Page's L trend test as appropriate.
Results
Thirty-one subjects volunteered, but eight fulfilled the exclusion criteria and so were not recruited: Of these, five were taking regular medication or had chronic health conditions, two were occasional smokers, and one had a body weight >100 kg. Another three subjects consented but subsequently decided they could not spare the time. Twenty subjects aged 21–27 years completed the study visit and the 1-wk follow-up.
There was little difference in baseline characteristics between dose cohorts (Table1). There were no grounds for noncontinuation, and the DMC recommended progression after each dose cohort. There were no serious adverse events and no adverse events attributable to the study drug. No subject reported nausea, headache, vomiting, diarrhea, or abdominal pain at any time during the study visit or the 1-wk follow-up period. There were statistically significant decreases in some biochemical parameters between baseline and 6 hr, but none were of clinical significance and there were no effects of melatonin dose (Table2). Analysis of physiological measurements over time revealed some statistically significant changes related to time (oxygen saturation, diastolic blood pressure) or dose (systolic blood pressure), but none of these changes were considered to be clinically significant or of any concern (Fig.1).
Table 1.
Age, weight, and height data by dose cohort
| Dose mg | Age years | Weight kg | Height cm |
|---|---|---|---|
| 20 | 25 [23–26] | 75 [73–94] | 179 [173–203] |
| 30 | 22 [21–22] | 73 [57–99] | 183 [170–191] |
| 50 | 21 [21–27] | 76 [73–86] | 180 [178–191] |
| 100 | 22 [21–22] | 80 [73–85] | 188 [179–193] |
Median [range], n = 5 per dose cohort.
Table 2.
Biochemistry/hematology data by dose cohort
| Measure | Melatonin dose (mg) | |||
|---|---|---|---|---|
| 20 | 30 | 50 | 100 | |
| Hemoglobin g/L | ||||
| Baseline | 147 [139–161] | 138 [127–142] | 149 [142–169] | 147 [145–148] |
| 6 hr | 145 [136–153] | 133 [128–148] | 145 [145–166] | 141 [139–147] |
| Leukocytesa 109/L | ||||
| Baseline | 5.0 [3.7–6.9] | 5.6 [4.3–8.7] | 4.9 [4.2–5.7] | 5.3 [5.3–6.7] |
| 6 hr | 5.0 [4.2–6.7] | 5.3 [4.2–6.6] | 5.7 [4.3–6.7] | 5.6 [4.2–6.4] |
| AST Units/L | ||||
| Baseline | 33 [24–34] | 25 [18–30] | 24 [20–51] | 23 [17–41] |
| 6 hr | 24 [17–25] | 21 [16–29] | 22 [17–33] | 23 [16–36] |
| Bilirubin μmol/L | ||||
| Baseline | 17 [14–29] | 16 [9–38] | 18 [7–23] | 11 [9–14] |
| 6 hr | 15 [12–29] | 17 [8–35] | 19 [9–23] | 14 [9–17] |
| Creatinine μmol/L | ||||
| Baseline | 83 [74–86] | 73 [70–96] | 71 [68–77] | 80 [62–85] |
| 6 hr | 73 [71–83] | 68 [60–92] | 66 [63–73] | 79 [56–84] |
| Glucoseb mmol/L | ||||
| Baseline | 5.0 [4.3–5.3] | 4.5 [3.7–5.6] | 4.1 [3.6–4.7] | 4.4 [3.9–5.0] |
| 6 hr | 4.4 [3.8–5.1] | 4.4 [4.4–5.4] | 4.0 [3.7–5.3] | 4.2 [3.9–5.8] |
| Potassiumc mmol/L | ||||
| Baseline | 4.0 [3.9–4.6] | 3.9 [3.5–4.2] | 3.7 [3.6–3.8] | 4.0 [3.6–4.0] |
| 6 hr | 3.8 [3.7–3.9] | 3.9 [3.4–4.3] | 3.8 [3.7–3.9] | 3.8 [3.7–4.0] |
| Sodiumd mmol/L | ||||
| Baseline | 140 [140–141] | 140 [138–140] | 142 [140–143] | 141 [140–143] |
| 6 hr | 141 [139–142] | 140 [139–142] | 141 [139–142] | 141 [140–141] |
Median [range], n = 5 per dose cohort.
Baseline >6 hr (P < 0.001) across all subjects, no effect of dose.
Baseline >6 hr (P = 0.013) across all subjects, no effect of dose.
Baseline >6 hr (P < 0.001) across all subjects, no effect of dose.
Baseline >6 hr (P = 0.001) across all subjects, no effect of dose.
Fig 1.
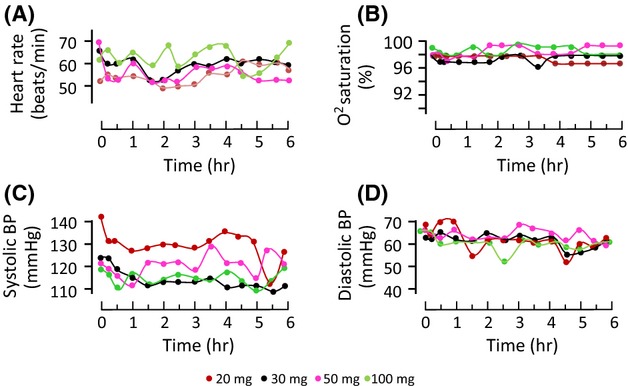
Physiological measures in healthy subjects over 6 hr after receiving an oral dose of melatonin. (A) Heart rate, (B) oxygen saturation, (C) systolic blood pressure, and (D) diastolic blood pressure. Median values shown for clarity, n = 5 subjects per dose cohort. Multilevel linear modeling showed that oxygen saturation and diastolic blood pressure decreased over time, unrelated to dose (P = 0.017 and P < 0.001, respectively), and systolic blood pressure increased with melatonin dose (P < 0.005).
