Abstract
Scientific appreciation for the subtlety of brain sulfur chemistry has lagged, despite understanding that the brain must maintain high glutathione (GSH) to protect against oxidative stress in tissue that has both a high rate of oxidative respiration and a high content of oxidation-prone polyunsaturated fatty acids. In fact, the brain was long thought to lack a complete transsulfuration pathway (TSP) for cysteine synthesis. It is now clear that not only does the brain possess a functional TSP, but brain TSP enzymes catalyze a rich array of alternative reactions that generate novel species including the gasotransmitter hydrogen sulfide (H2S) and the atypical amino acid lanthionine (Lan). Moreover, TSP intermediates can be converted to unusual cyclic ketimines via transamination. Cell-penetrating derivatives of one such compound, lanthionine ketimine (LK), have potent anti-oxidant, neuroprotective, neurotrophic and anti-neuroinflammatory actions and mitigate diverse neurodegenerative conditions in preclinical rodent models. This review will explore the source and function of alternative TSP products, and lanthionine-derived metabolites in particular. The known biological origins of lanthionine and its ketimine metabolite will be described in detail and placed in context with recent discoveries of a GSH- and LK-binding brain protein called LanCL1 that is proving essential for neuronal antioxidant defense; and a related LanCL2 homolog now implicated in immune sensing and cell fate determinations. The review will explore possible endogenous functions of lanthionine metabolites and will discuss the therapeutic potential of lanthionine ketimine derivatives for mitigating diverse neurological conditions including Alzheimer’s disease, stroke, motor neuron disease and glioma.
Keywords: Transsulfuration, lanthionine, LanCL1, LanCL2, CRMP2, free radical, glutathione, hydrogen sulfide, neuroinflammation, autophagy
Introduction
The mammalian transsulfuration pathway (TSP) is a well-known conduit for salvaging reduced sulfur from the folate cycle and incorporating it into cysteine, an essential precursor for the ubiquitous intracellular reducing agent, glutathione [1]. As such, the TSP has been long appreciated for its importance in redox biochemistry and antioxidant defense. In recent years the TSP has begun to receive renewed research interest because TSP enzymes have been discovered to exhibit promiscuous activities that result in the generation of the antioxidant and gasotransmitter, hydrogen sulfide (H2S) [2–4] as well as lanthionine metabolites that have their own inherent neuroprotective and neurotrophic properties [5]. Moreover, TSP products have been associated with improved longevity in species ranging from C. elegans [6] to Drosophila [7] to long-lived Dwarf mice [8]. TSP activity is required for the longevity-promoting effects of dietary restriction in Drosophila [7], and loss of activity can accelerate senescence of human cells [9]. In the aging human brain, TSP might be particularly vulnerable due to the relatively high brain demand for reduced sulfur combined with low inherent activity of neural TSP enzymes and chronic deficiency of B-complex vitamins essential to proper functioning of the folate cycle and TSP [10]. Indeed, dementia and Alzheimer’s disease risk increase strongly with high circulating homocysteine (hCys) – a marker for either impairment of the folate cycle or insufficient sulfur flux through the TSP [10–12].
The purpose of this review is to highlight aspects of TSP function that are typically omitted from basic biochemistry texts but which are now becoming appreciated for their potential biomedical importance. The review will emphasize alternative functions of TSP enzymes, with particular attention to TSP-mediated production of H2S and lanthionine. Emerging importance of the lanthionine ketimine metabolite will be presented in relation to its possible natural roles and its therapeutic potential in neurodegenerative disease. New hypotheses will be proposed for mechanisms underlying hCys association with age-related vascular and cognitive impairment. Gaps in our current knowledge concerning mechanisms of redox TSP regulation and switching between classical vs. alternative TSP functions will be indicated along with suggested directions for future research.
The classic transsulfuration pathway: A means to decrease neurotoxic homocysteine concentration while salvaging sulfur to maintain adequate glutathione concentration and redox buffering capacity
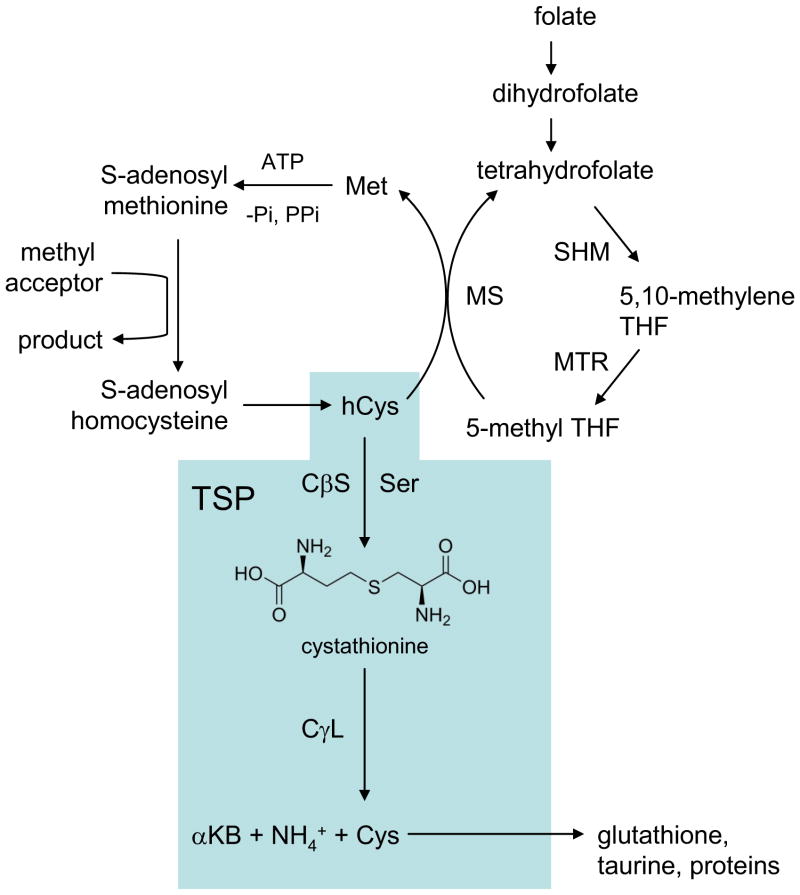
In mammalian biochemistry, the classic transsulfuration pathway (TSP; Fig. 1) is a well-known metabolic conduit that accepts homocysteine (hCys), a side product from the folate cycle, for the regeneration of useful cysteine [1,13]. Sometimes the mammalian TSP is referred to as the reverse TSP, to contrast with the situation in yeast and bacteria wherein cysteine is a precursor for conversion to methionine [14]. For simplicity, the mammalian pathway from hCys to Cys will be referred to herein as the TSP or classic TSP.
Figure 1. The classic transsulfuration pathway (TSP) in relationship to the folate cycle and the remethylation pathway.
The classic TSP whereby homocysteine (hCys) is converted to cysteine (Cys) is shaded.
The TSP serves two important purposes. First, it consumes hCys which is not proteogenic and is, in fact, a neurotoxin that allosterically activates the NMDA receptor leading to exacerbated glutamatergic signaling and downstream oxidative stress [15–16]. Indeed, elevated hCys is a well-known risk factor for both dementia and Parkinsonism [17]. Second, the TSP regenerates useful cysteine which can be incorporated into proteins or the ubiquitous cellular reductant, glutathione (GSH). In the mammalian brain, most cysteine derives from reduction of cystine imported by the Xc− cystine-glutamate exchanger but a significant amount of astrocytic cysteine is generated through TSP [18–19].
The classic TSP consists of two enzymatic steps, each catalyzed by a different pyridoxal 5′-phosphate (PLP, or vitamin B6)-dependent enzyme. In the first committed step, cystathionine-β-synthase (CβS; L-serine hydro-lyase; EC 4.2.1.22), catalyzes a beta-replacement reaction whereby the hydroxyl group of L-serine (Ser) is displaced by L-homocysteine (hCys) to yield cystathionine (Ser + hCys → cystathionine; Fig. 1) [1,4]. Amongst the PLP enzymes, eukaryotic CβS is unique in that it possesses a heme cofactor which is likely important in redox regulation [20–21]. Reduction of CβS to the ferrous state causes a 50% loss of activity that can be reversed by oxidation with ferricyanide, confirming the redox-sensitive regulation of this enzyme [22]. Reaction of the heme with endogenous CO likely occurs, and regulates the classic function of CβS [23] but the implications to variant CβS enzymologies such as those leading to lanthionine formation have not been explored.
Cystathionine serves as substrate for a second PLP-catalyzed reaction catalyzed by cystathionine-γ-lyase (CγL; L-cystathionine cysteine-lyase; CSE; EC 4.4.1.1). CγL catalyzes a β,γ-elimination replacement reaction unique amongst PLP-dependent enzymes, ultimately consuming cystathionine to yield cysteine (Cys), α-ketobutyrate, and ammonia (Fig. 1) [1,13,24].
The existence of a complete brain TSP was long doubted because brain CγL is very low and brain cystathionine is present at higher concentrations than in other organs [18,25]. Nonetheless, Bannerjee and colleagues have clearly demonstrated the presence of a functional TSP in human neurons and astrocytes and mouse brain organotypic preparations by incubating the cells with [35S] methionine and measuring radiolabeled glutathione [18]. Not only was glutathione synthesis sensitive to blockade at the CγL junction in these studies, but 40% of glutathione was depleted within 10h by CγL inhibition and this correlated with reduced cell viability under oxidative stress [18].
