Abstract
Bile acid amidation defects were predicted to present with fat/fat soluble vitamin malabsorption with minimal cholestasis. We identified and treated 5 patients (1 male/4 females) from 4 families with defective bile acid amidation due to a genetically confirmed deficiency in bile acid CoA:amino acid N-acyl transferase (BAAT) with the conjugated bile acid, glycocholic acid (GCA). Fast atom bombardment-mass spectrometry analysis of urine and bile at baseline revealed predominantly unconjugated cholic acid and absence of the usual glycine and taurine conjugated primary bile acids. Treatment with 15 mg/kg GCA resulted in total duodenal bile acid concentrations of 23.3 ± 19.1 mmol/L (mean ± SD) and 63.5 ± 4.0% of the bile acids were secreted in bile in the conjugated form of which GCA represented 59.6 ± 9.3% of the total biliary bile acids. Unconjugated cholic acid continued to be present in high concentrations in bile because of partial intestinal deconjugation of orally administered GCA. Serum total bile acid concentrations did not significantly differ between pretreatment and post-treatment samples and serum contained predominantly unconjugated cholic acid. These findings confirmed efficient intestinal absorption, hepatic extraction and biliary secretion of the administered GCA. Oral tolerance tests for vitamin D2 (1000 IU vitamin D2/kg) and tocopherol (100 IU/kg tocopherol acetate) demonstrated improvement in fat-soluble vitamin absorption after GCA treatment. Growth improved in 3/3 growth-delayed prepubertal patients. Conclusions: Oral glycocholic acid therapy is safe and effective in improving growth and fat-soluble vitamin absorption in children and adolescents with inborn errors of bile acid metabolism due to amidation defects.
Introduction
Inborn errors of bile acid metabolism may present in childhood with neonatal cholestasis, fat-soluble vitamin deficiency with rickets, or hypoprothrombinemia, chronic liver disease or growth failure (1). In adulthood these metabolic defects may present with chronic liver disease or neurologic impairment. To date, 9 enzymatic defects in the bile acid synthetic pathway have been reported with characteristic pathophysiologic findings. Almost one decade before we described the first defect in bile acid conjugation, the final step in hepatic bile acid synthesis, a bile acid amidation defect was predicted to present with fat/fat soluble vitamin malabsorption with minimal cholestasis (2). In 1997 we first reported the case of a child presenting with fat-soluble vitamin deficiency caused by the excessive production of unconjugated cholic acid and a failure to conjugate primary bile acids with glycine or taurine (3). Some years later, Carlton et al reported an Amish kindred with a bile acid-CoA: amino acid N-acyltransferase (BAAT) deficiency who had increased serum bile acids with only unconjugated bile acids in serum, growth failure, coagulopathy without jaundice and any longitudinal follow-up (4). We recently, described the clinical, biochemical, molecular and morphological features of a cohort of 10 patients with fat-soluble vitamin deficiency caused by a genetic defect in the BAAT gene resulting in defective bile acid amidation (5). The aim of the current study was to evaluate the effects of therapy with a conjugated bile acid, glycocholic acid (GCA), at a dose of 15 mg/kg/day, on biliary, serum and urine bile acid composition, and on fat-soluble vitamin absorption, and growth, in 5 of these children/adolescents with BAAT deficiency. We report on the safety and efficacy of GCA treatment in patients with this bile acid conjugation defect.
Methods
Study Design
All patients were definitively identified through mass spectrometry screening of urine for inborn errors of bile acid metabolism submitted for analysis by their attending gastroenterologist (PR, SH, DS, CP) who suspected an inborn error of bile acid metabolism. Molecular analysis confirmed mutations in the BAAT gene (5). Detailed descriptions of the phenotype and genotype, including pedigrees on available families, have been previously reported as subjects 4, 6, 7, 8, and 9 (5). When a subject was identified, the gastroenterologist providing care for the patient was contacted and the study protocol discussed. A copy of the protocol and informed consent document was sent to the referring physician and the family reviewed the informed consent document. Thereafter an oral description of the study was provided to the family by telephone by the Co-Prinicipal Investigator (JEH). When parents agreed to come to Cincinnati for study, transportation was arranged and the patient(s) and a parent were brought to Cincinnati. Upon arrival at the Cincinnati Children’s Hospital Medical Center (CCHMC) Clinical and Translational Research Center (CTRC), an additional verbal description of the study was provided by the PI and written informed consent obtained.