Fifteen of the 20 subjects reported drowsiness at some point during the study visit. There were 17 separate time points at which subjects in the 20 mg dose cohort reported feeling sleepy, six in the 30 mg cohort, five in the 50 mg cohort, and 17 incidences in the 100 mg cohort; thus, there were as many reports of drowsiness for the 20 mg dose cohort as the 100 mg cohort and no obvious relationship to dose. All subjects were fully recovered by 6 hr and were able to go home alone.
Sleep disturbance is measured as mid-sleep awakening, wake after sleep onset, movement during sleep, soundness of sleep, and quality of disturbance, while latency characteristics include sleep latency and quality of latency. The maximum available total score for the sleep disturbance domain is 700, and a lower score indicates less sleep disturbance. The median [range] domain totals are given in Table3. It can be seen that sleep disturbance was variable—but minimal—in most subjects and unrelated to the dose of melatonin. There was no difference in the time taken to fall asleep (sleep latency) or the sleep disturbance domain total between the three points at which sleep scales were completed.
Table 3.
Sleep scale domain total scores by dose cohort
| Dose | Timing of sleep scale | Disturbance domain | Efficiency domain | Supplementation domain |
|---|---|---|---|---|
| 20 mg | Before study visit | 59 [16–423] | 397 [310–461] | 2 [2–109] |
| Study visit | 46 [8–145] | 443 [324–490] | 22 [0–94] | |
| Week after | 99 [5–200] | 429 [400–468] | 2 [0–11] | |
| 30 mg | Before study visit | 72 [36–180] | 376 [313–420] | 16 [7–79] |
| Study visit | 37 [29–142] | 409 [272–487] | 59 [4–139] | |
| Week after | 81 [25–263] | 313 [272–412] | 36 [21–87] | |
| 50 mg | Before study visit | 59 [11–104] | 436 [268–456] | 7 [0–160] |
| Study visit | 77 [13–234] | 413 [312–458] | 13 [2–35] | |
| Week after | 60 [13–168] | 399 [369–430] | 1 [0–135] | |
| 100 mg | Before study visit | 65 [61–464] | 329 [317–453] | 9 [0–154] |
| Study visit | 21 [10–205] | 449 [251–458] | 82 [49–137] | |
| Week after | 95 [71–190] | 379 [319–469] | 18 [13–108] |
Median [range], n = 5 per dose cohort.
No significant changes between time points or dose cohorts.
Sleep efficiency is both the perceived quality and the duration of sleep. The maximum total score possible is 500 with a higher score representing greater sleep efficiency. Sleep efficiency was consistent and was similar in all subjects at all times, independent of melatonin dose (Table3). There was no change in perceived sleep quality or the sleep efficiency domain total between any of the completed sleep scales in any of the dose cohorts.
The sleep supplementation domain measures how much extra sleep time subjects had during the day. The maximum total score possible is 400, and the higher the total score was, the more supplemental sleep was needed. We found that supplementary sleep duration was variable between individuals but again was unrelated to melatonin dose (Table3). There was no difference in the total sleep periods between the sleep scales.
The technique for measuring melatonin was very sensitive with a lower limit of quantitation of 0.5 ng/mL. The mean intra-assay precision (percentage relative standard deviation, n = 6) was 4.7% at 1 ng/mL and 2.6% at 80 ng/mL. Interassay precision was 4.1% at 1 ng/mL and 1.0% at 80 ng/mL, and recovery of melatonin added to serum was 96% at 0.5 ng/mL and 105% at 100 ng/mL. For 6-hydroxymelatonin sulfate determination in serum, the mean intra-assay precision was 3.0% and the inter-assay precision was 5.0%. The precision of the urine 6-hydroxymelatonin sulfate assay was 4.1% intra-assay and 15.8% inter-assay.
In all subjects, melatonin was detectable in serum at 10 min after taking the oral dose and was rapidly cleared, with maximum levels reached at 30–60 min (Table4). Melatonin concentration was variable between individuals even after the same melatonin dose, but there was a significant effect of melatonin dose on both the area under the concentration curve (AUC, P = 0.0065) and maximum concentration (Cmax, P = 0.0018). Levels of 6-hydroxymelatonin sulfate were also detectable in serum by 10 min with maximum levels reached at a median of 120 min, but were less variable than melatonin, and remained broadly stable between 1 and 6 hr but were back at baseline levels at 24 hr (Fig.2). Fig.3 shows the relationship between melatonin and 6-hydoxymelatonin sulfate concentrations in individual subjects in the 50 mg dose cohort.
Table 4.
Melatonin pharmacokinetic data in individual subjects
| Dose and subject ID no. | AUCa ng/ml/min | Half-life mins | Cmaxb ng/ml | Tmax min |
|---|---|---|---|---|
| 20 mg | ||||
| 1 | 1102 | 62.01 | 12.80 | 30.00 |
| 2 | 3266 | 52.62 | 35.70 | 60.00 |
| 3 | 3405 | 58.76 | 30.33 | 60.00 |
| 4 | 1564 | 50.38 | 20.86 | 30.00 |
| 5 | 13616 | 52.07 | 147.68 | 30.00 |
| 30 mg | ||||
| 6 | 2491 | 48.33 | 30.09 | 30.00 |
| 7 | 1866 | 48.14 | 33.67 | 30.00 |
| 8 | 1304 | 71.62 | 12.95 | 30.00 |
| 9 | 822 | 63.22 | 6.25 | 60.00 |
| 10 | 1640 | 62.96 | 8.51 | 120.00 |
| 50 mg | ||||
| 11 | 6034 | 55.58 | 75.42 | 30.00 |
| 12 | 1812 | 45.06 | 24.53 | 30.00 |
| 13 | 8915 | 51.39 | 113.78 | 30.00 |
| 14 | 4234 | 41.75 | 67.84 | 30.00 |
| 15 | 6554 | 53.09 | 91.38 | 30.00 |
| 100 mg | ||||
| 16 | 9570 | 44.41 | 103.22 | 60.00 |
| 17 | 5639 | 29.47 | 94.00 | 60.00 |
| 18 | 18229 | 47.32 | 220.26 | 30.00 |
| 19 | 4458 | 45.18 | 47.71 | 60.00 |
| 20 | 11062 | 55.89 | 138.83 | 30.00 |
AUC, area under the concentration curve.