The classic TSP is known to be regulated at several levels. First, the committed step in TSP is regulated by substrate competition and allosteric feedback. Homocysteine also serves as substrate for transmethylation (remethylation) whereby hCys is converted back to methionine (Fig. 1). Methionine is convertible to the high-energy methyl donor, S-adenosylmethionine (SAM) which is an allosteric activator of CβS capable of stimulating activity by approximately three-fold [1,19]. Thus when Met concentrations are high, excess sulfur is channeled towards transsulfuration [19]. Second, Cys is a competitive inhibitor of CγL which may prevent accumulation of Cys to concentrations that would autoxidize to give excess cysteine [19,26]. Third, CβS and CγL are regulated at the expression level in various tissues though in vivo expression regulation in brain has been less well studied [26–27]. CβS expression can be activated by glucocorticoids and inhibited by insulin in liver [28–29] and in astrocytes, the inflammatory cytokine TNFα as well as epidermal growth factor (EGF) and cAMP all upregulate CβS [30]. In isolated glioma cells and primary astrocytes, CγL is reported to increase following depletion of GSH by diethylmaleate, and other studies have found that oxidative stress imposed by treatment with tert-butylhydroperoxide stimulates increased GSH synthesis via CγL upregulation [18–19,31]. These findings lead McBean to hypothesize that the astrocytic TSP may function in a reserve capacity that can be activated when Cys supply by the Xc− system is limited or when oxidative stress is imposed [19]. Post-translational regulation of CβS and CγL cannot be excluded but has not been well-studied in the brain, with the exception that CβS is documented to undergo some degree of modification by sumoylation both in SH-SY5Y neuroblastoma cells and in vivo in brain resulting in nuclear localization [32].
Redox regulation of the TSP and regulation by binding other proteins, specifically lanthionine synthase-like protein-1 (LanCL1), represent important themes that are emerging from study of TSP function in the brain and these will be discussed separately below.
Catalytic promiscuity of cystathionine β-synthase and cystathionine-γ-lyase: Imprecise enzymes or versatile mediators of alternative metabolic pathways that yield purposeful products?
PLP-dependent enzymes are well-known for their tendency to engage in promiscuous reaction chemistries. These enzymes can catalyze a diverse set of reactions including transamination, racemization, decarboxylation, elimination and replacements. Often the same enzyme can perform more than one class of reaction with alternative substrates [13]. The PLP-dependent TSP enzymes, CβS and CγL, are no exceptions [2,33]. For instance, in addition to the β– and γ–elimination reactions catalyzed by CβS and CγL described above, these two enzymes generate the important gasotransmitter hydrogen sulfide (H2S) by several mechanisms (Fig. 2) [3–4,24]. Chiefly, CβS generates H2S by β-replacement of cysteine by homocysteine [4]:
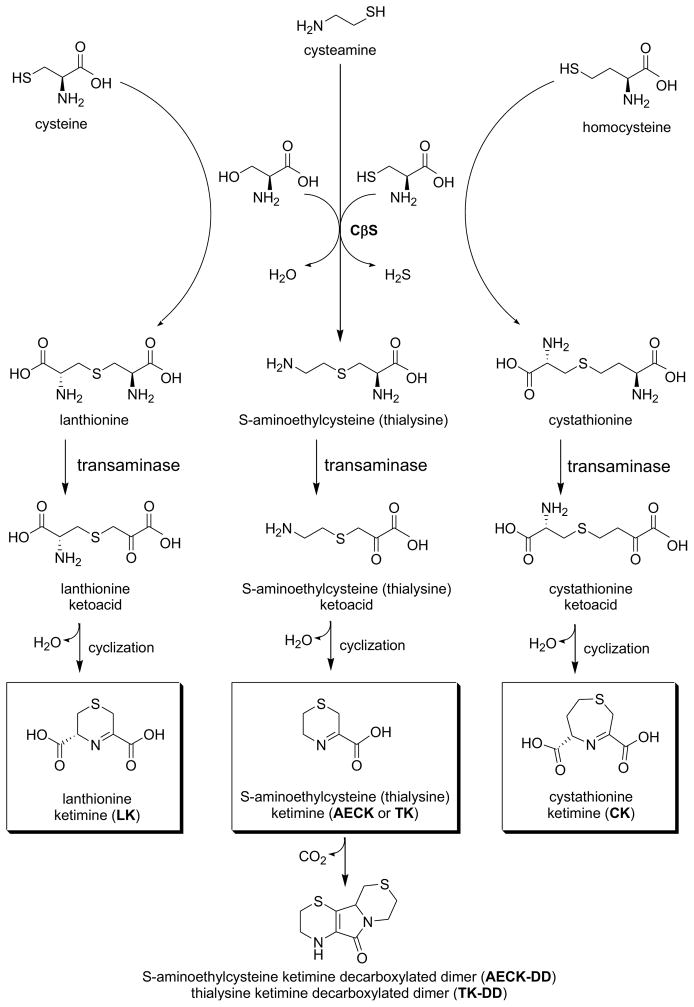
Figure 2. Formation of cyclic thioether ketimines from cystathionine and alternative transsulfuration products.
Lanthionine, thialysine and cystathionine formed via CβS undergo transamination to yield intermediate linear ketoacid amines. Cyclization of these species yields a family of cyclic thioether ketimines. Both the ketimines and decarboxylated dimers formed from their bimolecular oxidative breakdown are present in tissue and have appreciative antioxidant potential in vitro. Cell penetrating derivatives of lanthionine ketimine are becoming appreciated for their antioxidant, neuroprotective and neurotrophic properties and therapeutic potential. Note that the ketimines depicted here exist as tautomers with their corresponding enamines.
β-elimination (Cys → Ser) and condensation (2 Cys→ H2S + lanthionine) are quantitatively less significant [4]. CγL also can generate H2S from alternative reactions (Fig. 2) [24]:
Under normophysiological hCys concentrations, CβS is estimated to generate up to 70% of total H2S production through the TSP [24]. However, mathematical models suggest that under conditions of hyperhomocysteinemia, CγL may be even more important to H2S generation than CβS [24]. H2S also may be produced endogenously from the 3-mercaptopyruvate sulfotransferase (MST) catalyzed desulfuration of mercaptopyruvate (itself formed by the cysteine/aspartate-aminotransferase catalyzed reaction of cysteine and α-ketoglutarate) [3]. In this case, an MST-bound persulfide is formed which, in the presence of ambient reducing equivalents (particularly thioredoxin/thioredoxin reductase), releases H2S [3]. However, this is likely a very minor contribution to total H2S production relative to that of the TSP [3].
The emerging importance of H2S to redox biochemistry and pathophysiology is being treated elsewhere in excellent primary research and comprehensive reviews [3,34–35]. Suffice it to say that H2S is becoming widely appreciated for its natural roles in regulating critical biochemical pathways through reacting with oxidants, metals and thiol centers resulting in protein sulfhydration (formation of protein-S-SH adducts). Physiological ramifications include antioxidant effects, both direct and indirect; altered vasoactivity; neuromodulation through facilitation of hippocampal long-term potentiation (LTP); allosteric regulation of N-methyl D-aspartate (NMDA) receptors and their glutamatergic pathways; and induction of Ca2+ influx in astrocytes with significant downstream consequences [36]. In fairness, there are some valid arguments being put forth by Toohey and Cooper [37] that many biological roles now being ascribed to H2S based on in vitro H2S-generating systems are actually performed by sulfane sulfur (So). Further research to clarify this contention is warranted.
Given the presence of the unique heme center in CβS, a natural question arises as to whether H2S reaction with this prosthetic group affects CβS enzymology? Carballal et al. recently addressed this question and did not find evidence of an effect of exogenous H2S on classical CβS activity or heme binding [23], but a comprehensive study of this heme’s influence on alternative CβS chemistries (i.e. catalysis of reactions other than the classic conversion of Ser + hCys → cystathionine) has not yet been performed nor has the question been addressed under completely physiological conditions where other CβS-binding proteins such as LanCL1 (discussed below) could be important.
This brief consideration of H2S generation by TSP enzymes also makes clear that unusual amino acid products may also form through the normal, if underappreciated, biochemistries of CβS and CγL. As discussed above, free lanthionine (Lan) and homolanthionine (hLan) are byproducts of CγL-mediated H2S generation (Fig. 2) [4,24]. Additionally, free lanthionine can form as a product of alternative β-elimination wherein CβS catalyzes Cys (rather than hCys) reaction with Ser, in a reaction that would not generate H2S [24,38]:
Therefore free lanthionine and homolanthionine as well as cystathionine can be generated both through reaction pathways that generate H2S and other pathways that do not generate H2S, at least in vitro and in broken tissue preparations. Protein-bound lanthionine can be generated as a post-translational modification (PTM) by a variety of chemistries including dehydration of phospho-amino acid residues or aminoacrylate addition to protein-SH [39] but these topics will not be treated in this review which focuses on small molecule metabolites rather than protein PTMs.
The degree to which these alternative sulfur amino acid products of Lan and hLan form in vivo remains to be fully elucidated. Likewise, the possible biochemical functions of these thioether amino acids are by no means settled. Like cystathionine, Lan can act as a substrate for CγL at least in vitro [4]. However, these thioethers may be routed out of the TSP and used as substrates by aminotransferases usually associated with the kynurenine pathway [5,40–43]. It is with the alternative TSP products, lanthionine and cystathionine, and their downstream metabolites that the remainder of this review will be concerned.