After a complete physical examination of the patients (including heights and weights) an indwelling intravenous line was inserted for collection of blood samples and baseline laboratories including liver chemistries, prothrombin time (INR), and CBC. Baseline urine and serum was obtained for bile acid analysis by mass spectrometry. After an overnight fast, a nasoduodenal tube was passed under fluoroscopic control with topical anesthesia to the nare in which the tube was passed. Bile was collected by siphonage after intravenous administration of Kinevac® (Sincalide) (0.04 mcg/kg). On the second day, and after an overnight fast, 1,000 IU/kg vitamin D2 was given by mouth and blood collected at 0, +6, +12, +24, and +48 h as previously described (6, 7). On the 3rd day, 100 IU/kg tocopherol-acetate was administered by mouth and blood collected at 0, +6, +12, +18, and +24 h as previously described (8). After the final blood sample was collected, glycocholic acid (provide by Dr. Falk Pharma GmbH, Freiburg, Germany and administered under an FDA approved IND #50,046) 15 mg/kg/day by mouth was given and patients discharged from the CTRC with a supply of GCA. Patients were readmitted to the CTRC 5 to 12 months later for follow-up studies to assess the changes in anthropometrics, duodenal bile, serum and urinary bile acid composition, and vitamin E and D absorption using the same protocol as during the first admission. The study protocol was approved by the Institutional Review Board for Human Subjects and the Scientific Advisory Committee for the CTRC at the Cincinnati Children’s Hospital Medical Center.
Analytical Methods
Urine, duodenal bile and serum bile acids were measured by high pressure liquid chromatography-electrospray mass spectrometry (LC-ESI_MS) techniques previously described (5). Routine serum chemistries and prothrombin time (INR) were measured in the Clinical Laboratory of the Cincinnati Children’s Hospital Medical Center. Serum vitamin D2 was measured by HPLC in the laboratory of Ronald Horst (Iowa State University) (9). Serum tocopherol was measured by HPLC (10-13) in the Biochemistry Core Laboratory of the CTRC. The pharmacokinetics of vitamin D2 and tocopherol were computed from the serum concentration vs time profiles employing a non-compartmental approach using WinNonlin 4.1 (Pharsight Corporation, Cary, NC) computer software and the apparent bioavailability of each was measured as the area under the curve (AUCinf).
Data management and statistical methods
All data was stored in password-protected computers using Excel databases. All group data is expressed as mean ± SD. Since the sample size was too small to apply a paired Student’s T-test for statistical significance, we utilized the Wilcoxon signed rank exact test.
Results
Patients
The patients included 5 children/adolescents who were diagnosed with BAAT deficiency at age 5 mo-13 yrs. There were 4 females and 1 male. All patients were Caucasian with four Hispanic and 1 was Amish. Three of 5 patients (2 were adolescent females) presented with growth failure, three with cholestasis, and all with fat-soluble vitamin deficiencies.
Bile Acid Analysis
The bile acid composition of the pre-treatment urines of these patients has been described in detail previously (5) and is characterized by an absence of conjugated primary bile acids and the presence of high concentrations of unconjugated CA, apparent from the major ion m/z 407 in the FAB-MS mass spectrum. Sulphates and glucuronide conjugates of dihydroxy- and trihydroxy-C24 bile acids also feature in the mass spectra as revealed by ions at m/z 471, 567 and 583 (Fig. 1). During GCA treatment these ions are significantly reduced or absent in the urinary FAB-MS mass spectrum and the mass spectrum is unremarkable (Fig. 1).
Figure 1.