Significant effect of dose (P = 0.006).
Significant effect of dose (P = 0.006).
Fig 2.
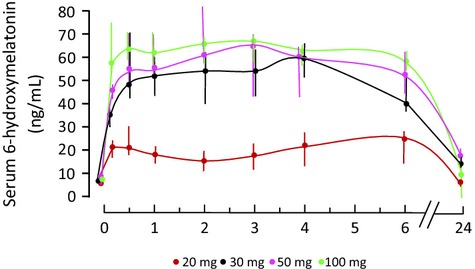
Seum 6-hydroxymelatonin sulfate levels in healthy subjects over 6 hr after an oral dose of melatonin. Median and full range shown, n = 5 per dose cohort. There was a significant effect of dose (P = 0.028), and levels after 30, 50, or 100 mg were significantly higher than after 20 mg (all P < 0.001).
Fig 3.
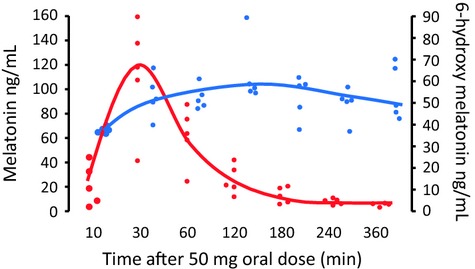
Serum melatonin (red) and serum 6-hydroxymelatonin (blue) in individual subjects following an oral dose of 50 mg melatonin (n = 5 per dose cohort). Lines show median values.
Urinary excretion of 6-hydroxymelatonin sulfate in the 6 hr after melatonin administration is shown in Table5. There was a significant dose effect (P = 0.0018) although there was considerable inter-individual variation.
Table 5.
Urine 6-hydroxymelatonin in individual subjects
| Dose and subject ID no. | Total 6-hydroxymelatonin excreted in 6 hra mg | Median dose cohort total 6-hydroxymelatonin excreted in 6 hr mg |
|---|---|---|
| 20 mg | ||
| 1 | 2.22 | 3.72 |
| 2 | 4.38 | |
| 3 | 3.72 | |
| 4 | 6.20 | |
| 5 | 2.96 | |
| 30 mg | ||
| 6 | 3.81 | 5.08 |
| 7 | 4.60 | |
| 8 | 8.80 | |
| 9 | 5.55 | |
| 10 | 5.08 | |
| 50 mg | ||
| 11 | 34.98 | 13.95 |
| 12 | 28.45 | |
| 13 | 13.95 | |
| 14 | 11.23 | |
| 15 | 5.82 | |
| 100 mg | ||
| 16 | 22.92 | 37.09 |
| 17 | 37.09 | |
| 18 | 47.09 | |
| 19 | 34.54 | |
| 20 | 66.03 | |
Significant effect of dose (P = 0.0018).
In the ex vivo study, IL-6 and IL-10 were both significantly increased in plasma from blood exposed to LPS/PepG (both P < 0.0001, Fig.4) compared with solvent control. Exposure of blood to LPS/PepG plus melatonin or 6-hydroxymelatonin caused a significant dose-dependent decrease in IL-6 (Fig.4A), but IL-10 was unaffected by either drug (Fig.4B). Likewise, LPO was significantly higher in blood exposed to LPS/PepG, and both melatonin and 6-hydroxymelatonin caused a significant dose-dependent decrease (Fig.4C). The respiratory burst also increased upon exposure to LPS/PepG, and this was significantly decreased when blood was co-treated with LPS/PepG plus melatonin or 6-hydroxymelatonin (Fig.5).
Fig 4.
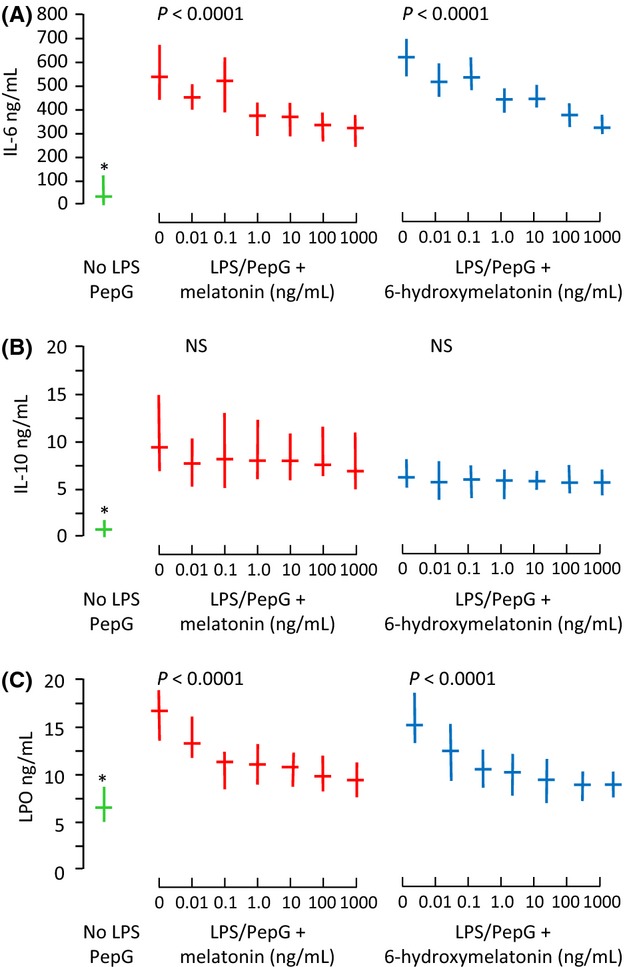
(A) Plasma interleukin-6 (IL-6), (B) IL-10, and (C) lipid hydroperoxide (LPO) from whole blood treated with solvent control (green), lipopolysaccharide and peptidoglycan G (LPS/PepG) plus melatonin (red) or 6-hydroxymelatonin (blue). Median and interquartile range, n = 20 subjects. P-value is Page's trend test. and * = significantly lower than with LPS/PepG alone (Wilcoxon Signed Ranks, P < 0.0001)
Fig 5.