Cystathionine and alternative TSP products can yield cyclic thioether ketimines through transamination
In the 1980–1990s Dorianno Cavallini and colleagues reported that lanthionine (Lan) exists in mammalian brain at concentrations similar to that of folate cycle intermediates, and his group began a series of basic science projects aimed at uncovering unappreciated roles for this unusual thioether [40–46]. Cavallini and his contemporaries determined that lanthionine and cystathionine are viable substrates for transaminase enzymes. To date, the main transaminase invoked for LK synthesis is kynurenine aminotransferase (KAT; now known to be synonymous with glutamine transaminase-K (GTK) and cysteine conjugate β-lyase) [43] which usually is discussed in reference to tryptophan biochemistry [47–49]. Consistent with the theme of substrate flexibility inherent to PLP enzymes, GTK catalyzes transamination between a large number of amino acids and α-keto acids with the highest Vmax/Km value for glutamine but will also accept large neutral, aromatic or sulfur amino acids and kynurenine [42–43]. As a caveat it should be noted that lanthionine is actually a rather poor GTK substrate relative to glutamine, which is present at mM concentrations in brain. Moreover, Cooper and colleagues have shown that a large number of PLP enzyme (aminotransferases) can catalyze cysteine-β-lyase reactions [50], so further research may reveal other transaminase activities to be quantitatively more important for lanthionine metabolism. In any event, the product of lanthionine transamination is a linear intermediate that spontaneously cyclizes to form lanthionine ketimine [LK; Figs. 2–3] [42–43]. Similar chemistry can be catalyzed by amino acid oxidase (AAO); however the transamination pathway probably predominates in the brain based on the relative levels of transaminase activity vs. AAO activity [43]. Cavallini and colleagues noted that besides lanthionine, cystathionine and other sulfurous amino acids (e.g. thialysine, aminoethylcysteine) could serve as substrates for GTK-mediated transaminations leading to an entire family of structurally-related compounds [41–46] (Fig. 2).
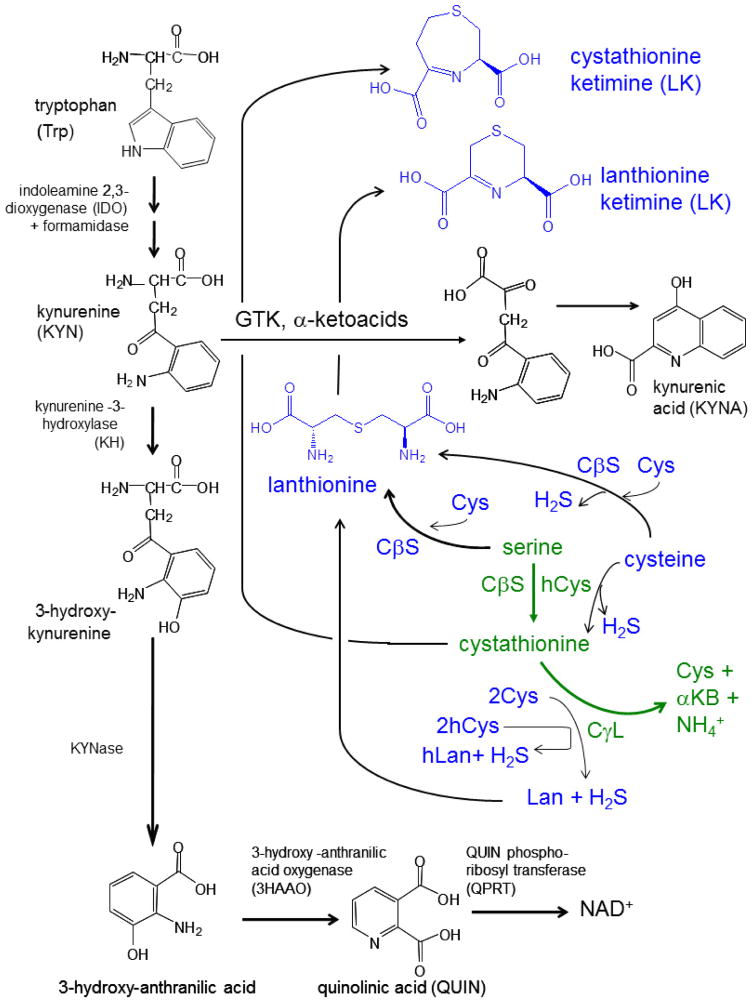
Figure 3. Summary diagram illustrating both classic and alternative chemistries catalyzed by TSP enzymes, and the possible relationship of their amino acid products to the kynurenine pathway of tryptophan catabolism.
The classic transsulfuration pathway (TSP; shown in green; see Fig. 1 also) is juxtaposed with “alternative” or variant reactions (blue) that are catalyzed by the same core enzymes, cystathionine β-synthase (CβS) and cystathionine-γ-lyase (CγL). Note that the alternative reactions utilize the same serine, cysteine and homocysteine substrates as the classic TSP reactions, but in different combinations. Products of these alternative TSP enzyme chemistries include the gasotransmitter, H2S as well as unusual thioethers, lanthionine and homolanthionine. Cystathionine, lanthionine and thialysine are amongst a set of brain sulfur amino acids that can be further converted to cyclic thioether ketimines by transaminase activities including (but not necessarily limited to) that of glutamine transaminase K (GTK; previously known as kynurenine amino transferase-1). GTK is a key enzyme in the kynurenine pathway of trypophan metabolism (black). The kynurenine pathway is necessary in the generation of NAD+ but when hyper-activated during neuroinflammation, can generate the excitotoxin, quinolinic acid (QUIN). Alternatively, diversion of kynurenine through GTK results in kynurenic acid (KYNA), a neuroprotectant and the only known endogenous NMDA receptor-acting endogenous anti-excitotoxin. Note that both the classic TSP intermediate cystathionine and the alternative product, lanthionine, can undergo transaminase-mediated conversion to cyclic thioether ketimines. A number of antioxidant, neuroprotective, neurotrophic, and therapeutic activities recently have been documented for brain-penetrating derivatives of lanthionine ketimine (Table 1).
To date, very little research attention has been focused on potential biological roles for these unusual thioethers. Using HPLC and gas-liquid chromatography, Cavallini et al. measured LK in mammalian brain at concentrations near 1 nmole/g tissue, similar to that of hCys and 10–20% the concentration of cystathionine [44]. Recognizing that the brain is very parsimonious with respect to conservation of reduced sulfur, Cavallini thought it highly unlikely that evolution would have failed to close such a wasteful sulfur “leak” unless lanthionine served a useful biochemical function. Despite significant research effort Cavallini and coworkers never discovered specific bioactivities for LK, however they did report that [35S]LK bound synaptosomal membranes tightly (Kd = 58 nM, in the range of typical neurotransmitter affinities) [45]. [35S]LK binding was saturable and reversible with cold LK, suggesting a receptor: ligand interaction [45]. The binding was specific because reduced LK (thiomorpholine dicarboxylate) could not displace [35S]LK [45]. However, despite this progress, these early researchers failed to document specific neurochemical activities inherent to LK or related cyclic thioether ketimines.
Cyclic thioether ketimines do themselves possess modest antioxidant activity, as one might expect from the nature of their thioether moiety, but certain of these (particularly aminoethylcysteine ketimine or AECK) can undergo spontaneous oxidative decarboxylation and dimerization to produce “decarboxylated dimers” that have greater antioxidant potential in vitro and in cell cultures [51–53] (Fig. 2). Evidence has been presented for the presence of aminoethylcysteine ketimine decarboxylated dimer (AECK-DD) in plasma and urine, but the extent to which AECK-DD forms through bimolecular reactions in their tissue of synthesis, and the biological relevance of the decarboxylated dimers to brain antioxidant balance, is a subject of some controversy [53].
GTK/KAT function in synthesis of the endogenous neuroprotectant, kynurenic acid, may suggest analogous neuroprotective roles for thioether ketimines
GTK/KAT activity is usually discussed in reference to tryptophan biochemistry. Normally, tryptophan is oxidized to kynurenine (Kyn) en route to formation of nicotinamide adenine dinucleotide (NAD+) (Fig. 3) [1,47–48]. These reactions occur within the mitochondrial matrix [47–49]. GTK / KAT catalyzes a shunt in which kynurenine reacts with the α-ketoacid, pyruvate, to form kynurenic acid (KYNA) (Fig. 3). KYNA is not a waste product, but binds to the glycine-b site of NMDA receptors thus acting as an anti-excitotoxin [54]. In fact, KYNA is the only widely recognized, endogenous anti-excitotoxin [55]. KYNA likely is neuroprotective in vivo because manipulation of endogenous KYNA supply by targeted deletion of the brain-specific form, renders rodents hypersensitive to intrastriatal injections of the excitotoxic NMDA receptor agonist quinolinate (QUIN; see Fig. 3), a classic model of HD [55]. Alterations in KYNA and QUIN also have been clinically implicated in diverse diseases including Huntington’s disease (HD) Alzheimer’s disease (AD); Parkinson’s disease (PD); and multiple sclerosis (MS) [reviewed in 56]. Accordingly, kynurenine pathway metabolites and derivatives, along with synthetic modulators of kynurenine pathway enzymes, are being researched aggressively in efforts to develop novel therapies for neurodegenerative diseases [56–57].
In 2004–2005 we began to hypothesize that LK and similar compounds caused by the flux of TSP thioethers through GTK / KAT might produce products with neuro-active functions, analogous to those of KYNA (Fig. 2). If true, such a hypothesis would point to an unexpected but functional metabolic linkage between the TSP pathway of sulfur amino acid metabolism and the kynurenine pathway of tryptophan metabolism. Even if LK synthesis eventually is found to rely on other transaminases, the emerging similarity between KYNA and neuroprotective functions (discussed below) is striking and may reflect a general, heretofore unappreciated importance of transaminase activities in maintaining brain health.
Cell and tissue-penetrating derivatives of lanthionine ketimine have potent antioxidant, neurotrophic and anti-neuroinflammatory activities and demonstrate preclinical activity in preclinical models of neurodegeneration or glioma
In order to test our hypothesis for neurochemical functionality of brain thioether ketimines, we synthesized compounds including LK and also novel ester and amide derivatives that were much more able to permeate cell membranes and mammalian tissues. This research has led to the documentation of potent neuro-activities for LK derivatives. Our first indication of lanthionine ketimine activity was the observation that it inhibited nitric oxide (•NO) production by cytokine-stimulated EOC-20 microglia with approximately the same potency as the classic microglial pacifier, minocycline [58]. Esterification increased potency, probably due to increased cell-penetrating ability [58].