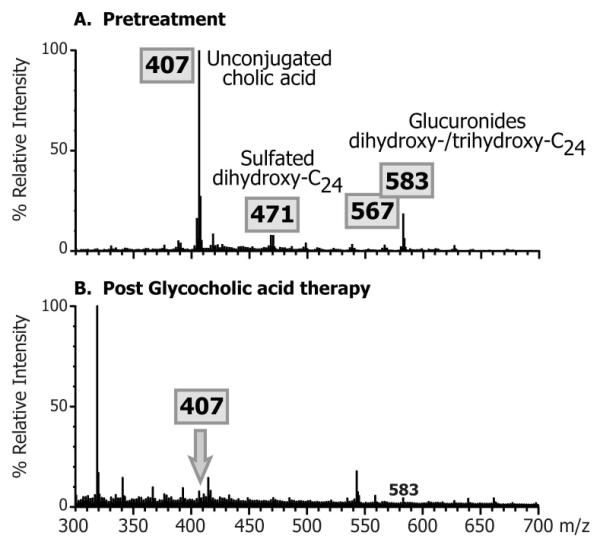
Typical negative ion fast atom bombardment ionization (FAB-MS) mass spectra comparing the urinary bile acid profiles pre-treatment and after treatment with glycocholic acid.
Biliary bile acids in duodenal aspirates measured at baseline revealed no identifiable taurine- or glycine-conjugated bile acids. Baseline mean (±SD) total biliary bile acid concentration for the 5 patients was 16.1 ± 12.5 mmol/L and only 8.4 ± 7.5% of the bile acids present were sulfate or glucuronide conjugates, and cholic acid accounted for 98.9 ± 0.7% of the bile acid species in bile (Table 2). During GCA treatment, the mass spectrum from FAB-MS analysis of the duodenal bile (Fig. 2) showed a dominant ion at m/z 464 consistent with the deprotonated molecular ion of glycine conjugated trihydroxy-cholanoic acid and consistent with the presence of GCA, thus confirming intestinal uptake, hepatic extraction and biliary secretion of the administered GCA, which became the major bile acid in bile. This was further confirmed by LC-MS analysis with selected ion recording (SIR) under negative ion mode of the deprotonated molecular ions for cholic acid (m/z 407) and glycocholic acid (m/z 464). Fig. 3 shows a typical combined SIR ion chromatograms for m/z 407 and m/z 464 for a duodenal bile aspirated from one of the patients at baseline, confirming the absence of GCA acid and the same patient after GCA therapy, which established the presence of GCA in bile during therapy. The mean total biliary bile acid concentration following GCA therapy was 23.1 ± 19.1 mmol/L and 61.1 ± 8.7% of the bile acids were secreted in the glycine conjugated form. GCA represented 59.6 ± 9.3% of the total bile acids secreted in bile. Unconjugated primary and secondary bile acids were observed in bile both at baseline and after GCA treatment (Fig. 3) but overall >96% of all bile acids were cholic acid species (Table 1).
Table 2.
Patient characteristics including age at diagnosis, duration of therapy, fat soluble vitamin levels before and after therapy and genotype
| Baseline | Follow up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age at Dx (Years) |
Age GC* started (Years) |
Duration GC (Years) |
25 OHD# (ng/ml) |
Retinol mcg/L |
Tocopherol mcg/ml |
PT/INR (secs) |
25 OHD (ng/ml) |
Retinol mcg/L |
Tocopherol mcg/ml |
PT/INR (secs) |
Nucleotide change |
| #4Δ | 1 | 2.75 | 7.6 | 35.5 | 222 | 5.0 | 12.7/0.93 | 34 | 299 | 6.7 | 12.7/0.94 | c.206A→T |
| #5 | 11 | 13 | 5.75 | 19.2 | 380 | ND | 13.0/1.07 | 24 | 339 | 1.1 | 14.1/1.04 | c.58C→T |
| #6 | 10 | 12 | 5.75 | 24.8 | 217 | 1.7 | 12.2/0.96 | 21.2 | 259 | 2.8 | 16.3/1.24 | c.58C→T |
| #8 | 0.25 | 0.8 | 7.8 | 14.5 | 589 | 7.1 | 12.0/0.84 | 34.9 | 299 | 2.2 | 12.2/0.88 | c.250C→A |
| #9 | 0.5 | 0.75 | 5 | 14.6 | 425 | ND | 13.3/0.96 | 16.3 | 314 | 3.2 | 13.9/1.05 | No mutation identified |
Glycocholic acid, serum 25 OH vitamin D (nl >20 ng/ml), Retinol (nl 200-490 mcg/L), tocopherol (nl 3.0-15.0 mcg/ml), ND= not detected,
Patient numbers correspond to those in reference 5
Figure 2.