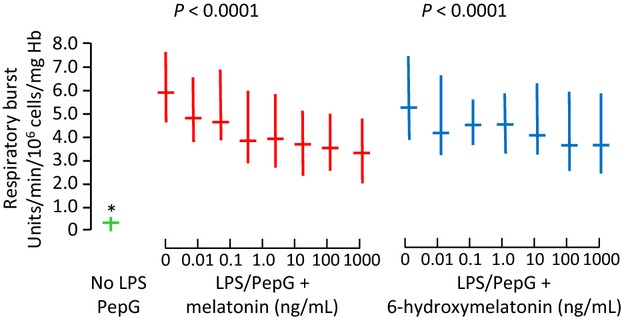
Respiratory burst in whole blood treated with solvent control (green), lipopolysaccharide and peptidoglycan G (LPS/PepG) plus melatonin (red) or 6-hydroxymelatonin (blue). Median and interquartile range, n = 20 subjects. P-value is Page's trend test. and * = significantly lower than with LPS/PepG alone (Wilcoxon Signed Ranks, P < 0.0001)
Exposure of isolated leukocytes to LPS/PepG resulted in a significant increase in mitochondrial membrane potential, total radical production, oxygen consumption, and mitochondrial superoxide production (Table6). Co-exposure of cells to LPS/PepG plus melatonin or 6-hydroxymelatonin resulted in decreased membrane potential, radical production, and oxygen consumption (Table6). When isolated neutrophils were cultured with E. coli, all bacteria were phagocytosed after 30 min regardless of treatment and there was no difference in the number of viable intracellular bacteria, showing that neither melatonin nor 6-hydroxymelatonin reduced neutrophil phagocytosis or killing of engulfed bacteria (data not shown).
Table 6.
Mitochondrial function in isolated leukocytes
| Melatonin or 6-hydroxymelatonin ng/ml | Mitochondrial membrane potential Red/green fluorescence ratio | Oxygen consumption nMoles O2/min/mg protein | Total radical production ΔFluorescence x 103/min/106 cells | Mitochondrial superoxide production Fluorescence units ×103 |
|---|---|---|---|---|
| Solvent control | 23.9 [18.3–27.0] | 17.9 [14.2–27.2] | 56 [34–61] | 260 [250–270] |
| Melatonin + LPS/PepG | P < 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.0029 |
| 0 | 28.6 [21.1–33.3] # | 36.1 [23.0–56.2] # | 88 [51–127] # | 290 [270–310] # |
| 0.01 | 21.6 [15.9–24.3]* | 27.4 [18.6–38.6]* | 59 [39–97]* | 280 [270–300]* |
| 0.1 | 19.9 [15.8–24.3]* | 22.6 [16.3–32.4]* | 62 [38–80]* | 270 [250–300]* |
| 1 | 21.0 [14.5–25.8]* | 24.3 [18.2–41.5]* | 62 [37–97]* | 270 [260–300]* |
| 10 | 19.6 [15.6–28.3]* | 25.6 [20.5–36.8]* | 60 [37–91]* | 270 [250–300]* |
| 100 | 23.2 [15.4–26.7]* | 32.4 [25.7–43.4] | 59 [35–111]* | 270 [250–290]* |
| 1000 | 24.5 [15.2–28.6]* | 37.7 [28.1–48.4] | 66 [43–105]* | 270 [270–280]* |
| Solvent control | 21.5 [16.1–23.3] | 19.0 [14.9–30.9] | 57 [41–70] | 250 [230–280] |
| 6-Hydroxymelatonin + LPS/PepG | P < 0.0001 | P = 0.0037 | P < 0.0001 | P = 0.0035 |
| 0 | 29.2 [21.8–32.8] # | 33.1 [21.1–53.4] # | 89 [60–122] # | 290 [260–300] # |
| 0.01 | 21.1 [16.1–23.3]* | 19.8 [13.8–26.7]* | 76 [45–117]* | 270 [250–300]* |
| 0.1 | 20.4 [17.2–23.9]* | 20.0 [12.9–25.2]* | 61 [46–108]* | 280 [270–300]* |
| 1 | 21.5 [19.1–26.7]* | 18.9 [15.1–25.2]* | 68 [40–93]* | 280 [260–300]* |
| 10 | 18.9 [15.8–24.4]* | 21.5 [14.8–25.9]* | 64 [50–110]* | 270 [250–300]* |
| 100 | 22.2 [18.9–25.0]* | 21.3 [15.3–26.7]* | 70 [54–109]* | 270 [260–300]* |
| 1000 | 24.6 [16.6–27.8]* | 19.4 [16.4–22.6]* | 61 [47–72]* | 270 [260–290]* |
6-OH Melatonin = 6-hydroxymelatonin. Median [interquartile range], n = 20. P-value shown is Friedman's test across LPS/PepG groups.
Significantly different from solvent control (Wilcoxon signed ranks) and *significantly different from LPS/PepG alone (Dunn's test) P < 0.05.
Discussion
We found that large oral doses of melatonin in healthy volunteers were very well tolerated with no safety concerns and no clinically relevant changes in any physiological or biochemical measures. The concentrations of melatonin in the blood were variable and were rapidly cleared, but levels of the metabolite 6-hydroxymelatonin sulfate were more consistent and remained stable for several hours, returning to baseline levels by 24 hr. The levels of both melatonin and 6-hydroxymelatonin achieved after oral dosing were shown to be bioactive in terms of antioxidant/anti-inflammatory effects in an ex vivo model of sepsis.
Sepsis can be defined as an uncontrolled immune and inflammatory response to an infectious insult, resulting in oxidative stress, massive cytokine release, and mitochondrial dysfunction. The role of damage to mitochondria in the pathophysiology of sepsis and subsequent organ dysfunction is widely accepted 3–5. Melatonin is known to have a plethora of antioxidant and anti-inflammatory effects likely to be of benefit in sepsis 4,5,7–9.