Our second indication of lanthionine ketimine activity stemmed from observation of NSC-34 motor neuron-like cells when these cells were exposed to medium conditioned by cytokine-stimulated EOC-20 cells. Medium conditioned by M1-activated microglia cause axon retraction and cell death in neurons, but LK-ester (LKE) prevented this process. Moreover, upon microscopic observation, it became apparent that LK or LKE alone was able to promote neurite extension in NSC-34 cells as these differentiated toward a motor neuron-like phenotype in cell culture [58]. We have built on this observation in subsequent studies of NSC-34 cells and also primary neurons including chick dorsal root ganglial neurons [59]. In the latter cell type, LKE promotes neurite extension at nanomolar concentrations [59; Fig. 4; Table 1], approaching the physiological concentration reported for brain LK [44] and near the reported Kd of [35S]LK binding to neural membranes [45]. LK and LKE also were found to be capable antioxidants that protect primary neurons and neuronal cell lines from hydrogen peroxide (H2O2) and tert-butyl-hydroperoxide, probably by direct reaction of the sulfur with the oxidant (Table 1) [58,60].
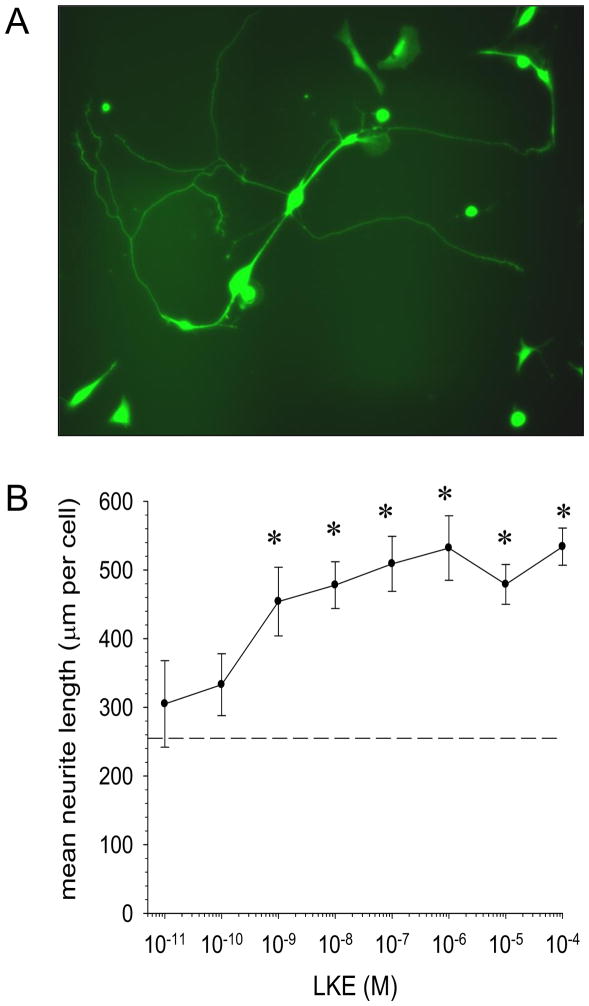
Figure 4. LKE is neurotrophic at nanomolar concentrations in chick dorsal root ganglia (DRG) cultures.
A: Typical chick DRG neurons at 4 days in vitro (DIV4), showing ample neuritic arborization (arrows). Cells were cultured, labeled with Promega Live/Dead® reagent and imaged as described previously [59]. Data in this figure represent updated data with inclusion of additional experiments performed since the initial report [59]. Neurites (arrows) were measured by a blinded analyst using MetaMorph® software (Molecular Devices). B: DIV3 neurons were treated 48h with vehicle or LKE, photomicrographed, and quantitatively assessed for morphometry by an analyst blinded to treatment group. Data indicate mean ± SEM for 10–30 microscopic fields at each LKE concentration. Control neurite length (no drug) was 239 ± 23 mm (dashed line). *p<0.05 by t-test.
Table 1.
Biological properties, bioactivities, and effects in animal models documented for lanthionine ketimine and its ethyl ester (LKE).
| Activity | Reference |
|---|---|
| bound CRMP2 (DPYSL2) | 58 |
| bound syntaxin binding protein 1 (STXBP1) | 58 |
| bound LanCL1 | 58 |
| bound synaptosomes (saturable, nM affinity) | 45 |
| antioxidant | 58, 60 |
| anti-neuroinflammatory (microglia) | 58, 60 |
| anti-proliferative | 64 |
| neuroprotective | 58, 60 |
| neurotrophic (neurite promoting) activity | 58–59, 62, this work |
| suppressed amyloid peptide production | 63 |
The potency of LKE and its ability to act on both microglia and neurons intrigued us enough to begin exploring possible mechanisms-of-action (MOA). Accordingly, LK was attached to a solid-phase support which was used to affinity-enrich LK-binding brain proteins [58]. Three proteins bound to the column in a fashion that was reversible by competition with free LK and which yielded sufficient protein for accurate mass spectrometry-assisted sequencing. These were: Lanthionine synthase-like protein-2 (LanCL1); collapsin response-mediator protein-2 (CRMP2; also known as dyhydropyrimidinase-like protein-2 / DPYSL2 / DRP2); and syntaxin binding protein-1 (STXBP1) [58]. The binding of LK to LanCL1 was an exciting but perhaps not entirely unexpected finding, because we had previously identified LanCL1 as a novel glutathione-binding protein with primary and tertiary homology to prokaryotic lanthionine cyclases [discussed in detail, below]. However, the finding that synthetic LK could selectively associate with LanCL1 in a matrix containing thousands of other brain proteins, strongly suggests that the eukaryotic LanCL1 is related to small-molecule lanthionine metabolites in a non-trivial fashion. The meaning and possible physiological purpose of both glutathione and LK binding to LanCL1 remain mysteries.
The second LK-binding protein, CRMP2, immediately suggested one plausible explanation for the neurotrophic (neurite growth-promoting) effects of LK and LKE. CRMP2 was originally identified as a microtubule-binding protein responsible for effecting axon growth and retraction in the developing CNS [reviewed in 59,61]. Increasing CRMP2 expression promotes neurite outgrowth whereas interference with CRMP2 expression or phosphorylation of specific residues, causes axon retraction [59,61]. Thus it was natural to hypothesize that LK (LKE) may functionally enhance CRMP2. Indeed, such hypothesis is corroborated by two findings. First, LK alters the stoichiometry of proteins that co-immunoprecipitate with CRMP2 in pull-down assays despite an apparently lack of effect on CRMP2 phosphorylation status [58]. Second, Holgado’s group has shown that LKE can promote the growth of cholinergic commissures in developing C. elegans nematodes and furthermore that LKE can partially rescue the developmental defect of incomplete commissure development that occurs in Unc-33 (CRMP2) mutant hypomorphs (Table 1) [62]. LKE does not, however, affect the phenotype of Unc-33 complete null mutants [62]. The precise nature of LK (LKE) binding to CRMP2 remains uncertain, but may involve binding to interfaces shared between CRMP2 and its many protein binding partners [59,61]. CRMP2 is an intriguing binding partner for LK (LKE) for another reason: It is becoming associated with a number of diverse nervous system pathologies including schizophrenia; juvenile neuronal ceroid lipofuscinosis (Batten disease); neuropathic pain; stroke; traumatic brain injury (TBI); epilepsy; and Alzheimer’s disease [59,61]. In fact, in Alzheimer’s disease, CRMP2 is very like the more well-known tau protein that forms diagnostic neurofibrillary tangles (NFTs): Both CRMP2 and tau are microtubule-binding proteins whose phosphorylation by GSK3β and Cdk5 leads to microtubule release and subsequent microtubule instability; and both CRMP2 and tau co-aggregate inside the NFTs [discussed in 59,61]. Therefore a small-molecule that functionally affects CRMP2 could be well worth exploring in the context of Alzheimer’s disease therapy development.
Syntaxin binding protein-1 (STXBP1, also known as MUNC-18) is part of the protein complex that regulates synaptic vesicle fusion and exocytosis. As such it is possible that LK (LKE) binding to STXBP1 could affect synaptic transmission, though no research has yet been conducted to explore possible consequences of LK (LKE) interaction with STXBP1 [58].
In the time period while we were documenting cellular activities of LKE and identifying potential protein binding partners, we began testing LKE for effects in preclinical animal models of neurodegeneration. This research was expedited by the fact that LKE is orally bioavailable and penetrates brain tissue readily when administered systemically or by feeding in food formulations [63]. To date, LKE has demonstrated in vivo efficacy in a number of preclinical rodent models (Table 1). Separate studies report LKE ability to (1) Slow disease progression and extend lifespan in the SOD1G93A mouse model of the motor neuron disease, amyotrophic lateral sclerosis (ALS) [5]; (2) Reduce infarct volume and promote functional recovery after permanent middle cerebral artery occlusion in a mouse model of stroke [60]; and (3) Reduce amyloid beta-peptide burden, lower phosphorylated tau accumulation, suppress microglial activation and protect against cognitive deficits in the triple-transgenic (3xTg-AD) mouse model of Alzheimer’s disease [63]. In the 3xTg-AD study, LKE was administered ad libitum, in food, for more than 8 months, without adverse effects, suggesting both safety and practicality of this compound as a potential therapeutic for chronic neurodegenerative pathologies.