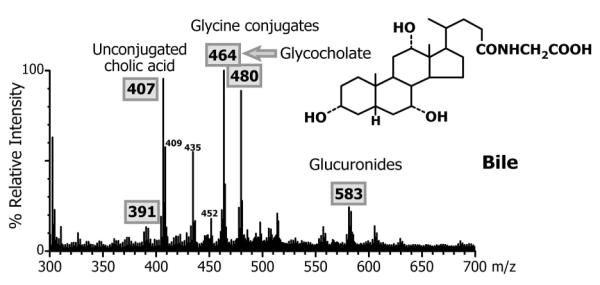
Negative ion fast atom bombardment ionization (FAB-MS) mass spectrum of the bile from a patient with BAAT deficiency treated with glycocholic acid.
Figure 3.
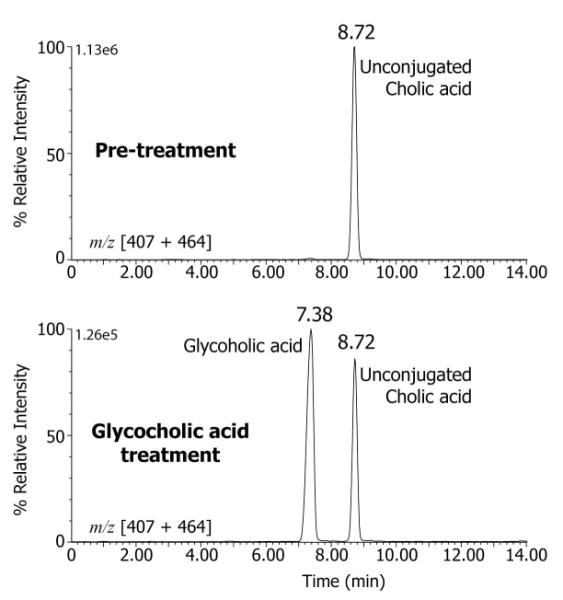
LC-MS-SIR analysis of the duodenal bile from a patient with BAAT deficiency at baseline (pre-treatment) and after treatment with glycocholic acid. Selected ion recordings are shown for the deprotonated ions m/z 407 and m/z 464 combined. Unconjugated cholic acid (retention time 8.72 min) was detected in both pre-treatment and post-treatment bile samples and glycocholic acid (retention time 7.38 min) was present only in the post-treatment sample.
Table 1.
Duodenal bile acid concentrations and composition for patients (n=5) with deficiency of bile acid-CoA;amino acid transferase (BAAT) enzyme at baseline and after treatment with glycocholic acid
| Patient* | Treatment | Total bile acid concentration (mmol/L) |
% Conjugated |
GCA (mmol/L) |
% GCA |
% CA |
% CDCA |
% DCA |
% LCA |
% UDCA |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Baseline | 22.70 | 1.5 | 0 | 0 | 99.3 | 0.6 | 0.0 | 0.1 | 0.0 |
| Post- treatment |
7.59 | 67.6 | 5.10 | 67.2 | 99.6 | 0.3 | 0.0 | 0.1 | 0.0 | |
| 5 | Baseline | 5.25 | 14.3 | 0 | 0 | 99.2 | 0.9 | 0.2 | 0.0 | 0.0 |
| Post- treatment |
32.72 | 72.2 | 23.52 | 71.0 | 99.6 | 0.2 | 0.2 | 0.0 | 0.0 | |
| 6 | Baseline | 1.38 | 17.7 | 0 | 0 | 97.8 | 2.2 | 0.0 | 0.0 | 0.0 |
| Post- treatment |
0.89 | 60.1 | 0.49 | 55.1 | 96.4 | 3.6 | 0 | 0.0 | 0.0 | |
| 8 | Baseline | 31.70 | 7.7 | 0 | 0.0 | 98.5 | 1.5 | 0.7 | 0.0 | 0.0 |
| Post- treatment |
47.93 | 54.1 | 24.59 | 51.3 | 98.7 | 0.6 | 0.7 | 0.0 | 0.0 | |
| 9 | Baseline | 19.60 | 0.8 | 0 | 0.0 | 99.7 | 0.4 | 0.0 | 0.0 | 0.0 |
| Post- treatment |
26.48 | 51.5 | 13.56 | 51.2 | 99.5 | 0.3 | 0.1 | 0.0 | 0.0 | |
| Mean ± SD |
Baseline | 16.1 ± 12.5 | 8.4±7.5 | 0 | 0 | 98.9± 0.7 |
1.1±0.7 | 0.2±0. 3 |
- | - |
| Mean ± SD |
Post- treatment |
23.1 ± 19.1 | 61.1±8.7 | 59.6 ± 9.3 |
59.2 ±9.3 |
98.7± 1.4 |
1.0±1.5 | 0.2± 0.3 |
- | - |
Patient numbers from reference #5.