Oral bioavailability of melatonin is low and has been estimated to be approximately 15% of the parent compound 22–24 with rapid clearance. The low bioavailability of melatonin and the marked interindividual variability that are reported here have been reported previously 24,25 and are a consequence of variable first-pass extraction in the liver, due to genetic differences in the activity of cytochrome P450 enzymes, notably CYP1A1 but also CYP1A2, which convert melatonin to the 6-hydroxymelatonin metabolite before it enters the systemic circulation 26–28. The majority of melatonin is converted to 6-hydroxymelatonin in the liver, then sulfated (∼80%) or glucuronidated (∼10%), and excreted in urine. The remainder can be demethylated back to N-actylserotonin 29. In addition, 6-hydroxylmelatonin sulfate can be formed at extrahepatic sites by CYP1B1 27. In addition to enzymatic processes, 6-hydroxymelatonin can also be generated by nonenzymatic means via reaction with peroxynitrite or hydroxyl radical such that during oxidative stress, persistent production of 6-hydroxymelatonin might be expected.
We and others have reported a protective effect of melatonin in animal models of sepsis 7,13–15,30, suggesting a potential beneficial effect of melatonin in patients with sepsis. It is now apparent that 6-hydroxymelatonin has similar antioxidant effects to melatonin and protects against various oxidative stress initiators 11,29,31–34 such that after oral dosing with melatonin, antioxidant effects may be mediated by 6-hydroxymelatonin. We undertook this dose escalation study to determine the tolerance of large oral doses of melatonin and the levels of melatonin and 6-hydroxymelatonin sulfate achieved and related this to a physiologically relevant ex vivo model of sepsis. Melatonin metabolism has been commonly monitored using measurement of urine excretion of 6-hydroxymelatonin sulfate, with maximum levels up to 2 mg/hr reported to occur between 04:00 and 08:00 but negligible amounts at other times 35; however, there are few reports of 6-hydroxymelatonin in the serum after oral doses of melatonin in humans. Data from a single subject after an oral dose of 1 mg melatonin showed that plasma 6-hydroymelatonin sulfate levels were elevated for 6 hr after melatonin administration, measured using radioimmunoassay, concurring with our findings 36. In another study by Härtter and colleagues 37, a maximum plasma level of total 6-hydroxymelatonin from a single subject after taking 25 mg oral melatonin was reported to be approximately 600 ng/mL, again persisting for over 6 hr. Härtter et al. undertook enzymatic conversion of both the sulfate and glucuronidate forms of 6-hydroxymelatonin before liquid chromatography–mass spectrometry, to give the total conjugated 6-hydroxymelatonin level, but this technique incurred large losses during the hydrolysis step 37. We found that 6-hydroxymelatonin was extremely unstable—hence our decision to measure 6-hydroxymelatonin in serum as the sulfated form using a validated enzyme immunoassay rather than using liquid chromatography. The long-term stability of 6-hydroxymelatonin sulfate has been previously reported 38.
In some countries such as the USA, melatonin is considered a food supplement, whereas in Europe, it is considered a neurohormone. The only licensed melatonin product in the UK is a slow-release melatonin agonist licensed for use in sleep disorders. In the present study, under EU legislation, melatonin was considered an investigational medicinal product and capsules were manufactured from chemically synthesized melatonin. Administration of melatonin in both preclinical and human studies, even at supraphysiological doses, has an excellent safety profile, although there is little in the way of robust toxicological investigation allowing an evaluation of risk in clinical trials 39. We found that there was no evidence of side effects after single doses of up to 100 mg oral melatonin in healthy young men. Minor decreases in leukocyte count, glucose, potassium, and sodium were probably related to the dilutional effects of drinking water in subjects who had been fasting. There were also minor changes in oxygen saturation and blood pressure over time, but again, no values were of clinical significance or concern at any time point. Small decreases in systolic blood pressure and heart rate have been reported after a single dose of 5 mg melatonin at 14:00 or 21:00 hr in healthy subjects, but values remained well within normal ranges 40,41. There have been previous anecdotal reports of nausea, headache, and itching in healthy subjects and various patient groups 42,43. However, there are few previous comprehensive reports of rigorous and comprehensive monitoring of physiological and biochemical parameters in different melatonin dose cohorts. Using a nonsuggestive approach, we had no reports of any symptoms at any dose, either immediately after dosing or up to 1 wk afterward. No effect of chronic administration of melatonin on a multitude of biochemical measures was also seen in previous randomized, double-blinded study of subjects taking 10 mg daily for a month 43, and there was also no difference in the incidence of adverse events such as nausea and headache between those taking melatonin and those given placebo 43.
Melatonin has been reported to cause drowsiness 44,45 and indeed has been used at doses of 1–5 mg to treat sleep disorders and jet lag 46. In our study, most subjects did report subjective drowsiness, and some, but not all, fell asleep, but the number of subjects who were drowsy or fell asleep appeared unrelated to the melatonin dose. All our subjects were lying supine on a bed in a warm and soporific environment; we therefore thought it likely that subjective drowsiness levels would be overestimated, and so we were somewhat surprised that more subjects did not report feeling drowsy after even the highest dose of melatonin. All subjects were alert and able to go home at the end of the study visit (6 hr after the melatonin dose). Melatonin also did not change the objective assessment of subsequent sleeping patterns in any subject. Although several subjects reported subjectively that they ‘slept very well’ the night of the study visit, this was not reflected in the recorded quality or duration of sleep in the completed sleep scales. The sleep scale used is a validated scale used extensively for assessing sleep efficacy and disturbance and the need for supplementary daytime sleep 16. However, the day of the week on which the sleep scales were completed may of course have had an impact on the data, and any alcohol intake the day after the study visit or a week later may also have affected results.