Aside from neurodegenerative diseases and injury, LKE has demonstrated some potential to treat brain neoplasias. In collaboration with Dr. Rheal Towner, we found that ad libitum LKE significantly slowed the growth of cortically-implanted C6 glioma cells in a rat xenograft model of glioma as measured by magnetic resonance imaging (MRI) volumetry [64]. This model is limited in relevance because C6 cells are not truly syngenic with any rat strain, such that a therapeutic effect in this model might not necessarily translate into efficacy against a true organic neoplasia. Nonetheless, the finding is remarkable in the field of oncology because most small-molecule chemotherapeutics are highly toxic molecules that kill rapidly-dividing cells (cancer cells) somewhat faster than the drugs harm other cell types. A non-cytotoxic, and even neuroprotective, small molecule that slows glioma growth could revolutionize the way gliomas are perceived and treated if the drug proves itself in other rigorous preclinical and clinical studies.
On the existence of mammalian lanthionine synthase-like proteins (LanCLs); their possible relationship to lanthionine metabolites; and their emerging importance in neurological disease
So far as is known, brain lanthionine is formed mainly through alternative TSP reactions as described above. Prokaryotes, however, synthesize lanthionine bridged peptides via lanthionine cyclase (LanC) enzymes that crosslink Cys and dehydrated Ser / Thr residues in specific antibiotic peptides, to form very potent anti-biotic allelochemicals termed “lantibiotics” [65–66]. Eukaryotes including mammals express homologous proteins, though none of these LanC-like proteins have yet been shown to catalyze lanthionine formation. In 2005 our lab purified brain LanC-like protein-1 (LanCL1) and showed this protein binds both reduced and oxidized glutathione with low micromolar affinity [67]. Subsequently, in collaboration with Dr. Cai Zhang we published the first X-ray crystal structure of GSH complexed to LanCL1 [68]. Curiously, LanCL1 retains structural homology to the active site of prokaryotic LanC enzymes including a central Zn2+ to which GSH appears ligated [68]. Over-expression of LanCL1 promoted nerve growth factor (NGF)-dependent neurite extension in PC12 cells whereas mutants of its SH3-binding domain lacked this ability [68]. To date, we have not been able to demonstrate an enzymatic function of either recombinant LanCL1 or the purified brain protein, though the methods we used may have damaged any inherent enzymatic potential. A very recent study by Huang et al. reports a GST-like activity inherent to a LanCL1 that they engineered [69; discussed further, below].
We have suggested that LanCL1 could, theoretically, catalyze GSH reaction with phospho-serine or phosphatidylserine to yield a glutathione-S-alanine intermediate (glutathione-lanthionine) [5] but empirical evidence for such reaction has been elusive. Such a putative glutathione conjugate could be converted to lanthionine and/or lanthionine ketimine-like metabolites through known enzyme pathways [5].
Alternatively, LanCL1 might bind to or catalyze chemistries involving thioether ketimines. In an unbiased proteomic study, lanthionine ketimine immobilized to a solid-phase support via the C-2 center (modification of which does not affect microglia-suppressing activity; personal observations) was able to bind a small set of brain proteins, specifically and competitively with free LK. Among the binding partners was LanCL1 [58]. That is to say, in an unbiased brain proteome-wide search, LanCL1 emerged as a major binding partner for LK, a finding that suggests that LanCL1 and brain LK may be functionally related. The nature of such a relationship naturally might be that LK is a substrate for LanCL1; a product of LanCL1-dependent catalysis; or an allosteric regulator for LanCL1 activity or binding interactions. Discrimination amongst these possibilities will require more research.
Besides glutathione and lanthionine ketimine, LanCL1 has been identified in several proteomics studies as an interaction partner for a diverse set of proteins (Table 2). Most notably, reported LanCL1 interaction partners include CβS, binding to which may negatively regulate classic transsulfuration in neurons [70; discussed in detail below]. LanCL1 also binds Eps8, an SH3 domain-containing protein involved in formation of Rac-specific guanine nuclecleotide exchange factor signaling complexes and microfilament stabilization [68]; the NAD+-dependent histone deacetylase sirtuin 1 (Sirt1) that is involved with cellular energy sensing and homoestasis [71]; stomatin, a transmembrane protein of uncertain function that is diminished in the erythrocyte hemolytic disease, overhydrated hereditary stomatocytosis [72–73]; and PfSBP1, an integral membrane protein associated with Maurer’s cleft structures in Plasmodium infected red blood cells [74]. In a yeast two-hybrid screen of potential LanCL1-interacting brain proteins, the only strong interactors that we identified for LanCL1 were mitochondrial 28S ribosomal protein S9 and actin-related protein-10 (ACTR10 / HARP11)(KH; personal observations). Our own attempts to purify binding partners using biotinylated, recombinant human LanCL1 as bait have yielded evidence for interaction with β-spectrin; synaptotagmin; adaptin-2 (AP2); oligodendrocyte CNPase; and tripartite motif-containing protein-3 (unpublished observations). It is difficult to find common functional or structural motifs common amongst these LanCL1-binding proteins, except that most of them are cell membrane-localized proteins associated with cytoskeletal dynamics and/or vesicular fusion events. This would be generally consistent with the observations that LanCL1 mutations perturb neuritigenesis in differentiating PC12 cells [68] and that LK derivatives strongly promote neurite development (discussed above).
Table 2.
Published LanCL1-interacting proteins, small molecules, and ligands.
| Protein binding partner | Reference |
|---|---|
| cystathionine β-synthase (CβS) | 70 |
| Eps8 (SH3 domain-containing protein) | 68 |
| sirtuin 1 (SIRT1), NAD+ dependent histone deacetylase | 71 |
| stomatin | 72–73 |
| PfSBP1 | 74 |
LanCL1 has been particularly associated with central nervous system disorders or sensitivity to toxins. For instance LanCL1 is up-regulated several-fold in the spinal cords of the SOD1G93A mouse model of ALS [67], suggesting an association of the protein with this neuropathology. More recently, Jones et al. colleagues identified LanCL1 as a major determinant in mouse strain variation in sensitivity to the Parkinsonian neurotoxin, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) [75] and Huang et al. report that LanCL1 knockout mice suffer severe neurodegeneration in the early adult period [69; discussed further, below].
LanCL1 reportedly binds to and negatively regulates the classic function of CβS in a GSSG/GSH-sensitive fashion, but implications to alternative TSP functions remain unexplored
In a 2012 study Zhong et al. [70] replicated our finding that LanCL1 binds GSH and GSSG. Subsequently these authors described a direct binding interaction between LanCL1 and CβS. The LanCL1:CβS binding was broken by increasing ambient GSSG/GSH above 1/7 which approximates the situation in neurons exposed to subtoxic concentrations of H2O2 [70] Separation of LanCL1 from CβS by modulating [GSSG/GSH] or with LanCL1 decoy peptide resulted in a 3-fold increase of CβS-catalyzed cystathionine formation in vitro, when the enzyme was assayed by a colorimetric technique under conditions specific to this one activity mode (high [Ser] + [hCys]) [70]. Interestingly, RNAi knock-down of LanCL1 or treatment of the cells with Tat-LanCL1 decoy peptides rendered the cells more resistant to H2O2 toxicity [70].
Zhong et al. interpret their data to indicate that increased activity of LanCL1-free CβS facilitates Cys synthesis and hence, GSH synthesis thus boosting antioxidant defense against the oxidative stress. We question this interpretation on several grounds. First, total [GSH + GSSG] is relatively high in brain (40 nmole/mg protein in neurons, or about 2–4 mM; and up to 8 mM in astroglia) [76–77] so that a neuron under oxidative stress doesn’t immediately need more GSH as much as it needs to reduce GSSG back to GSH. Increasing cystathionine would not accomplish this need, and moreover, any increase in [GSH + GSSG] would be slow because three more enzymatic steps lie between cystathionine and GSH. Second, neurons have very low CγL activity, which is the reason these cells must import Cys as cystine from the extracellular space (discussed above); so the cystathionine increase due to freeing CβS from LanCL1 inhibition is unlikely to translate either to increased [Cys] or [GSH]. Third, Zhong et al. do not actually report a change in total glutathione in LanCL1 knock-down cells. These considerations beg the question of whether the redox regulation of CβS via LanCL1 serves another function besides increasing “classic” CβS-catalyzed Cys regeneration to boost [GSH]. We suspect another function would be the allocation of reduced sulfur to LK or cystathionine ketimine products. To date, there has been no experiment reported that probes the effect of changing GSSG/GSH upon the alternative TSP reactions yielding lanthionine or H2S.
Gene knockout studies suggest an essential role for LanCL1 in neuronal antioxidant defense, neuronal survival, and neuroinflammation
Huang et al. have now published a description of the phenotype of mice bearing an engineered deletion of the LanCL1 gene [69]. These mice display spontaneous neuroinflammation, enhanced Iba1+ microglial immunolabeling characteristics, and neurodegeneration at 8–12 weeks of age [69]. Neurodegeneration in these mice is associated with progressive oxidative damage to lipids, proteins, DNA and mitochondria whereas increased LanCL1 expression confers neuroprotection [69]. By 4–8 weeks there was widespread immunoreactivity in cortical regions against 4-hydroxynonenal-protein epitopes, and a general increase in other antioxidant defense enzymes including SOD1 and catalase [69]. Moreover, neurons lacking LanCL1 had a diminished response to neurotrophic factors EGF and BDNF [69]. These observations affirm our own published findings that LanCL1 strongly modulates growth factor-dependent neurite outgrowth [68]. Huang et al. attribute the neuroprotective role of LanCL1 to a GST-like activity and report a Km of 2 mM for the substrate CDNB (chloro-dinitrobenzoic acid) which is quite similar to that of glutathione-S-transferase isoform P1 [69]. Other, indirect mechanisms of neuroprotection by LanCL1 through either its yet-undiscovered enzymology or its protein interactome cannot be ruled out at this time. If the findings reported by Huang et al. can be reproduced and extended, LanCL1 will assume an important place amidst the pantheon of critical antioxidant defense enzymes.