Serum bile acids were measured at baseline and after GCA treatment. The pre-treatment mean (±SD) total serum bile acid concentration was 78.7 ± 88.0 μmol/L and was comprised mainly of unconjugated cholic acid (43.1 ± 52.7 μmol/L). Following GCA administration the mean (±SD) total serum bile acid concentration did not significantly change (67.4 ± 7.9 μmol/L) and consisted mostly of unconjugated cholic acid. Analysis of the serum by LC-MS/MS with collision induced transition m/z 464.4 > 73.6 for GCA and m/z 407.4 > 407.4 for unconjugated cholic acid established an absence of glycocholic acid in the pre-treatment serum and the presence of both unconjugated and GCA during treatment (Fig. 4). However, the mean serum GCA concentration was low (0.5 ± 0.3 μmol/L) and accounted for <1% of the total bile acids in serum, consistent with efficient hepatic extraction of exogenously administered GCA.
Figure 4.
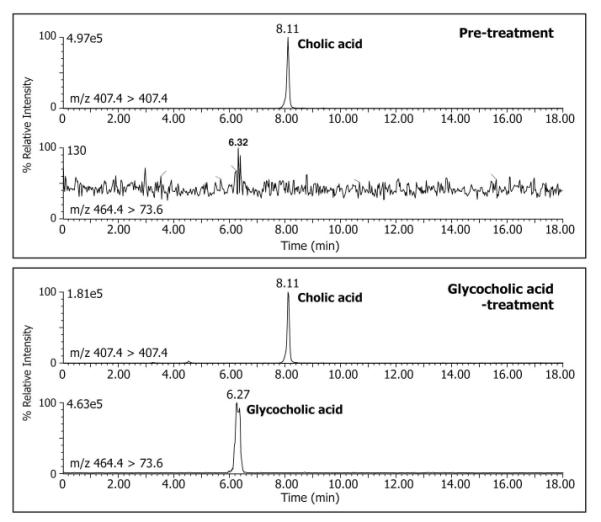
LC-MS-MRM analysis of serum from a patient with a genetic defect in bile acid amidation at baseline (pre-treatment) and after treatment with glycocholic acid. Separate negative ion MRM recordings are shown for the transitions m/z 407.4>407.4 for unconjugated cholic acid and m/z 464.4>73.6 for glycocholic acid, respectively. Unconjugated cholic acid (retention time 8.11 min) was detected in both pre-treatment and post-treatment serum samples and glycocholic acid (retention time 6.27 min) was present only in the post-treatment serum sample.
Vitamin D2 and tocopherol absorption
The pre- and post-GCA treatment absorption curves for vitamin D2 and tocopherol are shown in Fig. 5. Peak plasma concentrations (mean ± SD) attained following single oral bolus dosing of each vitamin were significantly higher for both Vitamin D2 and tocopherol with GCA treatment (vitamin D2, Cmax 17 ± 21 ng/mL at baseline vs 70 ± 17 ng/mL post-treatment (p=0.03); vitamin E, 23 ± 1 ng/mL at baseline vs 80 ± 9 ng/mL after GCA treatment, p=0.09). To assess differences in absorption the AUCinf for the plasma appearance/disappearance concentration curves was calculated using WINNONLIN after correction for baseline values. The AUCinf for plasma vitamin D2 after administration of the oral bolus of vitamin D2 of the 5 subjects was significantly lower (p=0.03) in the pre-treatment period (baseline, 795 ± 790 hr*ng/mL) when compared with responses during GCA treatment (2,538 ± 814 hr*ng/mL). The plasma tocopherol AUCinf for 5 subjects was reduced but not at a level of signficance (p=0.3) at baseline (1798 ± 424 hr*ng/mL) compared to after GCA treatment (2585 ± 257 hr*ng/mL).