It was reported previously that 9 mg oral melatonin at 09:30 reduced sleep latency (i.e., time to fall asleep), assessed using a multiple sleep latency test at 2-hr intervals, with subjects forced to stay awake in-between 47. Another double-blind trial of up to 40 mg melatonin given at 10:00 showed decreased sleep latency, increased sleep duration, and decreased wake after sleep onset 2 hr after all doses of melatonin, measured polygraphically 44. However, in a randomized double-blind study of 30 subjects given 10 mg melatonin daily at night for 28 day, polysomnographic recording revealed no effect of melatonin on sleep latency, total sleep time, amount of rapid eye movement (REM) sleep, sleep efficiency, or arousals 43. The only significant finding in terms of sleep was shorter duration of stage 1 of non-REM sleep in the subjects taking melatonin compared to the placebo group, but no changes within the melatonin group before and after melatonin 43. Similarly, in a double-blind study of 1 or 5 mg melatonin given at night, no effect on sleep latency or duration was found, recorded using EEG 48. These reports suggest that any sleep-promoting effects of melatonin are short lived and effects seem to be more pronounced when melatonin is given during the day 49. It is also possible that subjects in some previous studies may have had an expectation of the possible effects of melatonin, which may have resulted in subjective reports of somnolence. Two small studies of low-dose oral melatonin administration in patients on the intensive care unit reported minor effects on sleep, but no effect on sedation requirements 50,51.
We developed a whole blood model as a physiologically relevant representation of early events in sepsis. Modeling sepsis is not straightforward; animal models are fraught with controversy 52, and although possible, modeling sepsis in humans is unpleasant and at best can only actually model aspects of inflammation 53. The interaction between immune responses associated with sepsis is complicated and difficult to replicate in cell culture systems, although whole blood models are potentially more physiologically relevant than single-cell-type models in terms of reflecting complex immune cell–cell interactions. We found that the respiratory burst of whole blood exposed to LPS/PepG was decreased by both melatonin and 6-hydroxymelatonin, as reported previously in isolated neutrophils from patients with pancreatitis 54. Bacterial wall proteins such as LPS and PepG promote inflammation via engagement of toll-like receptors, leading to myD88-dependent inflammatory responses including the respiratory burst via NADPH oxidase activity 55,56 and resulting in activation of signaling pathways culminating in cytokine release. Treatment of blood with melatonin or 6-hydroxymelatonin also resulted in dose-dependent suppression of LPS/PepG-induced increases in IL-6 and lipid hydroperoxide levels, while IL-10 was unaffected, confirming previous findings in models of sepsis 7,14–16 and other disease models 57. The mechanism of the effect of melatonin/6-hydroymelatonin may be via antioxidant scavenging, but melatonin has also been reported to affect NADPH oxidase activity 58 and several transcription factor pathways involved in cytokine responses, possibly mediated through effects on mitogen-activated protein kinases (MAPK) 11,57–59.
We also investigated the effect of melatonin and 6-hydroxymelatonin on aspects of mitochondrial function in isolated leukocytes exposed to LPS/PepG. Sepsis is associated with mitochondrial damage, which is thought to contribute to the pathophysiology of organ dysfunction 3–6. In the present study, we exposed leukocytes to sepsis-like conditions for 18 hr to mimic the early stages of sepsis and found increased intracellular total radical production and intra-mitochondrial superoxide production, with concomitantly increased oxygen consumption, all of which were attenuated by melatonin and 6-hydroxymelatonin at all concentrations. We previously reported that melatonin and other related indoles, including 6-hydroxymelatonin, attenuated mitochondrial dysfunction induced by 7-day exposure to LPS/PepG in human endothelial cells 11. Similar protective effects of melatonin on mitochondrial function have been reported in models of sepsis in animals 7,30. Mitochondrial membrane potential in leukocytes in the present study increased upon LPS/PepG exposure and was reduced to that seen in control cells by melatonin and 6-hydroxymelatonin. The increase in mitochondrial membrane potential is required physiologically to enhance the bactericidal activity of phagocytes caused by the downregulation of mitochondrial uncoupling protein-2 60. The enhanced mitochondrial membrane potential is necessary to drive increased production of mitochondrial ROS (mROS) through boosting electron leak during oxidative phosphorylation. Evidence indicates that mROS is an important contributor to the bactericidal activity of the respiratory burst and innate immune signaling by augmenting pro-inflammatory cytokine production via nuclear factor κB (NFκB) and MAPK 61–64. Treatment with melatonin/6-hydroxymelatonin resulted in a return of mitochondrial membrane potential to baseline, thus presumably contributing to the blunting of subsequent inflammatory responses. Of course, this may have had an unwanted effect of reducing phagocytic cell killing of bacteria. However, in additional studies we found that the ability of neutrophils to both phagocytose and kill ingested E. coli was unaffected by melatonin or 6-hydroxymelatonin at concentrations up to 100 pg/mL.
Melatonin has been given previously to patients with sepsis at relatively low doses, with the aim of influencing sleeping patterns 50,51, but we suggest that higher doses of oral melatonin are likely to have beneficial effects on inflammatory responses and are probably primarily mediated via 6-hydroxymelatonin. A recently published study of 100 mg melatonin given as an intravenous infusion over an 8-hr period before injection of a very small dose of LPS in a human model of endotoxemia showed only minor effects of melatonin on cytokine levels and lipid peroxidation products 65. However, the changes seen in response to LPS administration were small compared with those seen both in other human models of endotoxemia and in patients with sepsis 53. Unfortunately, the levels of melatonin or its metabolites were not reported, nor the effects on sleep or other side effects, nor verification of the model in terms of inflammatory responses such as body temperature, hormone levels, and leukocyte counts 65.
In summary, we showed that the levels of both melatonin and 6-hydroxymelatonin achieved after oral dosing of melatonin were within the range of the doses at which antioxidant and anti-inflammatory effects were seen in blood cultured under conditions mimicking sepsis and that there were no side effects. The variable responses between individuals should be considered when melatonin is administered. We propose that administration of around 50 mg of melatonin would generate melatonin and—perhaps more importantly—6-hydroymelatonin levels that correspond to concentrations at which attenuation of inflammatory and oxidative stress biomarkers was seen ex vivo, even in those subjects in whom melatonin levels were lowest.