Recent discoveries point to a role for LanCL2 in fundamental cellular processes of nutrient sensing and adaptive response
Whereas LanCL1 is highly expressed in the central nervous system, a slightly longer LanCL2 homolog with an N-terminal myristoylation sequence is expressed at relatively higher levels outside the CNS [78–81]. LanCL2 also binds glutathione [68] but has been implicated primarily in the binding of absiscic acid, a plant hormone that may well be synthesized by mammals as well [82–83]. Research by Sturla’s group and independent work by Bassaganya-Riera and colleagues indicates that LanCL2 lies upstream in an absiscic acid-dependent pathway that regulates peroxisome proliferator-activated receptor-gamma (PPARγ) [79–81]. As such LanCL2 manipulation is being researched in the hopes of treating disorders including diabetes and certain types of infections [78–81]. Also, LanCL2 expression has been found to increase cell sensitivity to the chemotherapeutic adriamycin, apparently by regulating the expression of P-glycoprotein / MDR1, suggesting a possible role of this homolog in the detoxification or excretion of xenobiotics [84].
Most recently Zeng, van der Donk and Chen report that LanCL2 positively regulates Akt (protein kinase B) in liver cells as LanCL2 knock-down reduces cellular response to serum or insulin-stimulated Akt phosphorylation whereas over-expressing LanCL2 increases Akt sensitivity to these stimuli [85]. Apparently LanCL2 regulates Akt by promoting mTOR (mammalian target of rapamycin) complex 2 (mTORC2)-mediated Akt phosphorylation, with downstream effects on cell survival decisions [85]. The redox biological implications of LanCL2 interaction with the mTORC2→Akt axis remain to be investigated. Likewise, it remains to be seen whether LanCL1 performs a similar function in the central nervous system. Because mTORC2 (which regulates fundamentally anabolic cell growth/differentiation/survival decisions) intimately and reciprocally works with mTORC1 (which regulates the fundamental catabolic process of autophagy) [86], the possibility that LanCL1/2 and lanthionine metabolites also play a natural regulatory role in autophagy must not be overlooked. We are actively investigating this possibility.
Mammals also possess a truncated LanCL3 sequence but there is yet no evidence for expression or function suggesting this is probably a pseudogene.
Might alternative TSP functions suggest new hypotheses for hCys neurotoxicity and epidemiological association with neurodisease?
High circulating hCys is a well-accepted correlate to a variety of age-related problems including arteriosclerotic vascular disease, stroke, left ventricular hypertrophy, dementia and Alzheimer’s disease [10–12,87]. In general, circulating hCys increases with age, possibly due to age-related deficiency in uptake and utilization of B-complex vitamins [10,88–89]. At the moment it is not established whether high hCys is a contributor to particular age-related pathologies, or is merely a marker. In some cases high hCys may well be an epiphenomenon; for instance, hCys may become elevated secondary to kidney disease that is often integral to atherosclerotic disease [10]. In terms of possible metabolic explanations for elevated hCys, one can plausibly invoke either deficient remethylation by folic acid cycle (largely driven by vitamin B12-dependent enzymology of methionine synthase and subsequent action of methylene tetrahydrofolate reductase or MTFR) (Fig. 1); or deficiencies in vitamin B6-dependent action of TSP enzymes [12]. Deficient remethylation would prevent efficient conversion of hCys back to methionine, whereas suboptimal TSP function would diminish sulfur flow from hCys towards cysteine, lanthionine, and H2S (Figs. 1–2). Indeed, although elevated hCys is most often discussed in relationship to genetic variation in MTFR and the folate cycle, it is worth remembering that the first documentation of severe hyperhomocysteinemia-associated vascular pathology was a patient with genetic CβS deficiency [90]. Although quite rare, genetic CβS deficiency causes severe mental retardation, cerebral atrophy, and seizures though not all the pathology can be reasonably ascribed to the hyperhomocysteinemia [91].
Current hypotheses for mechanisms through which hCys might contribute to age-related cognitive impairment or neural disease, usually invoke the action of hCys as an allosteric agonist for N-methyl-D-aspartate (NMDA) receptors. Chronic over-stimulation of these ionotropic glutamate receptors could stimulate excessive intraneural Ca2+ accumulation with negative downstream consequences, including prominent oxidative stress phenomena [92]. Other mechanisms have been proposed whereby alterations in the ratio of S-adenosyl methionine/S-adenosyl homocysteine (SAM / SAH) may competitively impair SAM-dependent methylation of protein phosphatase-2A, with downstream consequences to increase phosphorylated tau protein, thus destabilizing microtubules and promoting formation of neurofibrillary tangles [93]. Certainly the topic of hCys neurotoxicity is still open to debate and research.
New hypotheses for hCys toxicity might be offered which invoke alternative action of brain TSP. For instance, H2S is a direct antioxidant but also increases astrocytic glutamate and stimulates microvessel dilation through sulfhydration and allosteric modulation of ATP-sensitive K+ channels [92]. However, excessive H2S has been linked to neuronal Ca2+ overload via protein kinase A (PKA-dependent) mechanisms [94–95]. Likewise, exogenous H2S increased intracellular Ca2+ in microglia [96]. Therefore a balance likely exists between whereas inhibition of microglial CγL lowered [Ca2+]in H2S production and utilization such that decrease or increase of steady-state H2S beyond optimal limits could impair neural function or even cause harm. At least theoretically, increased hCys could affect sulfur flow through the TSP by altering substrate availability to CβS and CγL. Depending on the relative local concentrations of Cys, Ser and hCys, high hCys would tend to favor H2S production by the TSP enzymes and possibly shift the balance of H2S production from CβS to CγL [4] (Fig. 2). Simultaneously, oxidative stress downstream from hCys-mediated NMDA receptor dysfunction could affect redox-dependent CβS regulation. The consequences of elevated hCys on alternative TSP-mediated H2S production, if any, remain unexplored but the possibility of a detrimental effect on neural health should be considered.
Elevated hCys could have profound consequences on lanthionine flux as well. As described above, Lan forms from CβS-catalyzed reaction between Cys and Ser. An increase in the ratio of hCys / Cys would be expected to favor formation of cystathionine (CβS chemistry) and homolanthionine (CγL chemistry) rather than lanthionine. Consequently, hCys elevation would probably reduce lanthionine ketimine production by GTK/KAT1. Although cystathionine can be converted to a cyclic ketimine by GTK [41–43,46], the neurochemical functions (if any) of CK are completely unexplored. Similarly, there is currently no knowledge regarding biological consequences of increased homolanthionine (Fig. 2). The degree to which alterations in endogenous cyclic thioether ketimines might affect brain cells remains a subject of speculation; however, given the potency and range of neuroprotective activities recently documented for lanthionine ketimine derivatives, this is a subject worthy of consideration and investigation.
Summary
Despite the wide acceptance that brain sulfur biochemistry is essential to the healthy function of neural tissue, there remains much to be understood about the origin and activities inherent to low-abundance metabolites stemming from non-canonical reactions of brain transsulfuration pathway enzymes. Recent discoveries that TSP-derived H2S is a functional neural gasotransmitter, combined with revelations that lanthionine metabolites are potently neurotrophic, mandate a serious reconsideration of this heretofore neglected topic in neurochemistry. Moreover the discovery that mammalian lanthionine synthase-like protein-1 is an essential antioxidant factor, strongly compels further research into the natural origin and physiological role of these molecules. In the event that alternative TSP products or their analogs formed through LanCL1-dependent chemistries do prove to be natural neural regulatory molecules, whole new paradigms will have to be constructed to integrate these species into our current understanding of brain health and neurodegenerative disease etiology. Regardless, an improved understanding of brain sulfur neurochemistry could prove of immense value in developing conceptually new strategies to combat neurodisease.
HIGHLIGHTS.
The mammalian transsulfuration pathway (TSP) can produce alternative metabolites.
Standard and alternative TSP reactions are subject to redox control.
Besides the usual cystathionine product, the TSP can make H2S and lanthionine.
H2S and further metabolites of lanthionine are important neuromodulators.
Glutathione and lanthionine metabolites are related via binding to LanCL proteins.
LanCL1 and -2 play roles in neural redox regulation, cell fate and autophagy pathways.
Acknowledgments
This work was supported in part by a University of Toledo Foundation Biomedical Innovation Award (KH); the Muscular Dystrophy Association (MDA217526; KH); the National Institutes of Health (NS082283; KH); and the Washington State University-Spokane Seed Grant Program (TTD). KH is the inventor on U.S. patent 7,683,055 covering composition and use of lanthionine ketimine derivatives including lanthionine ketimine-ester (LKE) and is co-founder of a company engaged in commercial development of the technology.
Abbreviations
- AECK
aminoethylcysteine ketimine
- AECK-DD
aminoethylcysteine ketimine decarboxylated dimer
- CβS
cystathionine-β-synthase; L-serine hydro-lyase
- CγL
cystathionine-γ-lyase; L-cystathionine cysteine-lyase; CSE
- CRMP2
collapsin response mediator protein-2; CRMP2; dihydropyrimidinase-like protein-2; DPYSL2
- DRG
dorsal root ganglia
- GTK / KAT
glutamine transaminase-K (GTK); kynurenine aminotransferase
- hCys
homocysteine
- KYNA
kynurenic acid
- Lan
lanthionine
- LK
lanthionine ketimine
- LK-DD
lanthionine ketimine decarboxylated dimer
- LKE
lanthionine ketimine-ethyl ester
- LanCL1
lanthionine synthetase-like protein-1
- LanCL2
lanthionine synthetase-like protein-2
- mTOR
mammalian target of rapamycin
- mTORC2
mTOR-complex-2
- PLP
pyridoxal 5′-phosphate
- TK
thialysine ketimine
- TK-DD
thialysine ketimine decarboxylated dimer
- TSP
transsulfuration pathway
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth Hensley, Email: Kenneth.Hensley@utoledo.edu, Associate Professor, Department of Pathology and Department of Neurosciences, University of Toledo Health Science Campus, 3000 Arlington Avenue, Toledo OH 43614, Tel. 419-383-3442.