Figure 5.
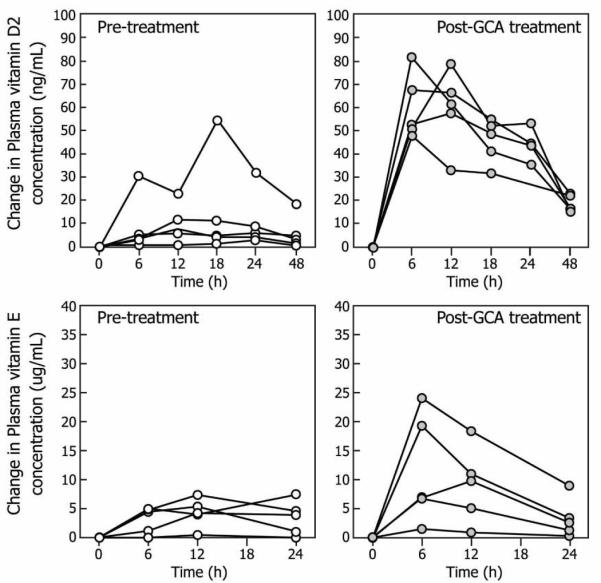
Changes from baseline in plasma vitamin D2 and tocopherol concentrations in response to a single oral bolus dose of vitamin D2 (1000 IU/kg bw) and tocopherol (100 IU/kg bw) in patients (n=5) with BAAT deficiency before and after treatment with glycocholic acid.
Growth, liver biochemstries, fat soluble vitamin levels
Anthropometric data was available on all participants at baseline and at follow up after GCA treatment. At baseline the 2 postpubertal patients had weights at the 25th% and 50th %iles, respectively. The 3 prepubertal patients had height and weight below the 5th ile and 5th % ile, at the 25th and 75th %iles(while on supplemental nasogastric tube feeding), and below the 5th %ile, which improved to the 50th and 50th %iles, 50th and 75th iles (supplemental feeding discontinued) and 50th%iles, and 25th iles and 75th iles, respectively, for each of the after GCA treatment. CDC growth charts for each of the prepubertal patients are available as a supplement to this manuscript online. All had normal or mild elevations of serum ALT and AST and normal GGT at presentation with no clear change in serum ALT, AST with GCA treatment. Baseline and follow-up fat soluble vitamins, onset and duration of treatment and genotype are presented in Table 2. Three patients had reduced serum 25 OH vitamin D levels at start of treatment with all but one normalizing with treatment. All had normal serum retinol before and after treatment. Serum tocopherol levels were reduced in 3 with normalization in 1 of 3 with one having reduced tocopherol levels after treatment. All had normal prothrombin times/INR prior to treatment and after treatment.
Compliance to long term treatment was monitored by assessing requests for additional study drug. Three months’ supply of study drug was provided to each patient and if a request for additional drug was not received when supplies should have been diminished, patients were contacted to reinforce the need for compliance and provision of additional study drug. The 3 prepubertal patients have been extremely compliant based upon these criteria while the postpubertal patients have required reinforcement to ensure adherence.
Discussion
The present report describes the effects of treatment with glycocholic acid for up to 92 months or a total of over 25 patient years in 5 children/adolescents with genetic defects in bile acid amidation. During the course of treatment, we observed an increase in the glycocholate content of duodenal bile concomitant with an improvement in fat-soluble vitamin absorption as assessed by oral tolerance testing. Furthermore, there have been no adverse events reported with the use of GCA therapy over this time period. Interestingly, we observed improved growth in the treated children who were prepubertal. All of these findings emphasize the importance of conjugated bile acids in the absorption of fat and fat-soluble vitamins on growth of the developing child with bile acid amidation defects. Although we did not directly assess fat absorption, it is presumed that improved growth and associated improvement in fat-soluble vitamin absorption could be causally linked to increasing the intraluminal conjugated bile acid concentration, which facilitated more efficient lipid absorption (5).