Author contributions
HFG conceived of the study, contributed to study design, took overall trial management responsibility, recruited subjects and acquired data, and drafted and revised the manuscript. NRW contributed to the design and conduct of the study and subject recruitment and critically revised the manuscript. LSA contributed to study design, undertook statistical analyses, and critically revised the manuscript. DAL contributed to study design, undertook sample preparation and laboratory analyses, and critically revised the manuscript. LA contributed to study design, conduct of the study, and data acquisition and critically reviewed the manuscript. GC developed and validated the melatonin assay and contributed to drafting of the manuscript. All authors read and approved the final article.
Acknowledgments
This study was funded by the Chief Scientist Office, NHS Scotland. We would like to thank all the volunteers who gave up their time and blood to take part in the study and the data monitoring committee and staff of the intensive care unit for their support. In addition, thanks to Dr Malachy Columb for performing Page's trend test for us and to Annette Fearnley at Nu-Pharm Ltd for her advice.
References
- 1.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the international sepsis forum. Crit Care Med. 2005;33:1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 2.Rinaldi S, Landucci F, de Gaudio AR. Antioxidant therapy in critically ill septic patients. Curr Drug Targets. 2009;10:872–880. doi: 10.2174/138945009789108774. [DOI] [PubMed] [Google Scholar]
- 3.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Galley HF. Bench to bedside review: Targeting antioxidants to mitochondria in sepsis. Crit Care. 2010;14:230. doi: 10.1186/cc9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Víctor VM, Espulgues JV, Hernández-Mijares A, et al. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 6.Lowes DA, Thottakam BMVJ, Webster NR, et al. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Lowes DA, Webster NR, Murphy MP, et al. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110:472–480. doi: 10.1093/bja/aes577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 9.Reiter RJ, Tan DX, Rosales-Corral S, et al. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem. 2013;13:373–384. doi: 10.2174/1389557511313030006. [DOI] [PubMed] [Google Scholar]
- 10.Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowes DA, Almawash AM, Webster NR, et al. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br J Anaesth. 2011;107:193–201. doi: 10.1093/bja/aer149. [DOI] [PubMed] [Google Scholar]
- 12.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites. AFMK and AMK. J Pineal Res. 2013;54:245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 13.Li Volti G, Musumeci T, Pignatello R, et al. Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp Biol Med. 2012;237:670–677. doi: 10.1258/ebm.2012.011425. [DOI] [PubMed] [Google Scholar]
- 14.Shang Y, Xu SP, Wu Y, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J (Engl) 2009;122:1388–1393. [PubMed] [Google Scholar]
- 15.Wu JY, Tsou MY, Chen TH, et al. Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. J Pineal Res. 2008;45:106–116. doi: 10.1111/j.1600-079X.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 16.Snyder-Halpern R, Verran JA. Instrumentation to describe subjective sleep characteristics in healthy subjects. Res Nurs Health. 1987;10:155–163. doi: 10.1002/nur.4770100307. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Ray P, Kanwar M, et al. A comparative analysis of antibody repertoire against Staphylococcus aureus antigens in patients with deep-seated versus superficial staphylococcal infections. Int J Med Sci. 2005;2:129–136. doi: 10.7150/ijms.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay P, Rajesh M, Yoshihiro K, et al. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collesa S, Chisolm GM. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J Lipid Res. 2000;41:1188–1198. [PubMed] [Google Scholar]
- 20.Smiley ST, Reers M, Mottola-Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petita C, Tri-Rouxela FP, Lesneb A, et al. Oxygen consumption by cultured human cells is impaired by a nucleoside analogue cocktail that inhibits mitochondrial DNA synthesis. Mitochondrion. 2005;5:154–161. doi: 10.1016/j.mito.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Demuro RL, Nafziger AN, Blask DE, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 23.Waldhauser F, Waldhauser M, Lieberman HR, et al. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–313. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 24.Fourtillan JB, Brisson AM, Gobin P, et al. Bioavailability of melatonin in humans after day-time administration of D(7) melatonin. Biopharm Drug Dispos. 2000;21:15–22. doi: 10.1002/1099-081x(200001)21:1<15::aid-bdd215>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Di WL, Kadva A, Johnston A, et al. Variable bioavailability of oral melatonin. N Engl J Med. 1997;336:1028–1029. doi: 10.1056/NEJM199704033361418. [DOI] [PubMed] [Google Scholar]
- 26.Lane EA, Moss HB. Pharmacokinetics of melatonin in man: first pass hepatic metabolism. J Clin Endocrinol Metab. 1985;61:1214–1216. doi: 10.1210/jcem-61-6-1214. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Idle JR, Krausz KW, et al. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- 28.Facciolá G, Hidestrand M, von Bahr C, et al. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur J Clin Pharmacol. 2001;56:881–888. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 29.Young IM, Leone RM, Francis P, et al. Melatonin is metabolized to n-acetyl serotonin and 6-hydroxymelatonin in man. J Clin Endocrinol. 1985;60:114–119. doi: 10.1210/jcem-60-1-114. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz F, García JA, Acuña-Castroviejo D, et al. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res. 2014;56:71–81. doi: 10.1111/jpi.12099. [DOI] [PubMed] [Google Scholar]