Travis T. Denton, Email: Travis.Denton@WSU.edu, Assistant Professor, Department of Pharmaceutical Sciences, Washington State University College of Pharmacy, P.O. Box 1495, Spokane, WA 99201, Tel. 509-368-6624.
References
- 1.Stryer L. Biochemistry. 3. 1988. [Google Scholar]
- 2.Dominy JE, Stipanuk MH. New roles for cysteine and transsulfuration enzymes: Production of H2S, A neuromodulator and smooth muscle relaxant. Nutr Review. 2004;62:348–353. doi: 10.1111/j.1753-4887.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochimica Biophysica Acta. 2014;1844:1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley K. Emerging Biological Importance of Central Nervous System Lanthionines. Molecules. 2010;15:5581–5594. doi: 10.3390/molecules15085581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Dinesh Kumar S, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, Moore PK. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5448. Epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabil H, Kabil O, Banerjee R, Harshman LG, Pletcher SD. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc Natl Acad Sci USA. 2011;108:16831–16836. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uthus EO, Bron-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albertini E, Koziel R, Durr A, Neuhaus M, Jansen-Durr P. Cystathionine beta synthase modulates senescence of human endothelial cells. Aging (Albany NY) 2012;4:664–673. doi: 10.18632/aging.100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selhub J, Troen A, Rosenberg IH. B vitamins and the aging brain. Nutr Rev. 2010;68(Suppl 2):S112–118. doi: 10.1111/j.1753-4887.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 12.Selhub J. The many facets of hyperhomocysteinemia: Studies from the Framingham cohorts. J Nutr. 2006;136:1726S–1730S. doi: 10.1093/jn/136.6.1726S. [DOI] [PubMed] [Google Scholar]
- 13.Aitken SM, Kirsch JF. The enzymology of cystathionine biosynthesis: Strategies for the control of substrate and reaction specificity. Arch Biochem Biophys. 2005;433:166–175. doi: 10.1016/j.abb.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Seiflein TA, Lawrence JG. Two transsulfuration pathways in Klebsiella pneumoniae. J Bacteriol. 2006;188:5762–5774. doi: 10.1128/JB.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler B. Homocysteine: Overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med. 2005;5:77–86. doi: 10.1055/s-2005-872394. [DOI] [PubMed] [Google Scholar]
- 16.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 18.Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 19.McBean GJ. The transsulfuration pathway: A source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 20.Taoka S, West M, Banerjee R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine beta-synthase reveals nonequivalent active sites. Biochemistry. 1999;38:2738–2744. doi: 10.1021/bi9826052. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee R, Evande R, Kabil O, Ojha S, Taoka S. Reaction mechanism and regulation of cystathione beta-synthase. Biochem Biophys Acta. 2003;1647:30–35. doi: 10.1016/s1570-9639(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee R, Zou CG. Redox regulation and reaction mechanism of human cystathionine-beta synthase: A PLP-dependent hemesensor protein. Arch Biochem Biophys. 2005;433:144–156. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Carballal S, Cuevasanta E, Marmisolle I, Kabil O, Gherasim C, Ballou DP, Banerjee R, Alvarez B. Kinetics of reversible reductive carbonylation of heme in human cystathionine β-synthase. Biochemistry. 52:4553–4562. doi: 10.1021/bi4004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallan HH, Moore S, Stein WH. L-cystathionine in human brain. Biol Chem. 230:707–716. [PubMed] [Google Scholar]
- 26.Martin JA, Pereda J, Martinez-Lopez I, Escrig R, Miralles V, Pallardo FV, Vento M, Vina J, Sastrea J. Oxidative stress is a signal to up-regulate gamma cystathionase in the fetal-to-neonatal transition in rats. Cell Mol Biol. 2007;53(Suppl):OL1010–OL1014. [PubMed] [Google Scholar]
- 27.Hwang SY, Sarna LK, Siow YL, Karmin O. High-fat diet stimulates hepatic cystathionine β-synthase and cystathionine γ-lyase expression. Can J Physiol Pharmacol. 2013;91:913–919. doi: 10.1139/cjpp-2013-0106. [DOI] [PubMed] [Google Scholar]
- 28.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulfide in the cardiovascular system and in inflammation – a tale of three gases! Pharmacol Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kimura H. Hydrogen sulfide: Its production, release and function. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 31.Krejsa CM, Franklin CC, White CC, Ledbetter JA, Schieven GL, Kavanagh TJ. Rapid activation of glutamate cysteine ligase following oxidative stress. J Biol Chem. 2010;285:16116–16124. doi: 10.1074/jbc.M110.116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabil O, Zhou Y, Banerjee R. Human cystathionine β-synthase is a target for sumoylation. Biochemistry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- 33.Jhee K-H, Kruger WD. The role of cystathionine β-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- 34.Paul BD, Snyder SH. H2S signaling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Lancaster JR., Jr Chemical foundation of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int. 2014;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Toohey JI, Cooper AJL. Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules. 2014;19:12789–12813. doi: 10.3390/molecules190812789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braunstein AE, Goryachenkova EV. The beta-replacement-specific pyridoxal-P-dependent lyases. Adv Enzymol Relat Areas Mol Biol. 1984;56:1–89. doi: 10.1002/9780470123027.ch1. [DOI] [PubMed] [Google Scholar]
- 39.Townsend DM, Lushchak VI, Cooper AJ. A comparison of reversible versus irreversible protein glutathionylation. Adv Cancer Res. 2014;122:177–198. doi: 10.1016/B978-0-12-420117-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavallini D, Ricci G, Federri G. The ketimine derivatives of thialysine, lanthionine, cystathionine, cysteine: Preparation and properties. In: Alan R, editor. Sulfur Amino Acids: Biochemical and Clinical Aspects. Liss Inc; NY, NY, USA: 1983. pp. 355–364. [PubMed] [Google Scholar]
- 41.Cavallini D, Ricci G, Dupre S, Pecci L, Costa M, Matarese RM, Pensa B, Antonuci A, Solinas SP, Fontana M. Sulfur-containing cyclic ketimines and imino acids. A novel family of endogenous products in search for a role. Eur J Biochem. 1991;202:217–223. doi: 10.1111/j.1432-1033.1991.tb16365.x. [DOI] [PubMed] [Google Scholar]
- 42.Cooper AJ, Anders MW. Glutamine transaminase K and cysteine conjugate β-lyase. Ann NY Acad Sci. 1990;585:118–127. doi: 10.1111/j.1749-6632.1990.tb28048.x. [DOI] [PubMed] [Google Scholar]
- 43.Cooper AJL. The role of glutamine transaminase K (GTK) in sulfur and alpha-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants. Neurochem Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Ricci G, Vesci L, Nardini M, Arduini A, Storto S, Rosato N, Cavallini D. Detection of 2H-1,4-thiazine-5,6-dihydro-3,5-dicarboxylic acid (lanthionine ketimine) in the bovine brain by a fluorometric assay. Biochim Biophys Acta. 1989;990:211–215. doi: 10.1016/s0304-4165(89)80036-4. [DOI] [PubMed] [Google Scholar]
- 45.Fontana M, Ricci G, Solinas SP, Antonucci A, Serao I, Dupre S, Cavallini D. [35S]Lanthionine ketimine binding to bovine brain membranes. Biochem Biophys Res Commun. 1990;171:480–486. doi: 10.1016/0006-291x(90)91418-r. [DOI] [PubMed] [Google Scholar]
- 46.Fontana M, Brunori A, Costa M, Antonucci A. Detection of cystathionine ketimine and lanthionine ketimine in human brain. Neurochem Res. 1997;22:821–844. doi: 10.1023/a:1022083809994. [DOI] [PubMed] [Google Scholar]
- 47.Moroni F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 48.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: When physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malherbe P, Alberati-Giani D, Kohler C, Cesura AM. Identification of a mitochondrial form of kynurenine aminotransferase / glutamine transaminase K from rat brain. FEBS Lett. 1995;367:141–144. doi: 10.1016/0014-5793(95)00546-l. [DOI] [PubMed] [Google Scholar]
- 50.Pinto JT, Krasnikov BF, Alcutt S, Jones ME, Dorai T, Villar MT, Artigues A, Li J, Cooper AJ. Kynurenine aminotransferase III and glutamine transaminase L are identical enzymes that have cysteine S-conjugate β-lyase activity and can transaminate L-selenomethionine. J Biol Chem. 2014 Sep 17; doi: 10.1074/jbc.M114.591461. pii: jbc.M114.591461. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macone A, Matarese RM, Gentili V, Antonucci A, Dupre S, Nardini M. Effect of aminoethylcysteine ketimine decarboxylated dimer, a natural sulfur compound present in human plasma, on tert-butyl hydroperoxide-induced oxidative stress in human monocytic U937 cells. Free Radical Res. 2004;38:704–714. doi: 10.1080/10715760410001705159. [DOI] [PubMed] [Google Scholar]
- 52.Macone A, Fontana M, Barba M, Botta B, Nardini M, Girga Fp, Calcaterra A, Laura P, Matarese RM. Antioxidant properties of aminoethylcysteine ketimine decarboxylated dimer: A review. Int J Mol Sci. 2012;12:3072–3084. doi: 10.3390/ijms12053072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsikas D, Evans CE, Denton TT, Mitschke A, Gutzki F-M, Pinto JT, Khomenko T, Szabo S, Cooper AJL. Stable-isotope GC-MS/MS determination of aminoethylcysteine ketimine decarboxylated dimer in biological samples. Anal Biochem. 2012;430:4–15. doi: 10.1016/j.ab.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leeson PD, Baker R, Carling RW, Curtis NR, Moore KW, Williams BJ, Foster AC, Donald AE, Kemp JA, Marshall GR. Kynurenic acid derivatives. Structure-activity relationships for excitatory amino acid antagonism and identification of potent and selective antagonists at the glycine site on the N-methyl-D-aspartate receptor. J Med Chem. 1991;34:1243–1252. doi: 10.1021/jm00108a002. [DOI] [PubMed] [Google Scholar]