Patients with defective amidation secrete predominantly unconjugated bile acids in bile and the reduced fat-soluble vitamin absorption observed is explained by the rapid non-ionic passive diffusion of unconjugated bile acids from the proximal small intestine thereby reducing their intraluminal effectiveness for lipid absorption(5). The differences in critical micellar concentration (CMC) between GCA (10 mM) used to treat these patients and unconjugated CA (11 mM) is relatively small but conjugated bile acids, by virtue of their lower pKa, are ionized at intraluminal pH and therefore retained within the upper small intestine with resultant high intraluminal bile acid concentration essential for efficient lipid absorption (5). Therefore, with GCA treatment, patients with bile acid conjugation defects either due to genetic defects in the genes encoding BAAT (4, 5), as described here, or the bile acid CoA ligase (SCL27A5) enzyme reported recently (14), can be expected to show improved lipolytic product and fat-soluble vitamin absorption and improved assimilation of dietary fat and fat-soluble vitamins with attendant effects on growth. These responses were observed in our patients.
Following oral administration of GCA mass spectrometric analysis revealed duodenal bile to contain high concentrations of GCA thus confirming intestinal absorption, hepatic extraction and canalicular secretion of the exogenously administered bile acid. Concentrations of total bile acids and GCA were variable among patients reflecting the sampling differences in duodenal aspirates; however, in all patients GCA represented 54.1 - 72.2% of the total bile acids in bile and the composition of bile was >96.4% cholic acid species (Table 1). Unconjugated cholic acid continued to be secreted in bile, and in serum was the major bile acid present.
Although the affected patients did not have evidence of clinical liver disease when we treated them with GCA, their serum bile acids were markedly elevated with the major component being unconjugated cholic acid. The high serum unconjugated bile acid concentrations, which is also observed in patients with bacterial overgrowth (17), reflects the combined effects of partial intestinal hydrolysis of GCA (15, 16) adding to the endogenous synthesis of cholic acid, combined with the more efficient hepatic extraction of the administered conjugated bile acid, GCA (17). Hepatic extraction of bile acids is also dependent upon the extent of protein binding and structure, with more polar bile acids being more efficiently taken up by the hepatocytes (18).
With effective treatment with GCA, we observed a marked improvement in absorption of vitamin D2 and tocopherol as assessed by the oral vitamin D2 and tocopherol tolerance tests (Fig. 3). The observed improvement in the serum appearance of vitamin D2 and tocopherol was quantitatively less than that previously observed in normal children (6, 8) but vitamin D2 was significantly improved over pre-treatment status and there was a trend toward improvement in tocopherol levels with treatment. The failure to demonstrate significant improvement in tocopherol most likely relates to the small sample size. From a biologic standpoint, the improvement in tocopherol levels with a challenge after treatment is significant. It is likely that with GCA treatment the intraluminal concentration of conjugated bile acids is still less than that observed in normal children and the micellar solubilisation of fat-soluble vitamins is likely quantitatively less than controls. Since patients with BAAT deficiency are dependent upon exogenous conjugated bile acids to ensure adequate intraluminal concentrations, it is likely that deconjugation by ileal and colonic bacterial hydrolases (16) compromises the ability to maintain higher intraluminal concentrations. Although we empirically treated our patients with 15 mg/kg/day of GCA, as we have previously dosed patients with other inborn errors of bile acid metabolism with cholic acid (1, 19), it is possible that higher exogenous doses of GCA may attain intraluminal conjugated bile acid concentrations similar to controls. Because of the high pill burden associated with increased dosing, we have not adjusted dosing based upon our findings. The improved growth in the prepubertal children and the improvement in fat-soluble vitamin absorption suggest that the dose is likely adequate to support normal growth and development in affected children. If patients had not had biochemical responses (normalization of fat-soluble vitamin levels or improved growth), increasing the dose might have been appropriate.