- 31.Maharaj DS, Walker RB, Glass BD, et al. 6-Hydroxymelatonin protects against cyanide induced oxidative stress in rat brain homogenates. J Chem Neuroanat. 2003;26:103–107. doi: 10.1016/s0891-0618(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 32.Duan Q, Wang Z, Lu T, et al. Comparison of 6-hydroxylmelatonin or melatonin in protecting neurons against ischemia/reperfusion-mediated injury. J Pineal Res. 2006;41:351–357. doi: 10.1111/j.1600-079X.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 33.Calvo JR, Reiter RJ, García JJ, et al. Characterization of the protective effects of melatonin and related indoles against alpha-naphthylisothiocyanate-induced liver injury in rats. J Cell Biochem. 2001;80:461–470. doi: 10.1002/1097-4644(20010315)80:4<461::aid-jcb1000>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Burillo S, Tan DX, Rodriguez-Gallego V, et al. Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and 6-hydroxymelatonin reduce oxidative DNA damage induced by Fenton reagents. J Pineal Res. 2003;34:178–184. [Google Scholar]
- 35.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- 36.Harthe C, Claustrat B, Brun J, et al. Direct radioimmunoassay of 6-sulfatoxymelatonin in plasma with use of an iodinated tracer. Clin Chem. 1991;37:536–539. [PubMed] [Google Scholar]
- 37.Härtter S, Morita S, Bodin K, et al. Determination of exogenous melatonin and its 6-hydroxy metabolite in human plasma by liquid chromatography-mass spectrometry. Ther Drug Monit. 2001;23:282–286. doi: 10.1097/00007691-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Griefahn B, Remer T, Blaszkewicz M, et al. Long-term stability of 6-hydroxy melatonin sulfate in 24-h urine samples stored at -20 degrees C. Endocrine. 2001;15:199–202. doi: 10.1385/ENDO:15:2:199. [DOI] [PubMed] [Google Scholar]
- 39.Guardiola-Lemaître B. Toxicology of melatonin. J Biol Rhythms. 1997;12:697–706. doi: 10.1177/074873049701200627. [DOI] [PubMed] [Google Scholar]
- 40.Harris AS, Burgess HJ, Dawson D. The effects of day-time exogenous melatonin administration on cardiac autonomic activity. J Pineal Res. 2001;31:199–205. doi: 10.1034/j.1600-079x.2001.310302.x. [DOI] [PubMed] [Google Scholar]
- 41.Sletten T, Burgess H, Savic N, et al. The effects of bright light and nighttime melatonin administration on cardiac activity. J Hum Ergol (Tokyo) 2001;30:273–278. [PubMed] [Google Scholar]
- 42.Markantonis SL, Tsakalozou E, Paraskeva A, et al. Melatonin pharmaco- kinetics in premenopausal and postmenopausal healthy female volunteers. J Clin Pharmacol. 2008;48:240–245. doi: 10.1177/0091270007311112. [DOI] [PubMed] [Google Scholar]
- 43.Seabra ML, Bignotto M, Pinto LRJR, et al. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 44.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- 45.Zhdanova I, Wurtman R. Efficacy of melatonin as a sleep-promoting agent. J Biol Rhythms. 1997;12:644–650. doi: 10.1177/074873049701200620. [DOI] [PubMed] [Google Scholar]
- 46.Herxheimer A, Petrie K. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2002;2:CD00152. doi: 10.1002/14651858.CD001520. [DOI] [PubMed] [Google Scholar]
- 47.Mishima K, Satoh K, Shimizu T, et al. Hypnotic and hypothermic action of daytime administered melatonin. Psychopharmacol. 1997;133:168–171. doi: 10.1007/s002130050387. [DOI] [PubMed] [Google Scholar]
- 48.James SP, Mendelson WB, Sack DA, et al. The effect of melatonin on normal sleep. Neuropsychopharmacology. 1987;1:41–44. doi: 10.1016/0893-133x(87)90008-x. [DOI] [PubMed] [Google Scholar]
- 49.Waterhouse J, Reilly T, Atkinson G, et al. Jet lag: trends and coping strategies. Lancet. 2007;369:1117–1129. doi: 10.1016/S0140-6736(07)60529-7. [DOI] [PubMed] [Google Scholar]
- 50.Mistraletti G, Sabbatini G, Taverna M, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–147. doi: 10.1111/j.1600-079X.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 51.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Poll T. Experimental human sepsis models. Drug Discov Today Dis Models. 2012;9:e3–e9. [Google Scholar]
- 53.Calvano SE, Coyle SM. Experimental human endotoxemia: a model of the systemic inflammatory response syndrome? Surg Infect. 2012;13:293–299. doi: 10.1089/sur.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HM, Hsu JT, Chen JC, et al. Delayed neutrophil apoptosis attenuated by melatonin in human acute pancreatitis. Pancreas. 2005;31:360–364. doi: 10.1097/01.mpa.0000180905.05494.9a. [DOI] [PubMed] [Google Scholar]
- 55.Miletic AV, Graham DB, Montgrain V, et al. Vav proteins control MyD88-dependent oxidative burst. Blood. 2007;109:3360–3368. doi: 10.1182/blood-2006-07-033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remer KA, Brcic M, Jungi TW. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol Lett. 2003;85:75–80. doi: 10.1016/s0165-2478(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 57.Mauriz JL, Collado PS, Veneroso C, et al. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Zhang S, Zhao X, et al. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1-42. J Pineal Res. 2008;45:157–165. doi: 10.1111/j.1600-079X.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 59.Xia MZ, Liang YL, Wang H, et al. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J Pineal Res. 2012;53:325–334. doi: 10.1111/j.1600-079X.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 60.Shi D, Xiao X, Wang J, et al. Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-kB, c/EBPB, and p300 signaling. J Pineal Res. 2012;53:154–165. doi: 10.1111/j.1600-079X.2012.00982.x. [DOI] [PubMed] [Google Scholar]
- 61.Kizaki T, Suzuki K, Hitomi Y, et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 2002;99:9392–9397. doi: 10.1073/pnas.142206299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emre Y, Hurtaud C, Nübel T, et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai Y, Onuma H, Bai X, et al. Persistent nuclear factor-κB activation in Ucp2–/– mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishio K, Qiao S, Yamashita H. Characterization of the differential expression of uncoupling protein 2 and ROS production in differentiated mouse macrophage-cells (Mm1) and the progenitor cells (M1) J Mol Histol. 2005;36:35–44. doi: 10.1007/s10735-004-2915-x. [DOI] [PubMed] [Google Scholar]
- 65.Alamili M, Bendtzen K, Lykkesfeldt J, et al. Melatonin suppresses markers of inflammation and oxidative damage in a human daytime endotoxemia model. J Crit Care. 2014;29:184. doi: 10.1016/j.jcrc.2013.09.006. [DOI] [PubMed] [Google Scholar]