- 55.Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp Neurol. 2005;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:121–127. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, Post J. Proteomic Identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J Neurosci. 2010;30:2979–2988. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hensley K, Christov A, Venkova K, Gunning W, Park J. Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- 60.Nada SE, Raghava A, Tulsulkar J, Hensley K, Shah ZA. A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem Int. 2012;61:1357–1363. doi: 10.1016/j.neuint.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khanna R, Wilson SM, Brittain JM, Weimer J, Sultana R, Butterfield DA, Hensley K. Opening Pandora’s jar: A primer on the putative roles of collapsin response mediator protein 2 (CRMP2) in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurology. 2012;7:749–771. doi: 10.2217/FNL.12.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubbard C, Benda E, Hardin T, Baxter T, StJohn E, O’Brien S, Hensley K, Holgado A. Lanthionine ketimine ethyl ester partially rescues neuro-developmental defects in unc-33 (DPYSL2/CRMP2) mutants. J Neurosci Res. 2013;91:1183–1190. doi: 10.1002/jnr.23239. [DOI] [PubMed] [Google Scholar]
- 63.Hensley K, Venkova K, Christov A, Johnson M, Eslami P, Gabbitta SP, Harris-White M. A derivative of the brain metabolite lanthionine ketimine improves cognition and diminishes pathology in the 3xTg-AD mouse model of Alzheimer’s disease. J Neuropath Exp Neurol. 2013;72:955–969. doi: 10.1097/NEN.0b013e3182a74372. [DOI] [PubMed] [Google Scholar]
- 64.Floyd R, Castro HC, Neto F, Zimmerman GA, Hensley K, Towner R. Nitrone-based therapeutics for neurodegenerative diseases. Their use alone or in combination with lanthionines. Free Rad Biol Med. 2013;51:931–941. doi: 10.1016/j.freeradbiomed.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chatterjee C, Paul M, Xie L, van der Donk WA. Biosynthesis and mode of action of lantibiotics. Chem Rev. 2005;105:633–683. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 66.Willey JM, Van der Donk WA. Lanthionines: Peptides of diverse structure and function. Ann Rev Microbiol. 2007;61:47–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- 67.Chung C, Kurien BT, Mehta P, Mhatre MC, Mou S, Pye QN, Stewart CA, West MS, Williamson KS, Post J, Liu L, Wang R, Hensley K. Identification of lanthionine synthase C-like protein 1 (LanCL1) as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochem. 2007;46:3262–3269. doi: 10.1021/bi061888s. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Wang L, Liu Y, Xu J, Zhu G, Cang H, Li X, Bartlam M, Hensley K, Li G, Rao Z, Zhang X. Structure of human lanthionine synthetase C-like protein-1 and its interaction with Eps8 and glutathione. Genes Development. 2009;23:1387–1392. doi: 10.1101/gad.1789209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C, Chen M, Pang D, Bi D, Zou Y, Zia X, Yang W, Luo L, Deng R, Tan H, Zhou L, Yu S, Guo L, Du X, Cui Y, Hu J, Mao Q, Worley PF, Xiao B. Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required for neuronal survival. Developmental Cell. 30:479–487. doi: 10.1016/j.devcel.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong WX, Wang YB, Peng L, Ge XZ, Zhang J, Liu SS, Zhang XN, Zu ZH, Chen Z, Luo JH. Lanthionine synthetase C-like protein 1 interacts with and inhibits cystathionine β-synthase: a target for neuronal antioxidant defense. J Biol Chem. 2012;287:34189–34201. doi: 10.1074/jbc.M112.383646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Law IK, Liu L, Xu A, Lam KS, Vanhoutte PM, Che CM, Leung PT, Wang Y. Identification and characterization of proteins interacting with SIRT1 and SIRT3: Implications in the anti-aging and metabolic effects of sirtuins. Proteomics. 2009;9:2444–2456. doi: 10.1002/pmic.200800738. [DOI] [PubMed] [Google Scholar]
- 72.Mayer H, Salzer U, Breuss J, Ziegler S, Marchler-Bauer A, Prohaska R. Isolation, molecular characterization, and tissue-specific expression of a novel putative G protein-coupled receptor. Biochim Biophys Acta. 1998;1395:301–308. doi: 10.1016/s0167-4781(97)00178-4. [DOI] [PubMed] [Google Scholar]
- 73.Mayer H, Bauer H, Breuss J, Ziegler S, Prohaska R. Characterization of rat LanCL1, a novel member of the lanthionine synthase C-like protein family, highly expressed in testis and brain. Gene. 2001;269:73–80. doi: 10.1016/s0378-1119(01)00463-2. [DOI] [PubMed] [Google Scholar]
- 74.Blisnick T, Vincensin L, Barale JC, Namane A, Braun BC. LanCL1, an erythrocyte protein recruited to the Maurer’s clefts during plasmodium falciparum development. Mol. Biochem Parisitol. 2005;141:39–47. doi: 10.1016/j.molbiopara.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Jones BC, Miller DB, O’Callaghan JP, Lu L, Unger EL, Alam G, Williams RW. Systems analysis of genetic variation in MPTP neurotoxicity in mice. Neurotoxicology. 2013;27:26–34. doi: 10.1016/j.neuro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 77.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 78.Langlinger C, Salzer U, Prohaska R. Myristoylation of human LanC-like protein-2 (LANCL2) is essential for the interaction with the plasma membrane and the increase in cellular sensitivity to Adriamycin. Biochim Biophys Acta. 2006;1758:1759–1767. doi: 10.1016/j.bbamem.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Lu P, Bevan DR, Lewis SN, Hontecillas R, Bassaganya-Riera J. Molecular modeling of lanthionine synthetase component C-like protein 2: a potential target for the discovery of novel type 2 diabetes prophylactics and therapeutics. J Mol Model. 2011;17:543–553. doi: 10.1007/s00894-010-0748-y. [DOI] [PubMed] [Google Scholar]
- 80.Bassaganya-Riera J, Guri AJ, Lu P, Climent M, Carbo A, Sobral BW, Horne WT, Lewis SN, Bevan DR, Hontecillas R. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2011;286:2505–2516. doi: 10.1074/jbc.M110.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu P, Hontecillas R, Horne WT, Carbo A, Viladomiu M, Pedragosa M, Bevan DR, Lewis SN, Bassaganya-Riera J. Computational modeling-based discovery of novel classes of anti-inflammatory drugs that target lanthionine synthetase C-like protein-2. PLoS One. 7(4):e34643. doi: 10.1371/journal.pone.0034643. Epub 2012 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sturla L, Fresia C, Guida L, Bruzzone S, Scarfi S, Usai C, Fruscione F, Magnone M, Millo E, Basile G, Grozio A, Jacchetti E, Allegretti M, De Flora A, Zocci E. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J Biol Chem. 2009;284:28045–28057. doi: 10.1074/jbc.M109.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sturla L, Fresia C, Guida L, Grozio A, Vigliarola T, Mannino E, Millo E, Bagnasco L, Bruzzone S, De Flora A, Zocci E. Binding of abscisic acid to human LANCL2. Biochem Biophys Res Commun. 2011;415:390–395. doi: 10.1016/j.bbrc.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 84.Park S, James CD. Lanthionine synthetase components C-like 2 increases cellular sensitivity to adriamycin by decreasing the expression of P-glycoprotein through a transcription-mediated mechanism. Cancer Res. 2003;63:723–727. [PubMed] [Google Scholar]
- 85.Zeng M, van der Donk WA, Chen J. Lantibiotic cyclase-like protein 2 (LanCL2) is a novel regulator of Akt. Mol Biol Cell. 2014 Oct 1; doi: 10.1091/mbc.E14-01-0004. pii: mbc.E14-01-0004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vadlakona L, Dash A, Pasupuleti M, Anil Kumar K, Reddanna P. The Paradox of Akt-mTOR interactions. Front Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 88.Hermann W, Quast S, Ullrich M, Schultze H, Bodis M, Geisel J. Hyperhomocysteinemia in high-aged subjects: relation of B-vitamins, folic acid, renal function and the methylenetetrahydrofolate reductase mutation. Atherosclerosis. 144:91–101. doi: 10.1016/s0021-9150(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 89.Obeid R, Schorr H, Eckert R, Hermann W. Vitamin B12 status in the elderly as judged by available biochemical markers. Clin Chem. 2004;50:238–241. doi: 10.1373/clinchem.2003.021717. [DOI] [PubMed] [Google Scholar]
- 90.McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 91.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wicken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Gnet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 92.Obeid R, Hermann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 93.Vafai SB, Stock JB. Protein phosphatase 2A methylation: A link between elevated plasma homocysteine and Alzheimer’s disease. FEBS Lett. 2002;518:1–4. doi: 10.1016/s0014-5793(02)02702-3. [DOI] [PubMed] [Google Scholar]
- 94.Wang JF, Li Y, Song JN, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Bereguiain MA, Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxid Redox Signal. 2008;10:31–42. doi: 10.1089/ars.2007.1656. [DOI] [PubMed] [Google Scholar]
- 96.Lee SW, Hu YS, Hu LF, Dawe GS, Moore PK, Wong PT, Blan JS. Hydrogen sulfide regulates calcium homeostasis in microglial cells. Glia. 2006;54:116–124. doi: 10.1002/glia.20362. [DOI] [PubMed] [Google Scholar]