Our findings indicate that GCA is efficacious in improving the intraluminal milieu to encourage improved fat-soluble vitamin absorption and enhanced growth in prepubertal children. GCA appears safe in this limited experience of over 30 patient years of treatment. Based upon our limited experience, we believe that GCA should be the standard of care and supplemental fat soluble vitamins should be the standard of care for affected patients. Clinicians should be vigilant to potential patients who present with neonatal cholestasis, growth failure or fat-soluble vitamin deficiencies (5) since glycocholic acid treatment for these patients offers clear therapeutic benefit without adverse effects.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Falk Pharma for generously providing glycocholic acid to the patients, their patients and families, the staff or the CTRC and Mekibib Ataye for his assistance with statistical analysis. This study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077-05 and a pilot project grant from the Cholestatic Liver Consortium CLiC NIH U54019455 and U01 DK62497. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Setchell KDR, Heubi JE. Defects in bile acid biosynthesis--diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S17–22. doi: 10.1097/01.mpg.0000226386.79483.7b. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann AF, Strandvik B. Defective bile acid amidation: predicted features of a new inborn error of metabolism. Lancet. 1988;2:311–313. doi: 10.1016/s0140-6736(88)92359-8. [DOI] [PubMed] [Google Scholar]
- 3.Setchell KDR, Heubi JE, O’Connell NC, Hofmann AF, Lavine JE. Identification of a unique inborn error in bile acid conjugation involving a deficiency in amidation. In: Paumgartner G, Stiehl A, Gerok W, editors. Bile Acids in Hepatobiliary Diseases: Basic Research and Clinical Application. Kluwer Academic Publishers; Dordrecht/Boston/London: 1997. pp. 43–47. [Google Scholar]
- 4.Carlton VEH, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nature Genetics. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 5.Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, Potter C, et al. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. 2013;144:945–955. e946–945. doi: 10.1053/j.gastro.2013.02.004. quiz e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heubi JE, Hollis BW, Specker B, Tsang RC. Bone disease in chronic childhood cholestasis. I. Vitamin D absorption and metabolism. Hepatology. 1989;9:258–264. doi: 10.1002/hep.1840090216. [DOI] [PubMed] [Google Scholar]
- 7.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42:644–649. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 8.Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983;85:1172–1182. [PubMed] [Google Scholar]
- 9.Horst RL, Hollis BW. Vitamin D assays and their clinical utility. In: Holick MF, editor. Vitamin D: Physiology, Molecular Biology and Clinical Applications (Nutrition and Health) 1st ed Humana Press; 1999. pp. 239–272. [Google Scholar]
- 10.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 11.Bortolotti A, Lucchini G, Barzago MM, Stellari F, Bonati M. Simultaneous determination of retinol, alpha-tocopherol and retinyl palmitate in plasma of premature newborns by reversed-phase high-performance liquid chromatography. J Chromatogr. 1993;617:313–317. doi: 10.1016/0378-4347(93)80505-x. [DOI] [PubMed] [Google Scholar]
- 12.Catignani GL, Bieri JG. Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983;29:708–712. [PubMed] [Google Scholar]
- 13.Lee BL, Chua SC, Ong HY, Ong CN. High-performance liquid chromatographic method for routine determination of vitamins A and E and beta-carotene in plasma. J Chromatogr. 1992;581:41–47. doi: 10.1016/0378-4347(92)80445-v. [DOI] [PubMed] [Google Scholar]
- 14.Chong CP, Mills PB, McClean P, Gissen P, Bruce C, Stahlschmidt J, Knisely AS, et al. Bile acid-CoA ligase deficiency - a new inborn error of bile acid metabolism. J Inherit Metab Dis. 2011;35:521–530. doi: 10.1007/s10545-011-9416-3. [DOI] [PubMed] [Google Scholar]
- 15.Hepner GW, Hofmann AF, Thomas PJ. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. II. Glycine-conjugated dihydroxy bile acids. J Clin Invest. 1972;51:1898–1905. doi: 10.1172/JCI106992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepner GW, Hofmann AF, Thomas PJ. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. I. Cholylglycine. J Clin Invest. 1972;51:1889–1897. doi: 10.1172/JCI106991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldini R, Roda A, Labate AM, Cappelleri G, Roda E, Barbara L. Hepatic bile acid uptake: effect of conjugation, hydroxyl and keto groups, and albumin binding. J Lipid Res. 1982;23:1167–1173. [PubMed] [Google Scholar]
- 18.Roda A, Cappelleri G, Aldini R, Roda E, Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res. 1982;23:490–495. [PubMed] [Google Scholar]
- 19.Gonzales E, Gerhardt MF, Fabre M, Setchell KD, Davit-Spraul A, Vincent I, Heubi JE, et al. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137:1310–1320. e1311–1313. doi: 10.1053/j.gastro.2009.07.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


